Abstract
There is little information regarding age‐related reference intervals (RIs) of carotid‐femoral pulse wave velocity (cfPWV) for large healthy populations in South America. The aims of this study were to determine cfPWV RIs and percentiles in a cohort of healthy children, adolescents, and adults and to generate year‐to‐year percentile curves and body‐height percentile curves for children and adolescents. cfPWV was measured in 1722 healthy participants with no cardiovascular risk factors (9–87 years, 60% men). First, RIs were evaluated for males and females through correlation and covariate analysis. Then, mean and standard deviation age‐related equations were obtained for cfPWV using parametric regression methods based on fractional polynomials and age‐specific (year‐to‐year) percentile curves that were defined using the standard normal distribution. Age‐specific first, 2.5th, 5th, 10th, 25th, 50th, 75th, 90th, 95th, 97.5th, and 99th percentile curves were calculated. Finally, height‐related cfPWV percentile curves for children and adolescents (<21 years) were established. After adjusting for age and blood pressure differences with respect to females, males showed higher cfPWV levels (6.60 vs 6.45 m/s; P < .01). Thus, specific RIs for males and females were reported. The study provides the largest database to date concerning cfPWV in healthy people from Argentina. Specific RIs and percentiles of cfPWV are now available according to age and sex. Specific percentiles of cfPWV according to body height were reported for people younger than 21 years.
Keywords: arterial stiffness, epidemiology, pulse wave velocity, reference intervals
1. INTRODUCTION
Normal and pathological values of arterial stiffness (AS) were first reported in 1893 and mentioned in textbooks for the next century.1 In 1983, Avolio and colleagues2 showed that AS evaluated through carotid‐femoral pulse wave velocity (cfPWV) in large populations was significantly correlated with age and mean arterial pressure. It was later demonstrated that hypertension, age, and diabetes mellitus were main determinants of cfPWV.3, 4 cfPWV is currently considered a predictor of cardiovascular risk and a prognostic marker of all‐cause mortality even for populations of different backgrounds and medical conditions.5, 6, 7, 8, 9 An increase of one standard deviation in cfPWV represents an increase in mortality and risk of cardiovascular events equivalent to 10 years of aging, or more than 1.5 times the risk determined by increases of 10 mm Hg of systolic blood pressure (SBP) values.10
Several clinical investigations have revealed the relevance of cfPWV measurement as a biomarker that characterizes cardiovascular risk and all‐cause mortality. However, these investigations have also shown that AS and cardiovascular risk factors (CRFs) are different in Europeans, Hispanics, Chinese, African, subcontinental Indian, and Amerindians from Brazil.11, 12, 13, 14, 15 Reference intervals (RIs) of cfPWV that characterize large healthy populations are still incomplete for the Latin American population, specifically in the Southern Cone. Furthermore, there are few population‐based studies that evaluate AS in populations from Brazil, Uruguay, and Argentina.13, 16, 17 Moreover, there are currently neither age‐related nor sex‐related cfPWV percentile curves and/or RIs for a large and healthy Argentinean population with no evident cardiovascular disease or CRFs. This is an important issue, since the lack of normal age‐related RIs is a limitation in clinical practice, particularly in our continent.
Existing studies show controversial results with respect to sex‐related differences in cfPWV. Moreover, the influence of sex on AS remains to be defined.9, 18, 19, 20 Considering the above‐mentioned discrepancies, the need for sex‐specific cfPWV percentiles and RIs obtained in a large healthy Argentinean cohort is evident.
Several studies have also shown the association between increased AS in children and adolescents and subclinical atherosclerosis21, 22 and high blood pressure (BP).23, 24, 25 Moreover, abnormal AS values have been associated with pediatric obesity,21, 22 insulin resistance,26, 27 and diabetes mellitus.28, 29 These findings point out the relevance of early detection of abnormal AS values beyond physiological limits. Consequently, the need for RIs for pediatric populations is evident.30, 31, 32
In this context, the development of guidelines and recommendations for standard AS assessment has been encouraged, with the aim of obtaining accurate data and allowing comparative analysis among different populations.33 Furthermore, the Arterial Stiffness’ Collaboration group34, 35, 36 reported the relevance of standardizing and defining both the methods and the statistical approaches for stiffness analysis (ie, providing the equations from which RIs are calculated). There is also lack of standardization of approaches used to analyze or interpret cfPWV data (ie, fixed cutoff points, percentile distribution by decade or 5‐year periods, percentiles for adults or for patients 40 years and older), which limits and hinders the clinical use of cfPWV.
Considering the lack of knowledge in terms of AS reference values for Argentinean patients, the aims of this work were: (1) to determine AS levels using cfPWV measurements in a cohort of healthy patients, not exposed to CRFs, from an Argentinean population to obtain age‐ and sex‐related cfPWV percentile curves and RIs; and (2) to determine body height–related cfPWV percentiles and RIs for patients younger than 21 years (children and adolescents.
2. MATERIALS AND METHODS
This study is part of a project that started in 2010 in Tandil, Buenos Aires Province, Argentina, aimed at investigating the prevalence of CRFs. Preliminary data have been published.37 Tandil is located 360 km to the south of Buenos Aires city (37°19′08″S9°08′05″W). According to the National Institute of Statistics and Census report of 2010, the population was 123 871. Ethnically, the population is a mix of European immigration influx and native population.
