1. INTRODUCTION
A new concept of the synergistic vicious cycle of blood pressure (BP) variability and vascular disease (systemic hemodynamic atherothrombotic syndrome, SHATS) has recently been proposed.1, 2, 3 There are 2 common types of arterial disease: endothelial damage‐initiated4, 5 atherosclerosis resulting in advanced atherosclerotic plaque and arteriosclerosis with advanced arterial stiffness of large arteries, which is linked to small artery remodeling.6 The former is predominantly promoted by metabolic risk factors (eg, diabetes, dyslipidemia), whereas the latter is most closely linked to aging and hypertension. Previous definitions of SHATS have included both types of vascular disease. However, here we clarify the concept of HATS (hemodynamic arteriosclerotic syndrome) in a narrower sense of a previous SHATS concept, by separating the well‐known atherothrombotic mechanism of cardiovascular disease from the increasingly important arteriosclerotic mechanism.
2. WHAT IS HATS?
HATS refers to an accelerated vicious cycle of hemodynamic stress (BP variability/surge) and arteriosclerosis (arterial stiffness), resulting in target organ damage and cardiovascular events.1, 2, 3 Figure 1 shows the clinical phenotypes of cardiovascular events associated with HATS and 3 mechanistic trigger pathways: (1) BP surge to atherosclerotic plaque, (2) Strain to strain to strain vessel, (3) afterload to left ventricle. In HATS, the exaggerated power of the pulse (BP surge) is not properly absorbed by conduit arteries (such as the aorta) and is transmitted to peripheral atherothrombotic plaques, resulting in plaque rupture, triggering atherothrombotic events associated with coronary artery disease and atherothrombotic stroke. The exaggerated BP surge transmits to strain vessels7 small and short vessels that are exposed to high‐pressure, and therefore have to maintain a strong vascular tone to provide a large pressure gradient in a short distance.7 The higher wall strain on these stain vessels is partly derived from the impedance mismatch that depends on size and stiffness, and from branching at a rectangular angle from the relatively larger arteries. These strain vessels are found in vital organs (brain, heart, kidney) and are highly affected by exaggerated BP surge strain, resulting in progressive small artery disease.7 Small artery diseases include vascular dementia, lacunar infarction, and chronic kidney disease (CKD; microalbuminuria, and/or decreasing glomerular filtration rate). Finally, HATS increases left ventricular (LV) afterload by increasing aortic stiffness and reducing the transit time of reflected pulse waves. Increased LV afterload promotes chronic LV hypertrophy (LVH) and diastolic dysfunction. Acutely elevated LV afterload secondary to exaggerated BP surges would increase LV pressure to trigger acute heart failure in high‐risk HATS patients with stiff arteries and LVH.
Figure 1.
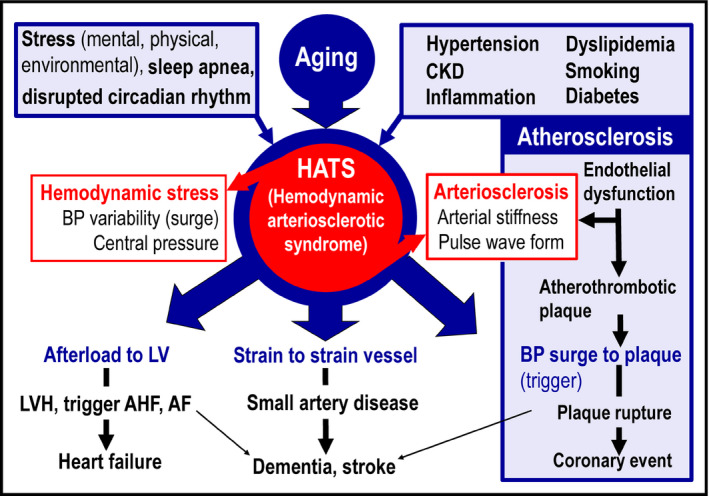
Concept of hemodynamic arteriosclerotic syndrome (HATS) and cardiovascular events. AHF, acute heart failure; AF, atrial fibrillation; BP, blood pressure; CKD, chronic kidney disease; LV, left ventricle; LVH, left ventricular hypertrophy
3. CLINICAL IMPLICATIONS
Figure 2 shows the long‐term development and progression of cardiovascular disease over the life span. Advancing HATS mirrors the aging process. Various cardiovascular risk factors (eg, hypertension, diabetes, dyslipidemia, smoking, CKD, hypoxia, inflammation) damage endothelial cells and advance both atherosclerosis and arteriosclerosis, promoting development of HATS (Figure 2). HATS can contribute to cardiovascular risk throughout life, although the clinical implications and importance of HATS might vary in younger vs older populations.In younger adults, the arteriosclerosis of HATS and atherosclerosis both increase the chronic risk of cardiovascular disease. HATS, the vicious cycle of hemodynamic stress and arteriosclerosis, precedes hypertension across all ages. Hypertension is defined using thresholds of average BP readings. The diagnostic threshold has gradually lowered over time and is currently 130/80 mm Hg according to the latest (2017) ACC/AHA guidelines.8 The current hypertension management strategy focusses on early and strict BP control to prevent cardiovascular disease. Early detection of HATS and lifestyle modification before hypertension diagnosis is important to prevent future cardiovascular disease.In older patients with advanced HATS‐related arterial stiffness, the associated hemodynamic stress could trigger cardiovascular events. Therefore, stricter 24‐hour BP control and suppression of exaggerated BP surge would be important to reduce cardiovascular events, including heart failure.9
Figure 2.
