Abstract
In the general population aortic stiffening, assessed by carotid femoral pulse wave velocity (cf‐PWV), is associated with cognitive dysfunction (CO/DY). Data in chronic kidney disease (CKD) are limited. This study tests the hypothesis that large artery stiffness and microvascular damage in CKD patients are related to the damage of brain microcirculation reflected by impaired cognitive function. A cross‐sectional study enrolled 151 patients (mean age 58.4 years; 64.5% males; 44 patients with CKD stage 1; 47 with stage 2; 25 with stage 3; and 35 with stage 4). Cognitive impairment, assessed by the Mini Mental State Examination (MMSE), the Clock – drawing test (Clock‐test), and the Instrumental Activity of Daily Living (IADL), was considered as primary outcome. We measured systolic and pulse pressures at the brachial and aortic sites and cf‐PWV. Our patients revealed a significant linear deterioration in all the domains of cognitive function according to CKD stages. High values of cf‐PWV (P = 0.029) and aortic pulse pressure (aPP) (P < 0.026) were independent determinants of cognitive decline assessed by the MMSE. The present trial supports the hypothesis of an interaction between the kidney, large artery damage, central pressure pulsatility, and the injury of brain microcirculation. In clinical practice, cf‐PWV and aPP measurements may help to predict cognitive decline. Whether the reduction in aortic stiffness following an aggressive treatment translates into improved cognitive outcomes remains to be determined.
Keywords: arterial stiffness, chronic kidney disease, cognitive dysfunction
1. INTRODUCTION
The kidney and the brain are affected in parallel during aging. Both organs are affected by similar aggressions through classical cardiovascular risk factors such as hypertension or diabetes mellitus (DM) but also through numerous other mechanisms such as inflammation, uremia, anemia, and oxidative stress. All of these mechanisms may be involved in both kidney injury and damage of the brain microcirculation, the later resulting in cognitive dysfunction (CO/DY).1, 2, 3, 4 Cognitive dysfunction in chronic kidney disease (CKD) patients is gaining widespread scientific interest. The prevalence of moderate‐to‐severe CO/DY is more than double compared to the general population; indeed up to 70% of hemodialysis patients aged ≥ 55 years old have moderate‐to‐severe cognitive impairment,5 with severe economic consequences such as increased cost of care, increased rate of hospitalization, and mortality.6 Thus, according to the current evidence and medical guidelines,7 detection and prevention of CO/DY in CKD are pivotal.
The hallmark of vascular aging is an increase in aortic stiffness, assessed by an increase in carotid‐femoral pulse wave velocity (cf‐PWV).8, 9, 10 Carotid‐femoral PWV is a major determinant of high central blood pressure and wave reflection. Large arteries are highly sensitive to early aging11 and their alteration is already linked with either CO/DY and CKD.12, 13 On the other hand, brain injury and related brain pathologies (cerebral stroke and cognitive decline) are associated with arterial stiffness.10, 14, 15, 16 Because increased central pulse pressure and wave reflections are considered the main cause of brain and kidney dysfunction, it is likely that these phenomena are emphasized in CKD patients.
Moreover, this may be of particular importance for kidney injury progression, because passive renal perfusion along with low resistance and input impedance in renal microvessels make kidneys particularly vulnerable to the damaging effect of systemic pulsatile pressure.17 However, data supporting the association between CO/DY and arterial stiffness in CKD patients are limited. Our purpose was to study the association between cognitive function assessed in different dimensions (praxis, memory, orientation, and executive function) and several indices of arterial stiffness in a cross‐sectional study in middle‐aged CKD population.
2. MATERIAL AND METHODS
2.1. Demographic data
Classification of CKD stages 1‐4 was done according to KDOQI (Kidney Disease Outcomes Quality Initiative classification stages 1‐4).6 Patients followed in the outpatient clinic of the nephrology department were eligible for the study if they presented stage 1‐4 CKD and no overt dementia or cardiovascular disease (stroke or myocardium infraction). Specific exclusion criteria for CO/DY assessment were (1) diagnosis of depression or delirium according to the history and neuropsychological tests, (2) history of prior stroke or transient ischemic attack documented in the medical chart, and (3) low hemoglobin (Hb) level <10 g/dL. Among the 244 patients screened for the study, 93 were excluded: 45 because of missing data of cf‐PWV or incomplete measurements values and 48 for missing data concerning the totality of cognitive tests. Finally, this study was composed of 151 patients (44 with CKD stage 1; 47 with stage 2; 25 with stage 3; and 35 with stage 4) with mean age 58.4 years, 64.5% being males. (Figure 1) The underlying diseases were glomerulonephritis 27%, diabetic nephropathy 16.5%, hypertensive nephropathy 23%, interstitial nephropathy 8%, polycystic disease 2.6%, and unknown 33%.
Figure 1.