The research protocol was approved by the institutional ethics committee in agreement with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Asymptomatic individuals from the community were considered for enrollment in this study. Participants were submitted to a clinical interview, blood sampling, and anthropometric assessment performed by the same group of physicians. Blood samples were obtained after 9 to 12 hours of fasting. Glycemia, lipid profile, and kidney functional parameters were determined. Anthropometric evaluation and a brief clinical interview allowed assessment of the exposure to CRFs. Patients included in the study met the following criteria: (1) normal BP at the time of examination (BP ≤ 140/90 mm Hg in adults and BP ˂90th percentile in patients 16 years and younger)32; (2) no history of cardiovascular, pulmonary, or renal disease; (3) not receiving any medication (antihyperlipidemic, antihypertensive, or antidiabetic drugs); (4) glycemia <6.11 mmol/L (<110 mg/dL), total blood cholesterol levels <5.17 mmol/L (<200 mg/dL),38 and normal serum triglycerides levels, defined as <1.69 mmol/L (<150 mg/dL), ≤1.5 mmol/L (<130 mg/dL), or ≤1.13 mmol/L (<100 mg/dL) for patients 18 years and older, between 10 and 17 years, and younger than 10 years, respectively.39
Patients who smoked, patients with diabetes mellitus, obese patients (body mass index [BMI] ≥ 30 kg/m2 for adults or BMI ≥97th percentile for patients younger than 18 years), patients with hypertension, or patients with averaged high BP levels at the time of the study were excluded. BP was measured using automatic sphygmomanometers (705IT; Omron Healthcare Inc.). BP values for adults were classified following guidelines for the management of arterial hypertension.38 In turn, BP levels in children and adolescents were categorized, considering sex, age, and body height.32
Based on inclusion and exclusion criteria, we defined a population that included 1722 participants (age range: 9–87 years, 60% male) used to define cfPWV RIs (Tables 1 and S1).
Table 1.
Distribution of the patient population among age groups: children and adolescents and older group divided into age quartiles
| Mean | SE | 95% CI lower and upper limit | SD | Minimum | p 25th | p 50th | p 75th | Maximum | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n = 365, male: 290 [79.5%]) | ||||||||||
| Age, y | 13.8 | 0.1 | 13.7 | 13.9 | 1.4 | 9.0 | 14.0 | 14.0 | 15.0 | 15.0 |
| Body weight, kg | 13.8 | 0.8 | 56.3 | 59.3 | 12.5 | 23.6 | 50.5 | 58.5 | 66.0 | 99.0 |
| Body height, cm | 165.5 | 0.7 | 164.1 | 166.9 | 11.4 | 131.0 | 159.0 | 168.0 | 173.0 | 194.0 |
| BMI, kg/m2 | 20.7 | 0.1 | 20.5 | 21.0 | 2.7 | 9.1 | 18.9 | 20.8 | 22.4 | 27.7 |
| Total cholesterol, mg/dL | 159.6 | 1.2 | 157.3 | 162.0 | 22.8 | 110.0 | 142.0 | 160.0 | 178.0 | 198.0 |
| Triglycerides, mg/dL | 73.3 | 1.2 | 71.0 | 75.7 | 22.7 | 40.0 | 56.0 | 70.0 | 88.0 | 142.0 |
| Glycemia, mg/dL | 82.0 | 0.5 | 81.1 | 82.9 | 8.9 | 63.0 | 75.0 | 83.0 | 90.0 | 97.0 |
| Creatinine, mg/dL | 0.8 | 0.0 | 0.8 | 0.9 | 0.1 | 0.6 | 0.8 | 0.8 | 0.9 | 1.1 |
| Hematocrit, % | 40.8 | 0.1 | 40.5 | 41.0 | 2.6 | 36.0 | 38.4 | 40.8 | 43.0 | 47.0 |
| SBP, mm Hg | 111.5 | 0.4 | 110.6 | 112.3 | 8.3 | 89.0 | 105.0 | 111.0 | 118.0 | 129.0 |
| MBP, mm Hg | 80.8 | 0.3 | 80.2 | 81.3 | 5.5 | 60.2 | 77.4 | 80.8 | 84.0 | 95.0 |
| DBP, mm Hg | 60.3 | 0.3 | 59.7 | 60.9 | 5.9 | 39.0 | 57.0 | 60.0 | 64.0 | 75.0 |
| PP, mm Hg | 51.2 | 0.4 | 50.3 | 52.1 | 8.6 | 29.0 | 44.0 | 51.0 | 57.0 | 72.0 |
| HR, beats per min | 69.2 | 0.7 | 67.8 | 70.5 | 13.1 | 44.0 | 59.0 | 67.0 | 77.0 | 111.0 |