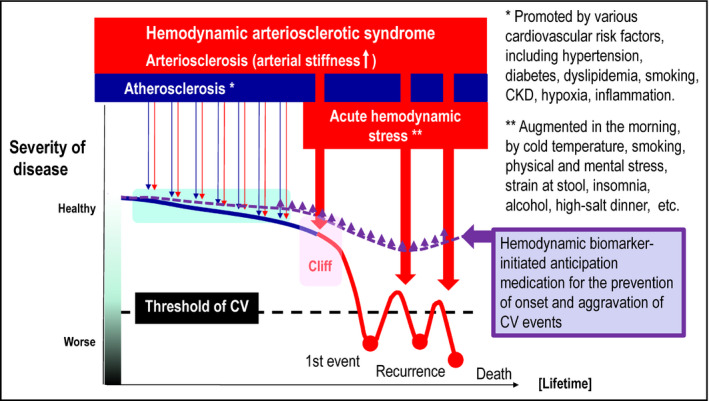
Aging and prognosis of cardiovascular disease throughout human life. BP, blood pressure; CKD, chronic kidney disease; CV, cardiovascular
4. EVALUATION
Evaluation of HATS should be based on 2 domains of cardiovascular risk: the assessment of hemodynamic stress (BP variability, BP surge), and the assessment of arteriosclerosis (arterial stiffness, some index of pulse waveform). Each could be assessed separately, but the novelty of the HATS assessment is the simultaneous evaluation of both domains, taking their synergistic effect on cardiovascular risk into consideration. The epidemiological evidence of HATS should be tested.
4.1. Hemodynamic stress
BP changes over time, from longer yearly, seasonal, day‐by‐day, diurnal, and trigger‐specific variation to shorter beat‐by‐beat changes.10, 11 Continuous BP represents the pulse wave at all time points, while the shortest BP variability is in the pulse waveform during one beat. Trigger‐specific BP changes occur in response to factors such as cold temperature, physical activity, and mental stress (Figure 2).11 Assessment of hemodynamic stress in current clinical practice includes clinic, home, and ambulatory BP variability (standard deviation [SD], coefficient of variation [CV], average real variability [ARV], and peak of BP readings). Recent prospective studies have shown that these visit‐to‐visit clinic, day‐by‐day home or ambulatory BP, and beat‐by‐beat clinic BP variabilities increase the risk of cardiovascular events (eg, stroke, coronary events) and age‐related disease (eg, heart failure, CKD, dementia), independently of average BP.12, 13, 14 BP surge is a measure of the pressor component of BP variability above the pathological threshold. Physiologically adequate BP variability (BP oscillation) is important for maintaining vascular health because the increased blood flow variability associated with BP oscillation increases shear stress to endothelial cells, resulting in increasing nitric oxide production. However, exaggerated BP surges can trigger cardiovascular events. This pathologically exaggerated BP surge (dynamic BP surge) could be augmented by synchronization of various BP surges with different time phases (resonance hypothesis of BP surge).15 Exaggerated BP surges occur more frequently in the morning compared with other times of the day because baroreflex sensitivity is reduced in the morning due to increased central sympathetic activation at this time. Both morning BP surge and morning home hypertension increase the risk of cardiovascular events, while morning home BP and increased day‐by‐day home BP variability increase the risk of stroke.13 Positional, mental, or physical (exercise) stress‐induced BP surge in the laboratory setting is one measure of BP surge. Exercise BP has been independently associated with impaired baroreflex sensitivity even in those with well‐controlled resting BP. Orthostatic hypertension (an increase in BP after standing) evaluated by clinic or home BP monitoring is associated with morning BP surge and is an independent risk factor for multiple silent cerebral infarcts detected by brain magnetic resonance imaging (MRI), which can precede dementia, depression, apathy, and falls in the elderly.1 Repeated assessment of BP surges triggered by specific events during daily life would facilitate clinically‐meaningful assessment of real‐world pathological BP surges. This could be achieved by using a recently developed ICT (information and communication technology)‐based multisensor ambulatory BP monitoring device equipped with actigraph, thermometer, and barometer.11 This approach calculates a new index of trigger‐specific BP surge: physical activity‐induced ambulatory BP increase (actisensitivity), which