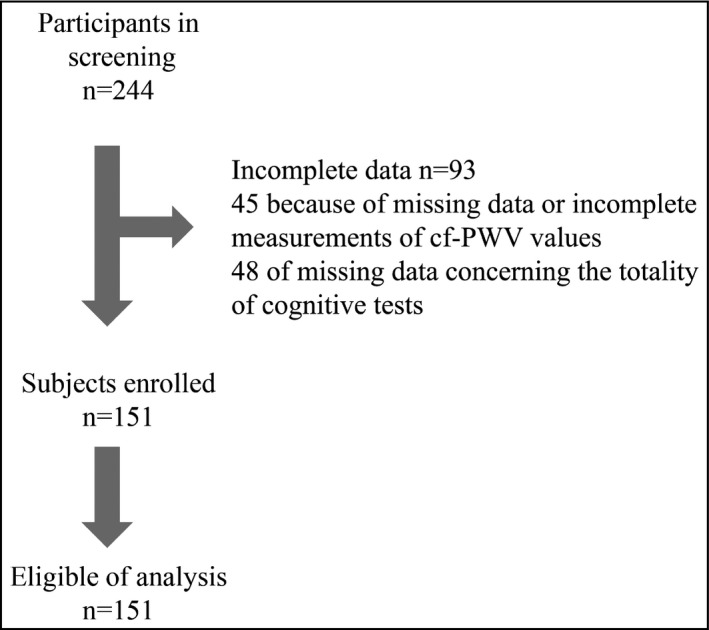
Flow diagram of patient enrollment
Demographic data were collected during a consultation. We recorded main demographic and anthropometric data, synthesized medical records, and usual blood tests during standardized workups. Blood pressure was measured at the brachial site (bSBP, bDBP, bMBP, bPP for systolic, diastolic, mean, and pulse pressures, respectively) using an automated sphygmomanometer (Omron Digital Blood Pressure Monitor 705‐IT) device. Blood pressure measurements were taken after a 5‐minute rest in a setting position. The mean values of the last 2 recordings for both brachial SBP and DBP were used for calculation.
Other cardiovascular risk factors such as history of diabetes mellitus (DM) and cardiovascular disease (CVD) were recorded. Finally, educational level was categorized as lower vs higher education, where the former refers to secondary and the latter to tertiary education.
2.2. Cognitive function assessment
Cognitive function was estimated by using 6 questionnaires, standardized for the general population of the country. All the participants in the study spoke the Greek language. Exclusion criteria were (1) diagnosis of depression or delirium according to the history and neuropsychological tests, (2) history of prior stroke or transient ischemic attack documented in the medical chart, and (3) low Hb level <10 g/dL. Three of these questionnaires, namely the Geriatric Depression Scale (GDS),18 the Neuropsychiatric Inventory‐Clinical rating scale (NPI‐C),19 and the Abbreviated Mental Test Score (AMTS),20 were used to assess the patients’ psychological status and to detect depression. These tests were not used in the statistical analysis. Patients who tested positive on one of these tests were excluded from the study because of prevalent neuropsychological disorders. For the global cognitive function, we used the Mini Mental State Examination (MMSE),21 range of 0 (worst score) to 30 (best score). Administration by a trained interviewer took approximately 10 minutes. For the assessment of executive and visual function we used the Clock‐test.22 Clock‐test is generally considered as an inexpensive, fast, qualitative tool for identifying dementia in clinical practice. The scale assigns up to 7 points based on 3 categories: time (3 points), numbers (2 points), and spacing (2 points) with a score of 7 being perfect. For the executive function, we used the Instrumental Activity of Daily Living (IADL) test.23 Questionnaires were administered by the same physician trained in psychological issues (D.K).
2.3. Arterial stiffness assessment
All patients were studied in a quiet room with controlled temperature as described previously.24 Brachial blood pressure was measured by an automated sphygmomanometer every 5 minutes (Omron Digital Blood Pressure Monitor 705‐IT). The mean values of the last 3 recording measurements were used for the analysis. Aortic pulse wave velocity was determined from carotid and femoral pressure waveforms obtained noninvasively by applanation tonometry (Millar tonometer; Millar Instruments, Houston, TX) using the Sphygmocor system (Atcor, Sydney, New South Wales, Australia). Details on pulse wave analysis are provided in Data S1.
Briefly, cf‐PWV was calculated from measurements of pulse transit time and the distance travelled by the pulse between 2 recording sites: cf‐PWV = distance (m)/transit time (seconds). Finally, values of cf‐PWV were adjusted using the 0.8 correction factor according to the new instructions.25 Aortic pressure waveforms were subjected to further analysis by the Sphygmocor software to calculate the aortic AIx. This is defined as the increment in pressure from the first systolic shoulder to the peak pressure of the aortic pressure. AIx is expressed as a percentage and provides a quantitative measure of the augmentation of central BP in response to wave reflection.
Brachial pulse pressure was calculated as SBP‐DBP. Mean blood pressure was calculated from the formula DBP + (SBP‐DBP)/3. Arterial tonometry was also performed at the right radial and common carotid arteries, in order to measure the pressure waveforms and calculate the local pulse pressure.
2.4. Statistical analysis
Statistical analyses were performed using the statistical package SPSS, Version 24.0 (IBM Corp, Released 2016, IBM SPSS Statistics for Windows, Armonk, NY, USA). Data were presented as absolute numbers and frequencies for binary and categorical variables and as median mean with standard deviation (SD) for continuous variables. Comparisons between patient groups were performed using chi‐square or Fisher's exact test for binary variables and one‐way analysis of variance (ANOVA) for continuous variables.
We performed univariate linear regression analysis for each variable using the cognitive indices, ie, MMSE, Clock‐test, and IADL as dependent variables for the entire population. We considered as independent covariates all the parameters we recorded in the previous paragraph of demographic data, ie, age, sex, and weight.