| cfPWV, m/s | 5.1 | 0.0 | 5.0 | 5.1 | 0.6 | 2.8 | 4.7 | 5.0 | 5.4 | 7.3 |
| Adolescents (n = 221, male: 159 [71.9%]) | ||||||||||
| Age, y | 17.5 | 0.1 | 17.3 | 17.8 | 1.7 | 16.0 | 16.0 | 17.0 | 19.0 | 21.0 |
| Body weight, kg | 67.6 | 0.9 | 65.7 | 69.4 | 11.1 | 48.0 | 60.0 | 67.0 | 75.3 | 99.0 |
| Body height, cm | 173.1 | 0.7 | 171.6 | 174.5 | 8.8 | 153.0 | 168.0 | 173.5 | 180.0 | 191.0 |
| BMI, kg/m2 | 22.5 | 0.2 | 22.2 | 22.9 | 2.5 | 16.7 | 20.7 | 22.5 | 24.3 | 28.4 |
| Total cholesterol, mg/dL | 158.1 | 1.7 | 154.8 | 161.4 | 24.7 | 100.0 | 138.0 | 163.0 | 178.0 | 198.0 |
| Triglycerides, mg/dL | 74.0 | 1.4 | 71.2 | 76.8 | 21.1 | 40.0 | 59.0 | 72.0 | 88.0 | 142.0 |
| Glycemia, mg/dL | 82.0 | 0.6 | 80.8 | 83.2 | 9.2 | 63.0 | 75.0 | 82.0 | 89.0 | 97.0 |
| Creatinine, mg/dL | 0.9 | 0.0 | 0.8 | 0.9 | 0.1 | 0.6 | 0.8 | 0.9 | 0.9 | 1.1 |
| Hematocrit, % | 40.5 | 0.2 | 40.2 | 40.9 | 2.5 | 36.0 | 39.0 | 40.0 | 42.0 | 47.0 |
| SBP, mm Hg | 119.6 | 0.6 | 118.5 | 120.8 | 8.6 | 98.0 | 113.0 | 120.0 | 126.0 | 138.0 |
| MBP, mm Hg | 86.7 | 0.5 | 85.7 | 87.6 | 6.9 | 72.8 | 81.8 | 86.0 | 91.6 | 106.8 |
| DBP, mm Hg | 64.7 | 0.5 | 63.6 | 65.7 | 7.9 | 49.0 | 60.0 | 62.0 | 70.0 | 86.0 |
| PP, mm Hg | 54.9 | 0.6 | 53.8 | 56.1 | 9.0 | 34.0 | 48.0 | 55.0 | 60.0 | 76.0 |
| HR, beats per min | 66.8 | 0.8 | 65.3 | 68.3 | 11.2 | 41.0 | 59.0 | 65.0 | 74.0 | 107.0 |
| cfPWV, m/s | 5.6 | 0.0 | 5.5 | 5.7 | 0.7 | 3.9 | 5.2 | 5.5 | 5.9 | 7.4 |
| Adults (quartile 1) (n = 283, male: 158 [55.8%]) | ||||||||||
| Age, y | 29.9 | 0.3 | 29.4 | 30.4 | 4.5 | 22.0 | 26.0 | 30.0 | 34.0 | 36.0 |
| Body weight (kg) | 69.1 | 2.3 | 64.4 | 73.7 | 14.0 | 30.0 | 62.0 | 71.1 | 78.8 | 99.0 |
| Body height, cm | 169.2 | 1.4 | 166.4 | 172.1 | 8.6 | 150.0 | 164.0 | 170.0 | 175.0 | 190.0 |
| BMI, kg/m2 | 24.0 | 0.2 | 23.6 | 24.3 | 3.1 | 13.7 | 21.8 | 24.3 | 26.0 | 29.9 |
| Total cholesterol, mg/dL | 157.7 | 1.4 | 154.9 | 160.5 | 23.9 | 100.0 | 140.0 | 157.0 | 178.0 | 198.0 |
| Triglycerides, mg/dL | 74.3 | 1.3 | 71.7 | 77.0 | 22.3 | 40.0 | 59.0 | 70.0 | 85.0 | 134.0 |
| Glycemia, mg/dL | 81.2 | 0.5 | 80.1 | 82.2 | 8.7 | 63.0 | 75.0 | 82.0 | 88.0 | 97.0 |
| Creatinine, mg/dL | 0.8 | 0.0 | 0.8 | 0.9 | 0.1 | 0.6 | 0.8 | 0.8 | 0.9 | 1.1 |
| Hematocrit, % | 40.9 | 0.1 | 40.6 | 41.1 | 2.4 | 36.0 | 39.0 | 41.0 | 43.0 | 47.0 |
| SBP, mm Hg | 118.7 | 0.7 | 117.4 | 120.0 | 11.1 | 90.0 | 110.0 | 119.0 | 129.0 | 139.0 |
| MBP, mm Hg | 90.4 | 0.5 | 89.5 | 91.4 | 8.0 | 72.0 | 84.6 | 90.2 | 97.8 | 106.8 |
| DBP, mm Hg | 71.6 | 0.4 | 70.8 | 72.5 | 7.3 | 57.0 | 66.0 | 70.0 | 78.0 | 88.0 |
| PP, mm Hg | 47.1 | 0.5 | 46.1 | 48.1 | 8.5 | 20.0 | 41.0 | 47.0 | 53.0 | 81.0 |
| HR, beats per min | 69.6 | 0.7 | 68.1 | 71.0 | 12.5 | 40.0 | 61.0 | 68.0 | 76.0 | 111.0 |
| cfPWV, m/s | 6.1 | 0.1 | 6.0 | 6.2 | 1.0 | 3.2 | 5.4 | 6.2 | 6.8 | 9.3 |
| Adults (quartile 2) (n = 285, male: 123 [43.5%]) | ||||||||||
| Age, y | 40.9 | 0.2 | 40.6 | 41.3 | 2.6 | 37.0 | 39.0 | 40.0 | 43.0 | 46.0 |
| Body weight, kg | 70.1 | 1.7 | 66.5 | 73.6 | 10.7 | 52.0 | 62.1 | 69.0 | 78.0 | 90.8 |
| Body height, cm | 166.7 | 1.2 | 164.2 | 169.2 | 7.5 | 154.0 | 161.0 | 165.0 | 169.0 | 185.0 |
| BMI, kg/m2 | 23.7 | 0.2 | 23.3 | 24.0 | 2.9 | 16.6 | 21.4 | 23.8 | 25.9 | 29.8 |
| Total cholesterol, mg/dL | 156.4 | 1.4 | 153.7 | 159.1 | 23.3 | 100.0 | 138.0 | 155.0 | 175.0 | 198.0 |
| Triglycerides, mg/dL | 75.6 | 1.3 | 73.0 | 78.2 | 22.5 | 40.0 | 57.0 | 75.0 | 88.0 | 142.0 |
| Glycemia, mg/dL | 82.7 | 0.5 | 81.6 | 83.7 | 9.0 | 63.0 | 76.0 | 84.0 | 90.0 | 97.0 |
| Creatinine, mg/dL | 0.8 | 0.0 | 0.8 | 0.8 | 0.1 | 0.6 | 0.8 | 0.8 | 0.9 | 1.1 |