is increased in winter vs summer.Trigger home BP monitoring has been developed to detect the risk of BP variability selectively trigged by specific triggers. In addition, hypoxia‐triggered nocturnal home BP monitoring could selectively measure sleep apnea‐induced BP elevations.16 The exaggerated sleep apnea‐induced nighttime BP surge might explain the increased frequency of sleep‐onset cardiovascular events seen in patients with obstructive sleep apnea. Current BP measurement is intermittent and based on the oscillometric method, whereas continuous pulse wave‐based BP measurement is ideal. Wearable continuous “beat‐by‐beat” surge BP monitoring devices are in development.10
4.2. Arterial stiffness
Arterial stiffness is a clinical measure of arteriosclerosis and is frequently evaluated using pulse wave velocity (PWV).17, 18 There are two methods for evaluating PWV, carotid‐femoral (cf) PWV (a measure of aortic stiffness), and brachial‐ankle (ba) PWV (a measure of both aorta and peripheral muscle artery stiffness). There is good evidence for both cf‐ and ba‐PWVs as predictors of cardiovascular events,19, 20 and these measures are commonly used in clinical practice. In addition, the cardio ankle vascular index (CAVI) is a promising BP‐independent index of arterial stiffness for predicting cardiovascular risk.21, 22 Other measures of arterial stiffness include intima‐media thickness and stiffness beta of the carotid artery evaluated by carotid echography, and aortic stiffness evaluated by MRI. Increased arterial stiffness detected using these measures reflects an increase in both structural stiffness due to changes in the matrix (smooth muscle cell hypertrophy, increased collagen, decreased elastin, and fibrosis), and functional stiffness due to neurohumoral activation (activation of sympathetic nervous system, renin‐angiotensin‐aldosterone system, endothelin system, etc.).
4.3. Pulse wave form
Pulse waveform (PWF) could be considered a measure of both hemodynamic stress and arteriosclerosis. Central pressure and arterial property indices (eg augmentation index, reflection magnitude) can be estimated from the PWF. A promising index of arterial property is reflection magnitude (RM), defined as the ratio of the amplitude of the backward wave to that of the forward wave.23, 24, 25 The RM is predominantly determined by the structure and function of total arterial tree, consisting of the central aorta and peripheral artery with various diameters. Prospective studies showed that RM is a strong predictor of incident heart failure and cardiovascular events.24, 25
5. CONCLUSIONS AND PERSPECTIVE
The concept of HATS will fit the goal of earlier and more stringent management of cardiovascular risk throughout the life‐span. Hemodynamic biomarker‐initiated anticipation medicine is a promising approach to prevent the onset and the aggravation of cardiovascular events. Pathological thresholds for the 2 main components of HATS (hemodynamic stress and arteriosclerosis) and their synergistic effects on cardiovascular events and organ damage need to be clarified. In addition, the feasible, optimal, and clinically meaningful combination of BP variability and arterial property measures for clinical practice needs to be determined.
CONFLICT OF INTEREST
K. Kario has received research funding from Teijin Pharma Limited, Omron Healthcare Co., Ltd., Fukuda Denshi, Bayer Yakuhin Ltd., A &D Co., Ltd., Daiichi Sankyo Company, Limited, Mochida Pharmaceutical Co., Ltd, EA pharma, Boehringer Ingelheim Japan Inc., Tanabe Mitsubishi Pharma Corporation, Shionogi & Co., Ltd., MSD K.K., Sanwa Kagaku Kenkyusho Co.,LTD., Bristol‐Myers Squibb K.K., and honoraria from Takeda Pharmaceutical Company Limited and Omron Healthcare Co., Ltd outside the submitted work.