In order to avoid either the interaction between homogenous variables or factors that interact in the multiple regression analysis (for example, weight, height, and body mass index [BMI] or SBP/DBP and pulse pressure [PP]), we created clusters as performed by Briet and colleagues.26 Clusters were constituted as follows: morphometric (sex, height, weight, BMI, body surface area [BSA]), blood pressure (MBP, PP, AIx), lipids (total cholesterol [t‐chol], high‐density lipoprotein cholesterol [HDL‐C], low‐density lipoprotein cholesterol [LDL‐C], triglycerides [TGL]), blood (hematocrit [Ht], hemoglobin [Hb], iron [Fe++], ferritin, total iron binding capacity [TIBC]), inflammation (fibrinogen, C‐reactive protein [CRP]), mineral metabolism [Calcium (Ca++)], phosphorus [PO4‐], Ca++x PO4‐ product, parathormone [PTH]), and treatment (angiotensin converting enzyme inhibitors [ACEIs], angiotensin II receptor blockers [ARBs], calcium channel blockers [CCBs], vitamin D [VitD], and intake of statins). Variables within each cluster that were significantly associated with the dependent variable, from each classification, were included in the multiple regression step analysis. When more than one variable in a given cluster was significantly associated with the dependent variable, then we chose the one with the largest R 2 or the most directly assessed one. In a second model, age was force‐introduced and then Glomerular‐Filtration Rate‐Modification of Diet in Renal Disease (GFR‐MDRD) was forced into the third model. Multiple regression analysis by stepwise selection was performed. Finally, in the last step of multiple linear regression analysis in order to further eliminate spurious results due to residual collinearities (for instance, age and GFR‐MDRD or cPP and cf‐PWV), we performed 2 subanalyses. Moreover, multiple regression (also known as multivariable regression) pertains to one dependent variable and multiple independent variables: y = f(x 1, x 2, … , x n).
Multivariate regression pertains to multiple dependent variables and multiple independent variables: y 1, y 2, … , y m = f(x 1, x 2,…, x n). Problems may be encountered where both the dependent and independent variables are arranged as matrices of variables (eg, y 11, y 12, … and x 11, x 12,…), so the expression may be written as Y = f(X), where capital letters indicate matrices. The purpose of our study was to find the interaction of one dependent variable (cf‐PWV) with multiple independent variables (MMSE, IADL, Clock‐test) demonstrating that multiple regression analysis is a more appropriate statistical solution for our study.
3. RESULTS
Sex ratio, education level, and incidence of DM were comparable among CKD groups. However, patients in advanced CKD stages 3‐4 were older and had an increased incidence of CVD disease (Table 1), together with more severe anemia, secondary hyperparathyroidism, and increased inflammation indices (Table 1). Patients in advanced CKD stages also exhibit significantly increased PP (brachial and aortic) by comparison with moderate CKD patients (Table 2). No significant differences between CKD groups were found for systolic BP, mean BP, cf‐PWV, and AIx (Table 2). The number of patients receiving antihypertensive agents and vitamin D analogs was significantly increased in parallel with the severity of CKD (Table 1).
Table 1.
Baseline characteristics of the population
| Parameters, n = 151 | CKD 1, n = 44 | CKD 2, n = 47 | CKD 3, n = 25 | CKD 4, n = 35 | Total population | P value |