| Hematocrit, % | 40.7 | 0.2 | 40.4 | 41.0 | 2.6 | 36.0 | 38.4 | 40.0 | 43.0 | 47.0 |
| SBP, mm Hg | 116.6 | 0.6 | 115.3 | 117.8 | 10.9 | 82.0 | 110.0 | 115.0 | 124.0 | 139.0 |
| MBP, mm Hg | 90.0 | 0.5 | 89.1 | 91.0 | 8.2 | 69.0 | 84.4 | 89.8 | 95.8 | 108.2 |
| DBP, mm Hg | 72.4 | 0.5 | 71.5 | 73.3 | 7.7 | 55.0 | 66.0 | 72.0 | 78.0 | 89.0 |
| PP (mm Hg) | 44.2 | 0.5 | 43.3 | 45.1 | 8.0 | 18.0 | 39.0 | 43.0 | 50.0 | 70.0 |
| HR, beats per min | 67.3 | 0.7 | 65.9 | 68.7 | 12.1 | 42.0 | 58.0 | 66.0 | 74.0 | 111.0 |
| cfPWV, m/s | 6.6 | 0.1 | 6.5 | 6.8 | 1.2 | 3.1 | 5.8 | 6.5 | 7.3 | 10.7 |
| Adults (quartile 3) (n = 291, male: 158 [54.3%]) | ||||||||||
| Age, y | 51.5 | 0.2 | 51.2 | 51.9 | 2.6 | 47.0 | 50.0 | 52.0 | 54.0 | 56.0 |
| Body weight, kg | 71.8 | 2.5 | 66.7 | 76.9 | 11.5 | 55.0 | 59.0 | 75.2 | 80.0 | 88.0 |
| Body height, cm | 168.9 | 1.6 | 165.5 | 172.3 | 7.6 | 156.0 | 162.0 | 169.0 | 175.0 | 183.0 |
| BMI, kg/m2 | 24.9 | 0.2 | 24.5 | 25.2 | 2.7 | 18.1 | 22.8 | 24.8 | 27.1 | 30.0 |
| Total cholesterol, mg/dL | 152.2 | 1.4 | 149.3 | 155.0 | 24.4 | 100.0 | 132.0 | 150.0 | 175.0 | 198.0 |
| Triglycerides, mg/dL | 75.0 | 1.3 | 72.4 | 77.5 | 21.8 | 40.0 | 59.0 | 74.0 | 87.0 | 142.0 |
| Glycemia, mg/dL | 80.9 | 0.5 | 79.8 | 81.9 | 9.0 | 63.0 | 73.0 | 82.0 | 88.0 | 97.0 |
| Creatinine, mg/dL | 0.8 | 0.0 | 0.8 | 0.8 | 0.1 | 0.6 | 0.7 | 0.8 | 0.9 | 1.1 |
| Hematocrit, % | 40.9 | 0.1 | 40.6 | 41.1 | 2.5 | 36.0 | 39.0 | 41.0 | 43.0 | 47.0 |
| SBP, mm Hg | 122.6 | 0.6 | 121.3 | 123.8 | 11.1 | 81.0 | 116.0 | 123.0 | 131.0 | 139.0 |
| MBP, mm Hg | 94.9 | 0.5 | 93.9 | 95.9 | 8.8 | 66.6 | 90.0 | 96.0 | 100.8 | 109.0 |
| DBP, mm Hg | 76.5 | 0.5 | 75.6 | 77.5 | 8.2 | 52.0 | 70.0 | 78.0 | 82.0 | 89.0 |
| PP, mm Hg | 46.0 | 0.4 | 45.2 | 46.9 | 7.4 | 24.0 | 40.0 | 47.0 | 51.0 | 72.0 |
| HR, beats per min | 67.4 | 0.7 | 66.0 | 68.8 | 12.2 | 42.0 | 59.0 | 65.0 | 74.0 | 111.0 |
| cfPWV, m/s | 7.7 | 0.1 | 7.5 | 7.8 | 1.4 | 4.1 | 6.1 | 7.5 | 8.6 | 12.5 |
| Adults (quartile 4) (n = 277, male: 137 [49.6%]) | ||||||||||
| Age, y | 64.7 | 0.4 | 64.0 | 65.4 | 6.1 | 57.0 | 60.0 | 64.0 | 68.0 | 87.0 |
| Body weight, kg | 66.2 | 2.0 | 62.2 | 70.3 | 13.2 | 30.0 | 57.0 | 68.0 | 74.0 | 99.0 |
| Body height, cm | 161.9 | 1.5 | 158.8 | 164.9 | 9.9 | 142.0 | 156.0 | 163.0 | 168.0 | 190.0 |
| BMI, kg/m2 | 25.3 | 0.2 | 25.0 | 25.6 | 2.7 | 14.5 | 23.5 | 25.7 | 27.4 | 29.9 |
| Total cholesterol, mg/dL | 154.0 | 1.5 | 151.1 | 156.8 | 24.4 | 100.0 | 133.0 | 151.0 | 175.0 | 198.0 |
| Triglycerides, mg/dL | 74.0 | 1.3 | 71.6 | 76.5 | 21.0 | 40.0 | 59.0 | 70.0 | 85.0 | 142.0 |
| Glycemia, mg/dL | 83.4 | 0.5 | 82.4 | 84.4 | 8.6 | 63.0 | 77.0 | 84.0 | 91.0 | 97.0 |
| Creatinine, mg/dL | 0.8 | 0.0 | 0.8 | 0.9 | 0.1 | 0.6 | 0.8 | 0.8 | 0.9 | 1.1 |
| Hematocrit, % | 40.9 | 0.2 | 40.6 | 41.2 | 2.6 | 36.0 | 39.0 | 40.0 | 43.0 | 47.0 |
| SBP, mm Hg | 125.2 | 0.6 | 124.0 | 126.5 | 10.5 | 90.0 | 119.0 | 126.0 | 135.0 | 139.0 |
| MBP, mm Hg | 96.4 | 0.5 | 95.3 | 97.4 | 8.8 | 69.2 | 90.6 | 98.0 | 103.0 | 109.0 |
| DBP, mm Hg | 77.1 | 0.5 | 76.0 | 78.2 | 9.2 | 50.0 | 71.0 | 79.0 | 84.0 | 89.0 |
| PP, mm Hg | 48.1 | 0.5 | 47.1 | 49.1 | 8.4 | 27.0 | 42.0 | 48.0 | 52.0 | 79.0 |
| HR, beats per min | 69.0 | 0.7 | 67.6 | 70.4 | 12.1 | 47.0 | 60.0 | 68.0 | 77.0 | 111.0 |
| cfPWV, m/s | 8.3 | 0.1 | 8.1 | 8.5 | 1.5 | 4.0 | 7.3 | 8.2 | 9.4 | 13.4 |
BMI, body mass index; cfPWV, carotid‐femoral pulse wave velocity; CI, confidence interval; DBP, diastolic blood pressure; HR, heart rate; MBP, mean blood pressure; PP, pulse pressure; SBP, systolic blood pressure; SD, standard deviation; SE, standard error.