REFERENCES
- 1. Kario K. Orthostatic hypertension‐a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol. 2013;9:726‐738. [DOI] [PubMed] [Google Scholar]
- 2. Kario K. Systemic hemodynamic atherothrombotic syndrome: a blind spot in the current management of hypertension. J Clin Hypertens. 2015;17:328‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kario K. Evidence and perspectives on the 24‐hour management of hypertension: hemodynamic biomarker‐initiated ‘anticipation medicine’ for zero cardiovascular event. Prog Cardiovasc Dis. 2016;59:262‐281. [DOI] [PubMed] [Google Scholar]
- 4. Kabutoya T, Hoshide S, Ogata Y, Iwata T, Eguchi K, Kario K. The time course of flow‐mediated vasodilation and endothelial dysfunction in patients with a cardiovascular risk factor. J Am Soc Hypertens. 2012;6:109‐116. [DOI] [PubMed] [Google Scholar]
- 5. Tomiyama H, Ishizu T, Kohro T, et al. Longitudinal association among endothelial function, arterial stiffness and subclinical organ damage in hypertension. Int J Cardiol. 2018;253:161‐166. [DOI] [PubMed] [Google Scholar]
- 6. Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116:1007‐1021. [DOI] [PubMed] [Google Scholar]
- 7. Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro‐cardiovascular risk. Hypertens Res. 2009;32:115‐121. [DOI] [PubMed] [Google Scholar]
- 8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127‐e248. [DOI] [PubMed] [Google Scholar]
- 9. Kario K. Global impact of 2017 AHA/ACC hypertension guidelines: a perspective from Japan. Circulation. 2018;137:543‐545. [DOI] [PubMed] [Google Scholar]
- 10. Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood‐pressure variability. Nat Rev Cardiol. 2013;10:143‐155. [DOI] [PubMed] [Google Scholar]
- 11. Kario K, Tomitani N, Kanegae H, et al. Development of a new ICT‐based multisensor blood pressure monitoring system for use in hemodynamic biomarker‐initiated anticipation medicine for cardiovascular disease: the national IMPACT program project. Prog Cardiovasc Dis. 2017;60:435‐449. [DOI] [PubMed] [Google Scholar]
- 12. Kikuya M, Ohkubo T, Metoki H, et al. Day‐by‐day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52:1045‐1050. [DOI] [PubMed] [Google Scholar]
- 13. Hoshide S, Yano Y, Haimoto H, et al. Morning and evening home blood pressure and risks of incident stroke and coronary artery disease in the Japanese general practice population: the Japan morning surge‐home blood pressure study. Hypertension. 2016;68:54‐61. [DOI] [PubMed] [Google Scholar]
- 14. Cho N, Hoshide S, Nishizawa M, Fujiwara T, Kario K. Relationship between blood pressure variability and cognitive function in elderly patients with well blood pressure control. Am J Hypertens. 2018;3:293‐298. [DOI] [PubMed] [Google Scholar]
- 15. Kario K. New insight of morning blood pressure surge into the triggers of cardiovascular disease‐synergistic resonance of blood pressure variability. Am J Hypertens. 2016;29:14‐16. [DOI] [PubMed] [Google Scholar]
- 16. Kuwabara M, Hamasaki H, Tomitani N, et al. Novel triggered nocturnal blood pressure monitoring for sleep apnea syndrome: distribution and reproducibility of hypoxia‐triggered nocturnal blood pressure measurements. J Clin Hypertens. 2017;19:30‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Rourke MF, O’Brien C, Edelman ER. Arterial stiffening in perspective: advances in physical and physiological science over centuries. Am J Hypertens. 2016;29:785‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheng CS, Li Y, Li LH, et al. Brachial‐ankle pulse wave velocity as a predictor of mortality in elderly Chinese. Hypertension. 2014;64:1124‐1130. [DOI] [PubMed] [Google Scholar]
- 20. Ohkuma T, Ninomiya T, Tomiyama H, et al. Brachial‐Ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta‐analysis. Hypertension. 2017;69:1045‐1052. [DOI] [PubMed] [Google Scholar]
- 21. Miyoshi T, Doi M, Hirohata S, et al. Cardio‐ankle vascular index is independently associated with the severity of coronary atherosclerosis and left ventricular function in patients with ischemic heart disease. J Atheroscler Thromb. 2010;17:249‐258. [DOI] [PubMed] [Google Scholar]
- 22. Park JB, Park HE, Choi SY, Kim MK, Oh BH. Relation between cardio‐ankle vascular index and coronary artery calcification or stenosis in asymptomatic subjects. J Atheroscler Thromb. 2013;20:557‐567. [DOI] [PubMed] [Google Scholar]
- 23. Westerhof BE, Guelen I, Westerhof N, Karemaker JM, Avolio A. Quantification of wave reflection in the human aorta from pressure alone: a proof of principle. Hypertension. 2006;48:595‐601. [DOI] [PubMed] [Google Scholar]
- 24. Wang KL, Cheng HM, Sung SH, et al. Wave reflection and arterial stiffness in the prediction of 15‐year all‐cause and cardiovascular mortalities: a community‐based study. Hypertension. 2010;55:799‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chirinos JA, Kips JG, Jacobs DR Jr, et al. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60:2170‐2177. [DOI] [PMC free article] [PubMed] [Google Scholar]