|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 49.4 ± 12.7 | 56.2 ± 12.8 | 59.9 ± 11.1 | 66.6 ± 12.1 | 57.08 ± 13.7 | <0.001 |
| Sex, (male/female), n (%) | 28 /16 (63.5/36) | 32/15 (68/32) | 18/7 (72/28) | 22/13 (50/30) | 101/51 (66.4/33.6) | 0.97 |
| Body mass index, (kg/m2) | 27.9 ± 4.4 | 28.9 ± 6.5 | 27.6 ± 4.5 | 27.8 ± 4.1 | 28.2 ± 5.1 | 0.03 |
| Body surface area, (m2) | 4.1 ± 0.8 | 3.7 ± 0.8 | 3.7 ± 0.8 | 3.4 ± 0.9 | 3.8 ± 0.83 | 0.03 |
| Higher education level, n (%) | 11 (25) | 16 (34) | 5 (22) | 9 (25) | 41 (61.9) | 0.58 |
| History of diabetus mellitus, n (%) | 4 (9) | 7 (15) | 6 (24) | 8 (23) | 25 (37.7) | 0.19 |
| History of cardiovascular disease, n (%) | 1 (2.3) | 0 (0) | 3 (12) | 7 (20) | 10 (16.6) | 0.001 |
| Laboratory characteristics | ||||||
| Hematocrit, (%) | 43.1 ± 3.4 | 41.8 ± 4.5 | 38.5 ± 4.1 | 36.8 ± 3.7 | 40.4 ± 4.5 | <0.001 |
| Hemoglobin, (g/dL) | 13.7 ± 1.4 | 13.7 ± 1.5 | 12.7 ± 1.4 | 11.8 ± 1.3 | 13.1 ± 1.6 | 0.000 |
| Total cholesterol, (mg/dL) | 205.8 ± 37.1 | 216.5 ± 51.2 | 201.1 ± 46.1 | 190.9 ± 51.8 | 204.7 ± 46.4 | 0.09 |
| Triglycerides, (mg/dL) | 124.4 ± 45.1 | 136.7 ± 71.2 | 145.4 ± 55 | 146.6 ± 63.4 | 136.8 ± 60.3 | 0.09 |
| High‐density lipoprotein cholesterol, (mg/dL) | 53.9 ± 12.2 | 52.7 ± 13.9 | 52.6 ± 1.3 | 52 ± 11.6 | 52.8 ± 13.2 | 0.55 |
| Low‐density lipoprotein, (mg/dL) | 234.4 ± 38.6 | 241 ± 50.3 | 224 ± 56.3 | 213 ± 57.5 | 230.2 ± 50.8 | 0.59 |
| Calcium, (mg/dL) | 9.7 ± 0.5 | 9.6 ± 0.3 | 9.4 ± 0.6 | 9.3 ± 0.7 | 9.5 ± 0.5 | 0.03 |
| Phosphorus, (mg/dL) | 4.1 ± 0.4 | 4.1 ± 0.5 | 4.1 ± 0.9 | 4.6 ± 0.8 | 4.2 ± 0.8 | 0.01 |
| Product of calcium × phosphorus | 39.9 ± 6.2 | 39.4 ± 6.1 | 37.7 ± 8.4 | 43.3 ± 8.8 | 4.2 ± 9.3 | 0.01 |
| Parathormone, (pg/mL) | 38.7 ± 13.9 | 44.8 ± 31.8 | 72.7 ± 71 | 156 ± 89.3 | 74.5 ± 73.2 | <0.001 |
| Serum albumin, (g/dL) | 4.5 ± 0.3 | 4.3 ± 0.4 | 4.5 ± 0.4 | 3.9 ± 0.5 | 4.2 ± 0.4 | <0.001 |
| C‐reactive protein, (mg/L) | 1.98 ± 1.03 | 2.3 ± 1.1 | 3.2 ± 1.5 | 2.9 ± 1.4 | 2.5 ± 1.4 | <0.001 |
| Fibrinogen, (mg/dL) | 349 ± 86.3 | 388 ± 137 | 391 ± 129 | 433 ± 125 | 387 ± 128 | 0.005 |
| Iron, (mg/dL) | 85.3 ± 37.08 | 92.2 ± 34.4 | 81.5 ± 27.2 | 71.9 ± 23.5 | 83.1 ± 32.4 | 0.001 |
| Ferritin, (mg/dL) | 110.4 ± 75.01 | 142.2 ± 102.9 | 137.4 ± 104.4 | 146.2 ± 136.2 | 133.4 ± 106 | 0.171 |
| Total iron binding capacity, (mg/dL) | 304.4 ± 76.7 | 289.8 ± 88.2 | 273.3 ± 80.2 | 290.8 ± 63.8 | 291.8 ± 77.5 | 0.459 |
| Urine total protein excretion, (mg/dL) | 212 ± 308 | 935 ± 850 | 922 ± 985 | 1826 ± 2822 | 908 ± 1784 | <0.001 |
| GFR‐MDRD (mL/min/1.7 m2) | 100 ± 14.1 | 71.9 ± 7.3 | 45.4 ± 8.9 | 21.1 ± 7.02 | 64.4 ± 31.4 | <0.001 |
| Pharmacological agents | ||||||
| Medications, n (%) | ||||||
| ACEIs/ARBs | 28 (63) | 39 (83) | 15 (60) | 29 (82) | 111 (73) | 0.01 |
| Calcium channel blockers | 5 (11) | 15 (32) | 22 (88) | 28 (80) | 70 (46) | 0.01 |
| Central blocker | 24 (55) | 5 (10) | 7 (28) | 2 (5) | 38 (57) | 0.01 |
| β‐blocker | 6 (13) | 7 (15) | 3 (12) | 4 (11) | 20 (13) | 0.22 |
| Statin | 13 (30) | 15 (32) | 23 (92) | 18 (52) | 69 (45) | 0.05 |
| Diuretics | 4 (9) | 11 (23) | 22 (88) | 31 (88) | 68 (45) | 0.01 |
| Vitamin D analogs | 2 (4) | 2 (4) | 3 (12) | 12 (34) | 19 (12) | 0.01 |
P value represents ANOVA analysis for all groups.
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blockers; GFR‐MDRD, Glomerular‐Filtration Rate‐Modification of Diet in Renal Disease; SD, standard deviation.
Table 2.