2.1. cfPWV measurements
All parameters were measured in patients after 15 minutes of rest in the supine position in a temperature‐controlled (≈22°C) room, in order to reach steady hemodynamic conditions. cfPWV was measured using two high‐fidelity strain gauges mechanotransducers (Motorola MPX 2050, Motorola Inc.) connected to an electronic signal amplifier device (Arteriometer, model V100), as described in other works.17, 37, 40, 41 The mechanotransducers were positioned on the skin over the carotid and femoral arteries to record, simultaneously, the arterial BP waves. Pressure waves were continuously recorded while monitored on the computer screen by using software that works in a Windows environment. The software calculates the time delay, or pulse transit time, between end‐diastole pressures (the foot of the waveform) of both recorded arterial pressure waves using a maximal upstroke algorithm. The direct distance between the carotid and femoral sensors was considered as the path length used for calculating cfPWV. In each patient, several cfPWV calculations were made from a single continuous recording, which included at least 10 cardiac cycles. All recordings were duplicated.33 The cfPWV values assigned to each patient were the means of the measurements, which were multiplied by a distance scaling factor of 0.8.18, 38 cfPWV data were considered valid only if the coefficient of variation of the individual measurements was <10%. When technical mistakes were made or low‐quality signals were observed, cfPWV was recalculated.
2.2. Data analysis
Continuous and categorical variables are expressed as mean ± standard deviation or as percentage, respectively. Data analysis was performed using MedCalc Statistical Software (version 14.8.1.) and IBM SPSS software (version 20). The differences between groups were analyzed by determining the P value (statistical threshold <0.05), and by analyzing the differences in mean value, standard error, and their 95% confidence interval.
A stepwise data analysis was performed. First, we evaluated whether separate RIs for males and females were needed. To this end, linear regression and analysis of covariance (ANVOCA) were performed, and sex influence was examined before and after adjustment for cofactors (ie, age and BP). Thus, correlations (Table 2) were performed to identify demographic, anthropometric (ie, body height, body weight, and BMI), blood (ie, total cholesterol), and/or hemodynamic (ie, heart rate and BP) variables that should be considered as cofactors in ANCOVA. Once the variables significantly associated with cfPWV were identified, ANCOVA was performed. As a result, specific RIs for males and females were necessary (Table 3).
Table 2.
Simple and point biserial correlations between cfPWV and demographic, anthropometric, blood, and hemodynamic factors
| cfPWV, m/s | Sex (1: female; 0: male) | Age, y | |
|---|---|---|---|
| cfPWV, m/s | |||
| R | – | .073* | .732* |
| P value | – | .003 | .000 |
| Sex (1: female; 0: male) | |||
| R | .073* | – | .213* |
| P value | .003 | – | .000 |
| Age, y | |||
| R | .732* | .213* | – |
| P value | .000 | .000 | – |
| Body height, m | |||
| R | .126* | −.466* | .090** |
| P value | .003 | .000 | .035 |
| Body weight, kg | |||
| R | .383* | −.290* | .238* |
| P value | .000 | .000 | .000 |
| BMI, kg/m2 | |||
| R | .406* | −.211* | .456* |
| P value | .000 | .000 | .000 |
| Total cholesterol, mg/dL | |||
| R | −.089* | .003 | −.096* |
| P value | .000 | .907 | .000 |
| Triglycerides, mg/dL | |||
| R | .012 | .030 | .018 |
| P value | .610 | .216 | .455 |
| Glycemia, mg/dL | |||
| R | .022 | −.016 | .029 |
| P value | .359 | .514 | .230 |
| Creatinine, mg/dL | |||
| R | .011 | .027 | .030 |
| P value | .655 | .259 | .215 |
| Hematocrit, % | |||
| R | .052** | .008 | .037 |
| P value | .030 | .732 | .122 |
| SBP, mm Hg | |||
| R | .442* | −.180* | .338* |
| P value | .000 | .000 | .000 |
| MBP, mm Hg | |||
| R | .567* | −.035 | .524* |
| P value | .000 | .148 | .000 |
| DBP, mm Hg | |||
| R | .570* | .079* | .579* |
| P value | .000 | .001 | .000 |
| PP, mm Hg | |||
| R | −.084* | −.311* | −.223* |
| P value | .000 | .000 | .000 |
| HR, beats per min | |||
| R | −.002 | .078* | −.010 |
| P value | 0.944 | .001 | .689 |
BMI, body mass index; DBP, diastolic blood pressure; HR, heart rate; MBP, mean blood pressure; PP, pulse pressure; cfPWV, carotid‐femoral pulse wave velocity; SBP, systolic blood pressure.
* and ** correlation is significant at the 0.05 level and 0.01 level (two‐tailed), respectively.
Table 3.
Analysis of sex‐related independent differences in cfPWV levels (ANCOVA)
| cfPWV after adjustment value, m/s | 95% CI for mean difference | Covariates in the model were evaluated at the following values | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MV ± SE | Lower limit | Upper limit | P value | Mean difference | SE difference | Lower limit | Upper limit | Age, y | BMI, kg/m2 | Total cholesterol, mg/dL | Hematocrit, % | MBP, mm Hg | |
| Model 1 | |||||||||||||
| Male | 6.59 ± 0 .032 | 6.522 | 6.649 | 0.002 | 0.165 | 0.052 | 0.063 | 0.268 | 35.89 | – | – | – | 89.538 |
| Female | 6.40 ± 0.040 | 6.342 | 6.498 | ||||||||||
| Model 2 | |||||||||||||
| Male | 6.588 ± 0.033 | 6.523 | 6.653 | 0.003 | 0.167 | 0.055 | 0.058 | 0.275 | 35.89 | 23.424 | 156.39 | 40.78 | 89.55 |
| Female | 6.422 ± 0.041 | 6.341 | 6.503 | ||||||||||
ANCOVA, analysis of covariance; BMI, body mass index; cfPWV, carotid‐femoral pulse wave velocity; CI, confidence interval; MV, mean value; SE, standard error.
P value obtained using unpaired two‐tailed test comparing male and female groups. Model 1 adjusted for age and mean blood pressure (MBP) as performed by Boutouyrie et al (2010). Model 2: adjusted by cofactors determined in Table 2, avoiding variables that introduced error by collinearity (simple and point‐biseral correlation analysis).
As a second step, mean and standard deviation age‐related equations (for males and females) were obtained for cfPWV. Parametric regression methods based on fractional polynomials, as described by Royston and Wright42 and previously used to construct RIs for arterial parameters in the Arterial Stiffness’ Collaboration group methodological strategy,34, 35, 36 were implemented using the MedCalc Software (for further details see Supplementary Material). Then, using the equations obtained for mean and standard deviation, age‐specific percentiles were defined using the standard normal distribution (z) (Table 4). Age‐specific first, 2.5th, 5th, 10th, 25th, 50th, 75th, 90th, 95th, 97.5th, and 99th percentile curves were calculated.
Table 4.
cfPWV (m/s) percentiles for females and males
| Age, y | 1st | 2.5th | 5th | 10th | 25th | 50th | 75th | 90th | 95th | 97.5th | 99th |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | |||||||||||
| 10 | 3.36 | 3.54 | 3.70 | 3.88 | 4.19 | 4.52 | 4.86 | 5.16 | 5.34 | 5.50 | 5.68 |
| 15 | 3.81 | 4.02 | 4.20 | 4.40 | 4.74 | 5.12 | 5.50 | 5.84 | 6.04 | 6.22 | 6.42 |
| 20 | 3.99 | 4.23 | 4.43 | 4.67 | 5.06 | 5.50 | 5.94 | 6.33 | 6.57 | 6.78 | 7.01 |
| 25 | 4.04 | 4.32 | 4.55 | 4.83 | 5.28 | 5.79 | 6.30 | 6.75 | 7.03 | 7.26 | 7.54 |
| 30 | 4.06 | 4.37 | 4.64 | 4.95 | 5.47 | 6.05 | 6.62 | 7.14 | 7.45 | 7.72 | 8.04 |
| 35 | 4.07 | 4.42 | 4.72 | 5.07 | 5.65 | 6.29 | 6.94 | 7.52 | 7.87 | 8.17 | 8.52 |
| 40 | 4.10 | 4.49 | 4.82 | 5.20 | 5.84 | 6.55 | 7.26 | 7.90 | 8.28 | 8.61 | 9.00 |
| 45 | 4.18 | 4.59 | 4.95 | 5.37 | 6.06 | 6.83 | 7.60 | 8.29 | 8.70 | 9.06 | 9.48 |
| 50 | 4.30 | 4.75 | 5.13 | 5.57 | 6.31 | 7.13 | 7.96 | 8.69 | 9.14 | 9.52 | 9.97 |
| 55 | 4.49 | 4.96 | 5.36 | 5.83 | 6.61 | 7.47 | 8.34 | 9.12 | 9.59 | 9.99 | 10.46 |
| 60 | 4.74 | 5.23 | 5.65 | 6.14 | 6.95 | 7.85 | 8.76 | 9.57 | 10.06 | 10.48 | 10.97 |
| 65 | 5.06 | 5.57 | 6.00 | 6.50 | 7.34 | 8.28 | 9.21 | 10.05 | 10.55 | 10.98 | 11.49 |
| 70 | 5.46 | 5.98 | 6.42 | 6.93 | 7.79 | 8.74 | 9.69 | 10.55 | 11.06 | 11.50 | 12.02 |
| 75 | 5.93 | 6.46 | 6.91 | 7.42 | 8.29 | 9.25 | 10.21 | 11.08 | 11.60 | 12.04 | 12.57 |