Cognitive and arterial parameters
| Parameters | CKD 1, n = 44 | CKD 2, n = 47 | CKD 3, n = 25 | CKD 4, n = 35 | P value |
|---|---|---|---|---|---|
| Cognitive | |||||
| Mini Mental State Examination (best score 30) | 21.8 ± 3.3 | 20.7 ± 4.8 | 20.5 ± 4.6 | 18.7 ± 6.7 | 0.08 |
| Clock‐test (best score 7) | 6.8 ± 0.5 | 6.6 ± 0.9 | 5.8 ± 1.1 | 5.3 ± 1.8 | <0.001 |
| Instrumental Activity of Daily Living (best score 9) | 9.8 ± 2.7 | 11.7 ± 3.9 | 14.1 ± 4.2 | 15.1 ± 5.7 | <0.001 |
| Arterial parameters | |||||
| Brachial blood pressure | |||||
| Systolic BP, (mm Hg) | 137.3 ± 9.8 | 139.4 ± 12.7 | 142.8 ± 12.8 | 137.2 ± 18.1 | 0.65 |
| Diastolic BP, (mm Hg) | 84.7 ± 9.8 | 81.8 ± 10.7 | 78.6 ± 22.4 | 77.4 ± 11.3 | 0.01 |
| Pulse pressure, (mm Hg) | 52.5 ± 12.2 | 57.5 ± 13.8 | 64.2 ± 22.4 | 59.8 ± 10.4 | 0.03 |
| Mean BP, (mm Hg) | 102.2 ± 10.3 | 101.9 ± 9.4 | 100.5 ± 10.3 | 97.3 ± 10.4 | 0.58 |
| Aortic blood pressure | |||||
| Systolic BP, (mm Hg) | 128.3 ± 14.8 | 128.1 ± 23.1 | 131.9 ± 13.3 | 130.2 ± 17.5 | 0.53 |
| Pulse pressure, (mm Hg) | 41.9 ± 12.8 | 47.7 ± 21.2 | 53.1 ± 17.2 | 53.4 ± 17.1 | 0.01 |
| Carotid blood pressure | |||||
| Systolic BP, (mm Hg) | 138.7 ± 16.5 | 140.6 ± 16.1 | 139.4 ± 14.2 | 137.9 ± 15.9 | 0.89 |
| Pulse pressure, (mm Hg) | 53.9 ± 15.9 | 58.1 ± 16.2 | 56.6 ± 17.4 | 60.1 ± 17.6 | 0.54 |
| cf‐PWVa, (m/s) | 6.3 ± 1.5 | 6.7 ± 1.8 | 6.1 ± 1.9 | 6.9 ± 2.3 | 0.97 |
| Augmentation index (%) | 26.8 ± 12.2 | 24.7 ± 11.2 | 25.8 ± 12.3 | 22.3 ± 12.8 | 0.21 |
P value represents ANOVA analysis for all groups.
cf‐PWV, carotid femoral pulse wave velocity; BP, blood pressure.
The cf‐PWV values are adjusted according to new instructions.
A major result is that cognitive function was profoundly altered in parallel with the severity of CKD. This interested the different dimensions of cognitive function, either assessed by MMSE (loss of 3.1 points between CKD1 and CKD4, P = 0.08), Clock – drawing test (Clock‐test), more specific for praxis (loss of 1.5 points between CKD1 and CKD 4, P < 0.001) or IADL, more specific of adaptation in daily life (gain of 5.3 points between CKD 1 and 4, P < 0.001) (Table 2 and Figure 2).
Figure 2.
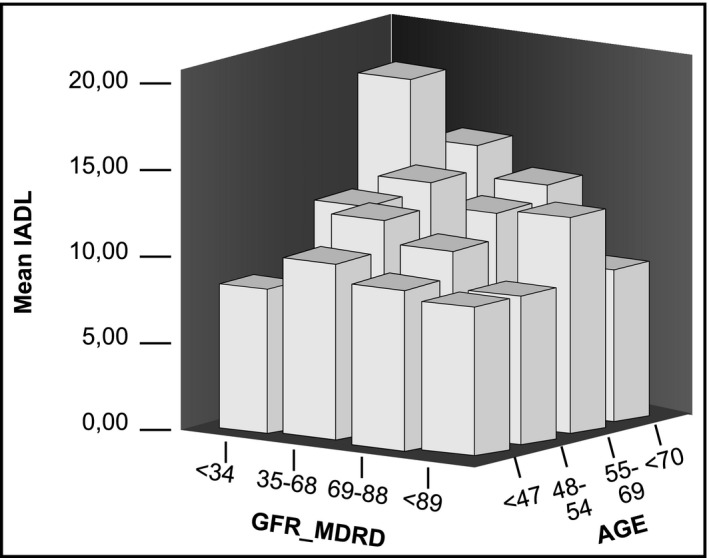
3D plot showing the interaction of IADL, GFR‐MDRD, and age. IADL, Instrumental activity of daily living; GFR‐MDRD, glomerular filtration rate ‐modification of diet in renal disease
Univariate linear regression analysis for each variable using the cognitive indices, ie, MMSE, Clock‐test, and IADL, are provided in Table S1.
The analyses with MMSE, Clock‐test, and IADL as dependent variable, respectively, yielded the following results:
For MMSE (lower scores depicting altered performance), age (beta coeff. −0.102, 95% confidence interval [−0.142 to −0.062], P < 0.001), and cf‐PWV (−0.209, [−0.409 to −0.010], P = 0.040) remained independent negative determinants. In addition, education level (7.159, [6.137 to 8.354], P < 0.001) and Ht levels (0.139, [0.022 to 0.257], P = 0.020) remained independent positive determinants. We further analyzed the data in 2 models that excluded age and either aortic pulse pressure (aPP) or cf‐PWV, in order to take into account possible interactions between age, aPP and cf‐PWV. When age and aPP were excluded from the model, cf‐PWV remained negatively and significantly associated with MMSE (−0.233, [−0.446 to −0.019], P = 0.029; Table 3) and Glomerular ‐ Filtration Rate‐Modification of Diet in Renal Disease (GFR‐MDRD) (0.020, [0.001 to 0.039], P = 0.032) positively and significantly associated with MMSE. When age and cf‐PWV were excluded from the model, aPP (−0.043, [−0.081 to −0.005], P = 0.026) was independently associated with cognitive decline (Table 3).
Table 3.