| 80 | 6.49 | 7.01 | 7.46 | 7.98 | 8.85 | 9.81 | 10.77 | 11.64 | 12.15 | 12.60 | 13.13 |
| 85 | 7.13 | 7.65 | 8.09 | 8.60 | 9.46 | 10.41 | 11.37 | 12.23 | 12.74 | 13.18 | 13.70 |
| Males | |||||||||||
| 10 | 3.67 | 3.83 | 3.97 | 4.13 | 4.40 | 4.70 | 5.00 | 5.27 | 5.43 | 5.57 | 5.73 |
| 15 | 3.83 | 4.06 | 4.25 | 4.48 | 4.85 | 5.26 | 5.68 | 6.05 | 6.28 | 6.47 | 6.70 |
| 20 | 3.89 | 4.17 | 4.41 | 4.69 | 5.16 | 5.68 | 6.20 | 6.66 | 6.94 | 7.19 | 7.47 |
| 25 | 3.91 | 4.25 | 4.53 | 4.86 | 5.41 | 6.02 | 6.64 | 7.19 | 7.52 | 7.80 | 8.13 |
| 30 | 3.94 | 4.31 | 4.64 | 5.01 | 5.64 | 6.34 | 7.03 | 7.66 | 8.03 | 8.36 | 8.73 |
| 35 | 3.98 | 4.39 | 4.75 | 5.17 | 5.86 | 6.63 | 7.40 | 8.10 | 8.51 | 8.87 | 9.29 |
| 40 | 4.04 | 4.50 | 4.89 | 5.34 | 6.09 | 6.93 | 7.77 | 8.52 | 8.97 | 9.36 | 9.81 |
| 45 | 4.14 | 4.62 | 5.04 | 5.53 | 6.33 | 7.23 | 8.12 | 8.93 | 9.41 | 9.83 | 10.31 |
| 50 | 4.27 | 4.79 | 5.23 | 5.74 | 6.59 | 7.53 | 8.48 | 9.33 | 9.84 | 10.28 | 10.80 |
| 55 | 4.45 | 4.99 | 5.45 | 5.98 | 6.87 | 7.86 | 8.85 | 9.73 | 10.27 | 10.73 | 11.27 |
| 60 | 4.67 | 5.22 | 5.70 | 6.25 | 7.17 | 8.19 | 9.22 | 10.14 | 10.69 | 11.17 | 11.72 |
| 65 | 4.93 | 5.50 | 5.99 | 6.55 | 7.50 | 8.55 | 9.60 | 10.55 | 11.11 | 11.60 | 12.17 |
| 70 | 5.24 | 5.82 | 6.32 | 6.89 | 7.85 | 8.93 | 10.00 | 10.96 | 11.53 | 12.03 | 12.62 |
| 75 | 5.59 | 6.18 | 6.68 | 7.27 | 8.24 | 9.32 | 10.41 | 11.38 | 11.96 | 12.47 | 13.05 |
| 80 | 5.99 | 6.58 | 7.09 | 7.67 | 8.65 | 9.74 | 10.83 | 11.80 | 12.39 | 12.90 | 13.49 |
cfPWV, carotid‐femoral pulse wave velocity.
Finally, a similar approach was used to determine height‐related cfPWV percentile curves for the entire population and specifically for children and adolescents (<21 years) and adults (divided in four quartiles of age). It is known that sex steroids modulate large artery stiffness in the prepuberty and postpuberty periods.43, 44 In consequence, the upper limit of the analyzed population of adolescents was set up to age 21 years to ensure that body growth and development were completed and that adulthood was undoubtedly reached.44 First, considering age and body height as covariates in ANCOVA, we observed that it was not necessary to define body height–related cfPWV RIs for males and females separately (Table 5). Then, following the approach described to obtain age‐related percentile curves for cfPWV, body height–related cfPWV percentile curves were constructed (Tables S5–S8).
Table 5.
Analysis of sex‐related differences in cfPWV levels (ANCOVA) after adjust by age and body height
| Age group | cfPWV, m/s | Before adjustment age, y | Body height, cm | After adjustment by age and body height | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cfPWV, m/s | 95% CI for mean difference | |||||||||||||||
| MV ± SE | P value | MV ± SE | P value | MV ± SE | P value | MV ± SE | 95% CI lower limit | 95% CI upper limit | P value | Mean difference | SE difference | Lower limit | Upper limit | Age, y | Body height, cm | |
| All (9–87 y) | ||||||||||||||||
| Male | 6.43 ± 0.05 | .004 | 32.71 ± 0.58 | <.001 | 170.96 ± 0.50 | <.001 | 5.73 ± 0.04 | 5.65 | 5.81 | 0.180 | −0.113 | 0.084 | −0.278 | 0.052 | 23.07 | 167.78 |
| Female | 6.65 ± 0.06 | 40.77 ± 0.67 | 160.13 ± 0.66 | 5.84 ± 0.07 | 5.71 | 5.97 | ||||||||||
| Children and adolescents (<21 y) | ||||||||||||||||
| Male | 5.31 ± 0.04 | .030 | 15.04 ± 0.09 | .259 | 170.32 ± 0.57 | <.001 | 5.29 ± 0.03 | 5.22 | 5.35 | 0.729 | −0.027 | 0.077 | −0.179 | 0.125 | 15.04 | 168.15 |
| Female | 5.14 ± 0.07 | 14.77 ± 0.31 | 158.94 ± 1.11 | 5.31 ± 0.07 | 5.18 | 5.45 | ||||||||||