Multivariate analysis of the study
| Parameters | In/Out | R 2 increment% | Beta coeff. | Lower CI | Upper CI | P value |
|---|---|---|---|---|---|---|
| Dependent variable MMSE | ||||||
| Age | In | 9.7 | −0.102 | −0.142 | −0.062 | <0.001 |
| Education | In | 58.1 | 7.159 | 6.137 | 8.354 | <0.001 |
| cf‐PWV | In | 1.0 | −0.209 | −0.409 | −0.010 | 0.040 |
| Ηt | In | 1.2 | 0.139 | 0.022 | 0.257 | 0.020 |
| GFR‐MDRD | Out | ‐ | ‐ | ‐ | ‐ | |
| R 2 = 0.700 | ||||||
| (a) Dependent variable MMSE without age and aPP | ||||||
| Education | In | 57.9 | 8.031 | 6.891 | 9.171 | <0.001 |
| Ht | In | 4.5 | 0.174 | 0.037 | 0.311 | 0.014 |
| cf‐PWV | In | 1.5 | −0.233 | −0.446 | −0.019 | 0.029 |
| GFR‐MDRD | In | 1.1 | 0.020 | 0.001 | 0.039 | 0.032 |
| R 2 = 0.650 | ||||||
| (b) Dependent variable MMSE without age and cf‐PWV | ||||||
| Education | In | 58 | 7.949 | 6.776 | 9.123 | <0.001 |
| aPP | In | 1.1 | −0.043 | −0.081 | −0.005 | 0.026 |
| LDL | In | 1.3 | 0.012 | 0.001 | 0.023 | 0.035 |
| Statin | In | 1.5 | −1.375 | −2.622 | −0.129 | 0.031 |
| GFR‐MDRD | In | 4.3 | 0.018 | 0.001 | 0.037 | 0.050 |
| R 2 = 0.663 | ||||||
| Dependent variable Clock‐test | ||||||
| Education | In | 15.4 | 1.058 | 0.663 | 1.454 | <0.001 |
| aPP | In | 2.2 | −0.012 | −0.023 | 0.001 | 0.035 |
| GFR‐MDRD | In | 24.2 | 0.019 | 0.014 | 0.025 | <0.001 |
| Age | Out | ‐ | ‐ | ‐ | ‐ | |
| R 2 = 0.419 | ||||||
| Dependent variable IADL | ||||||
| Age | In | 32.5 | 0.327 | 0.039 | 0.138 | 0.001 |
| Education | In | 6.3 | −0.493 | −3.755 | −1.155 | <0.001 |
| bPP | In | 1.1 | 0.541 | 0.003 | 0.069 | 0.032 |
| GFR‐MDRD | In | 7.1 | −0.525 | −0.056 | −0.015 | <0.001 |
| R 2 = 0.678 | ||||||
aPP, aortic pulse pressure; bPP, brachial pulse pressure; cf‐PWV, carotid femoral pulse wave velocity; CI, confidence interval; GFR‐MDRD, Glomerular Filtration Rate‐Modification of Diet in Renal Disease; Ht, hematocrit; LDL, low‐density lipoprotein.
For Clock‐test (lower scores depicting altered performance), aPP (−0.012, [−0.023 to 0.001], P < 0.035) showed a negative association whereas education level (1.058, [0.663 to 1.454], P < 0.001) and GFR‐MDRD (0.019, [0.014 to 0.025], P < 0.001) showed a positive association (the lower the GFR‐MDRD, the lower the Clock‐test, Table 3). Age and carotid‐femoral PWV were not significantly associated with Clock‐test.
For IADL (larger scores depicting better performance), age (0.327, [0.039 to 0.138], P < 0.001) and brachial (bPP) (0.541,[0.003 to 0.069], P < 0.032) were positively and significantly associated with CO/DY. Education level (−0.493, [−3.755 to −1.155], P < 0.001) and GFR‐MDRD (−0.525, [−0.056 to −0.015], P < 0.001) were negatively and significantly associated with CO/DY. Neither cf‐PWV nor central BP parameter was associated with IADL.
4. DISCUSSION
The present study in CKD patients showed that cognitive function was profoundly altered in parallel with the severity of CKD. In addition, high levels of arterial stiffness indices, either directly measured as cf‐PWV or indirectly estimated as aortic PP, were correlated with MMSE, an index of cognitive decline. This study is the first one to report such relationships between large artery damage and brain injury in CKD patients. These results support our hypothesis that increased cf‐PWV and aPP could play a pathophysiological role in the occurrence of CO/DY in CKD patients.
Several studies have examined the association between arterial stiffness and cognitive function in the general population but none in CKD patients. In the general population, the Baltimore study showed that cf‐PWV and pulse pressure were associated with prospective cognitive decline.16 Moreover, a growing body of evidence from longitudinal studies indicated that high values of cf‐PWV predicted lower MMSE score.14, 27, 28 In the general population of the Rotterdam study,29 arterial stiffness was not reported as an independent risk factor for cognitive decline during follow‐up. However, a significant association was observed between stiffness and cognitive decline at baseline. Our findings are in agreement with the aforementioned studies as we showed that 2 indices of arterial stiffness, cf‐PWV and aPP, are associated with lower cognitive performance.
The design of our study does not allow us to penetrate into causality and investigate the pathophysiological mechanisms. Macro and microcirculation in the kidney and the brain are unique and highly sensitive to increased pulsatility, which may lead to progressive organ failure.4 Increased aortic stiffness may promote the transmission of high pulsatile flow in the small arteries network and deteriorate them through hypertrophic remodeling, rarefaction, and finally chronic ischemia.4 Indeed high pressure pulsatility in the brain leads to such qualitative alterations in the structure and the arterial wall as an increase in extracellular matrix turnover, proliferation of smooth muscle cells, and apoptosis, which in turn may lead to the loss of cognitive function.2, 4, 24 Because high cf‐PWV and aPP are factors associated with elevated pressure pulsatility both in the kidney30 and cerebrovascular microcirculation,4 our data suggest that the latter could play a significant pathophysiological role in cognitive dysfunction. It is also possible that uremic toxins have a similar toxic effect on brain and arterial tissues. Indeed, a specific maladaptive remodeling of large arteries has been shown in CKD patients.26, 31 Mild forms of cognitive impairment may already appear at earlier stages of CKD and extreme manifestations, such as severe dysfunction, very likely appear in CKD stage 4. Thus, our findings suggest that cognitive injury appears along with kidney injury and, as we previously described, in every CKD stage, the risk of CO/DY is increased by more than 2‐fold.32 Meanwhile, data from the Heart, Estrogen/Progesterone study showed that, in menopausal women, each 10 mL/min/1.73 m2 decrement in eGFR corresponds to an approximately 15%‐25% increase in the risk of CO/DY.33
Recently, the concept of early vascular aging (EVA) was introduced.34 It generalized the prior considerations that arterial aging and cardiovascular risk are associated with arterial stiffness and pulse pressure.11 There is evidence of an intimate relationship between early brain aging and early vascular aging.13 Mild cognitive decline can be assimilated to an early brain aging in patients with increased cardiovascular risk factor and presence of arterial lesions (atheroma and arteriosclerosis) in micro and macrocirculation, as established in CKD patients.4
In our CKD patients, high hematocrits were associated with better cognitive performance. Our observation confirms previous evidence that anemia is a risk factor for CO/DY in CKD patients.35, 36 Interventions aiming at restoring normal hematocrit in the early stages of CKD could improve both cerebral and renal vasculature2 and could prevent cognitive decline.35, 37
However, we did not find a significant difference in cf‐PWV values between CKD groups. This could simply reflect a lack of power of our study, but each group included 25‐47 patients, with a total number of 151. Alternatively, this could result from the progressive arterial calcifications that characterize patients with CKD37 and the middle‐aged patients of our study population.26 Moreover, changes in cf‐PWV are related to hemodynamic changes (blood pressure load, salt intake, and volume excess in CKD patients) but may also result from the active management of our patients35 that may have attenuated the expected rise in cf‐PWV. Our results are in accordance with the Nephrotest study12 and confirm that aortic stiffness was relatively stable during CKD progression. Finally, we observed an important heterogeneity according to the different dimensions of the cognitive tests that were used in our patients; only 2 tests assessing the variety of mental mechanisms involved in cognitive impairment (MMSE and Clock‐test) showed associations with arterial parameters independently of confounding factors (age, GFR‐MDRD).
The study suffers from limitations, among them its cross‐sectional nature, and the risk of residual confounding variables. As underlined previously, we cannot infer causality and pathophysiology and only suggest possible mechanisms.
According to the European Society of Hypertension guidelines,7 it is necessary to prevent and detect CO/DY. Our findings suggest that, apart from cf‐PWV that is a direct index of arterial stiffness, indirect indices of arterial stiffness, such as aPP, may be useful clinical tools for predicting cognitive decline in CKD patients. Nevertheless, larger clinical trials remain to be done in order to confirm this result. For more than half a century, nephrologists have been focusing on cardiovascular morbidity and mortality concerning CKD patients. However, over the last decade, evidence is growing that these patients’ brains are also affected because of CKD. Cognitive tests should be used for monitoring either vascular or nonvascular dementia in those patients in order to detect and prevent CO/DY and its consequences. Future studies are required to evaluate whether interventions aiming at reducing arterial stiffness would be an effective strategy in order to delay or attenuate cognitive decline in CKD patients.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Supporting information
Karasavvidou D, Boutouyrie P, Kalaitzidis R, et al. Arterial damage and cognitive decline in chronic kidney disease patients. J Clin Hypertens. 2018;20:1276–1284. 10.1111/jch.13350
REFERENCES
- 1. Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension. 2004;43:163‐168. [DOI] [PubMed] [Google Scholar]
- 2. O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200‐204. [DOI] [PubMed] [Google Scholar]
- 3. Laurent S, Briet M, Boutouyrie P. Large and small artery cross‐talk and recent morbidity‐mortality trials in hypertension. Hypertension. 2009;54:388‐392. [DOI] [PubMed] [Google Scholar]
- 4. Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116:1007‐1021. [DOI] [PubMed] [Google Scholar]
- 5. Madero M, Sarnak MJ. Does hemodialysis hurt the brain? Semin Dial. 2011;24:266‐268. [DOI] [PubMed] [Google Scholar]
- 6. United States Renal Data System . Annual data report: Atlas of end ‐stage renal disease in the United States Bethesda, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2014.
- 7. Mancia G, Fagard R, Narkiewicz K, et al. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159‐2219. [DOI] [PubMed] [Google Scholar]
- 8. Gasecki D, Rojek A, Kwarciany M, et al. Pulse wave velocity is associated with early clinical outcome after ischemic stroke. Atherosclerosis. 2012;225:348‐352. [DOI] [PubMed] [Google Scholar]
- 9. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236‐1241. [DOI] [PubMed] [Google Scholar]
- 10. Scuteri A, Brancati AM, Gianni W, et al. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J Hypertens. 2005;23:1211‐1216. [DOI] [PubMed] [Google Scholar]
- 11. Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: a tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension. 2009;54:3‐10. [DOI] [PubMed] [Google Scholar]
- 12. Briet M, Collin C, Karras A, et al. Arterial remodeling associates with CKD progression. J Am Soc Nephrol. 2011;22:967‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanon O, Haulon S, Lenoir H, et al. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193‐2197. [DOI] [PubMed] [Google Scholar]
- 14. Benetos A, Watfa G, Hanon O, et al. Pulse wave velocity is associated with 1‐year cognitive decline in the elderly older than 80 years: the PARTAGE study. J Am Med Dir Assoc. 2012;13:239‐243. [DOI] [PubMed] [Google Scholar]
- 15. Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203‐1206. [DOI] [PubMed] [Google Scholar]
- 16. Waldstein SR, Rice SC, Thayer JF, et al. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99‐104. [DOI] [PubMed] [Google Scholar]
- 17. Georgianos PI, Sarafidis P, Liakopoulos V. Arterial stiffness : a novel risk factor for kidney injury progression? Am J Hypertens. 2015;28:958‐965. [DOI] [PubMed] [Google Scholar]
- 18. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37‐49. [DOI] [PubMed] [Google Scholar]
- 19. de Medeiros K, Robert P, Gauthier S, et al. The Neuropsychiatric Inventory‐Clinician rating scale (NPI‐C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr. 2010;22:984‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schofield I, Stott DJ, Tolson D, et al. Screening for cognitive impairment in older people attending accident and emergency using the 4‐item Abbreviated Mental Test. Eur J Emerg Med. 2010;17:340‐342. [DOI] [PubMed] [Google Scholar]
- 21. Teng EL, Chui HC, Schneider LS, et al. Alzheimer's dementia: performance on the Mini‐Mental State Examination. J Consult Clin Psychol. 1987;55:96‐100. [DOI] [PubMed] [Google Scholar]
- 22. Tuokko H, Hadjistavropoulos T, Miller JA, et al. The Clock Test: a sensitive measure to differentiate normal elderly from those with Alzheimer disease. J Am Geriatr Soc. 1992;40:579‐584. [DOI] [PubMed] [Google Scholar]
- 23. Barberger‐Gateau P, Commenges D, Gagnon M, et al. Instrumental activities of daily living as a screening tool for cognitive impairment and dementia in elderly community dwellers. J Am Geriatr Soc. 1992;40:1129‐1134. [DOI] [PubMed] [Google Scholar]
- 24. Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end‐organ damage. J Appl Physiol. 2008;105:1652‐1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laurent S, Cockcroft J, Van BL, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588‐2605. [DOI] [PubMed] [Google Scholar]
- 26. Briet M, Bozec E, Laurent S, et al. Arterial stiffness and enlargement in mild‐to‐moderate chronic kidney disease. Kidney Int. 2006;69:350‐357. [DOI] [PubMed] [Google Scholar]
- 27. Scuteri A, Tesauro M, Appolloni S, et al. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens. 2007;25:1035‐1040. [DOI] [PubMed] [Google Scholar]
- 28. Muela HCS, Costa‐Hong VA, Yassuda MS, et al. Higher arterial stiffness is associated with lower cognitive performance in patients with hypertension. J Clin Hypertens (Greenwich).2018; 20:22‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poels MM, van Oijen M, Mattace‐Raso FU, et al. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke. 2007; 38:888‐892. [DOI] [PubMed] [Google Scholar]
- 30. Bidani AK, Griffin KA, Williamson G, et al. Protective importance of the myogenic response in the renal circulation. Hypertension. 2009;54:393‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karras A, Haymann JP, Bozec E, et al. Large artery stiffening and remodeling are independently associated with all‐cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60:1451‐1457. [DOI] [PubMed] [Google Scholar]
- 32. Kalaitzidis RG, Karasavvidou D, Tatsioni A, et al. Risk factors for cognitive dysfunction in CKD and hypertensive subjects. Int Urol Nephrol. 2013;45:1637‐1646. [DOI] [PubMed] [Google Scholar]
- 33. Kurella M, Yaffe K, Shlipak MG, et al. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005;45:66‐76. [DOI] [PubMed] [Google Scholar]
- 34. Nilsson PM. Early vascular aging (EVA): consequences and prevention. Vasc Health Risk Manag. 2008;4:547‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grimm G, Stockenhuber F, Schneeweiss B, et al. Improvement of brain function in hemodialysis patients treated with erythropoietin. Kidney Int. 1990;38:480‐486. [DOI] [PubMed] [Google Scholar]
- 36. London G, Covic A, Goldsmith D, et al. Arterial aging and arterial disease: interplay between central hemodynamics, cardiac work, and organ flow‐implications for CKD and cardiovascular disease. Kidney Int Suppl. 2011;1:10‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang WK, Mok V, Chan SS, et al. Screening of dementia in stroke patients with lacunar infarcts: comparison of the Mattis Dementia Rating Scale and the Mini‐Mental State Examination. J Geriatr Psychiatry Neurol. 2005;18:3‐7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