| Adults (>21 y) | ||||||||||||||||
| Male | 7.32 ± 0.07 | .001 | 46.37 ± 0.55 | .399 | 174.05 ± 0.79 | <.001 | 7.24 ± 0.20 | 6.85 | 7.63 | 0.758 | 0.088 | 0.285 | −0.476 | 0.652 | 47.33 | 166.66 |
| Female | 7.02 ± 0.06 | 47.04 ± 0.58 | 161.15 ± 0.75 | 7.15 ± 0.15 | 6.86 | 7.45 | ||||||||||
| Adults (quartile 1) | ||||||||||||||||
| Male | 6.27 ± 0.08 | .040 | 29.88 ± 0.36 | .952 | 175.82 ± 1.39 | <.001 | 6.47 ± 0.26 | 5.95 | 7.00 | 0.357 | 0.372 | 0.397 | −0.438 | 1.181 | 29.78 | 169.11 |
| Female | 5.94 ± 0.07 | 29.91 ± 0.40 | 163.60 ± 1.42 | 6.10 ± 0.22 | 5.65 | 6.55 | ||||||||||
| Adults (quartile 2) | ||||||||||||||||
| Male | 6.79 ± 0.11 | .024 | 41.16 ± 0.25 | .278 | 173.60 ± 1.68 | <.001 | 6.93 ± 0.38 | 6.16 | 7.69 | 0.908 | −0.064 | 0.550 | −1.181 | 1.053 | 41.32 | 166.67 |
| Female | 6.47 ± 0.09 | 40.82 ± 0.20 | 162.15 ± 0.80 | 6.99 ± 0.28 | 6.42 | 7.56 | ||||||||||
| Adults (quartile 3) | ||||||||||||||||
| Male | 7.74 ± 0.12 | .309 | 51.77 ± 0.21 | .115 | 173.70 ± 1.26 | .04 | 7.88 ± 0.29 | 7.26 | 8.49 | 0.083 | 0.794 | 0.433 | −0.115 | 1.703 | 50.86 | 168.91 |
| Female | 7.57 ± 0.12 | 51.29 ± 0.23 | 164.92 ± 2.23 | 7.08 ± 0.26 | 6.53 | 7.63 | ||||||||||
| Adults (quartile 4) | ||||||||||||||||
| Male | 8.52 ± 0.13 | .028 | 63.82 ± 0.46 | .018 | 172.54 ± 1.80 | <.001 | 7.85 ± 0.58 | 6.69 | 9.02 | 0.663 | −0.343 | 0.780 | −1.926 | 1.239 | 66.90 | 163.20 |
| Female | 8.13 ± 0.11 | 65.55 ± 0.57 | 157.23 ± 1.30 | 8.20 ± 0.34 | 7.50 | 8.90 | ||||||||||
ANCOVA, analysis of covariance; cfPWV, carotid‐femoral pulse wave velocity; CI, confidence interval; MV, mean value; SE, standard error.
P value obtained using unpaired two‐tailed test comparing male and female groups.
Considering a 95% and 90% reference limit and confidence interval (two‐sided), respectively, and a 95% and 10% reference range and relative margin of error, respectively, the minimum required sample size was 377 patients.45
3. RESULTS
3.1. General characteristics of the analyzed population
A total of 1722 healthy patients were included in this research. Table 1 and Table S1 summarize the characteristics of the patients and discriminates values for males and females. As shown in Table S1, the mean age for the population was 36 ± 19 years (range 9–87 years). Females were significantly older than males (P < .001). Males showed higher weight, height, and BMI values (P < .001). SBP and pulse pressure (PP) mean values of males were significantly higher than those for females (P < .001), while diastolic BP (DBP) levels were higher in females (P < .01). Total cholesterol, triglycerides, glycemia, and creatinine mean values were within physiological ranges, as established by the exclusion criteria.
The cfPWV value for the entire population (N = 1722) was 6.5 ± 1.6 m/s. cfPWV increased with aging, with less dispersion among younger patients (Figures 1, S3, and S4).
Figure 1.
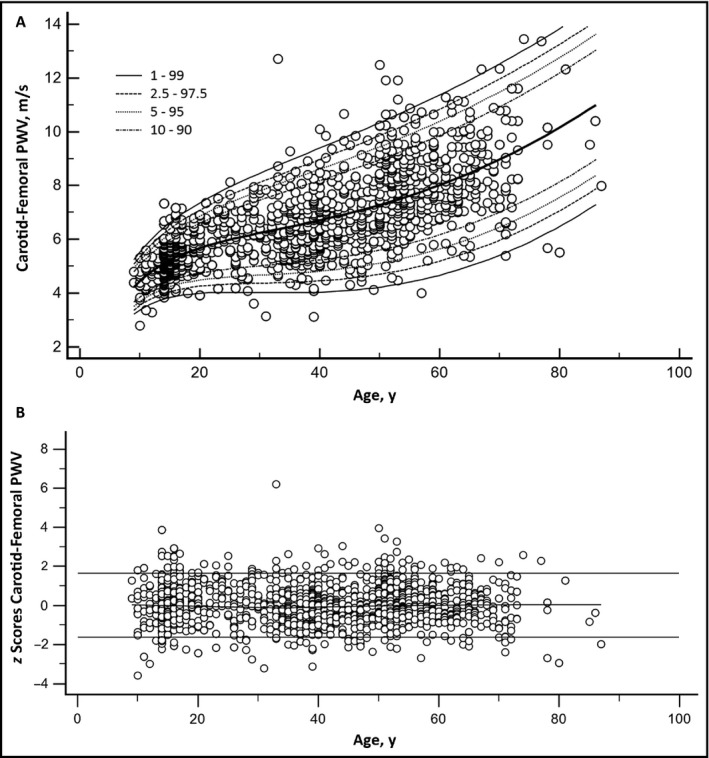
Panel 1‐A: Age‐specific carotid‐femoral pulse wave velocity (PWV) percentiles for the entire population (N = 1722). Panel 1‐B: Residual distribution (z scores) for the carotid‐femoral PWV–age analysis for the entire population
3.2. Analysis of the need for sex‐based RIs characterization
Tables 2 and 3 show correlation and covariance (ANCOVA) analysis, respectively, performed to determine whether an analysis by sex was needed in this research. The correlation analysis in Table 2 shows variables potentially associated with values of cfPWV, age, and sex. This allowed individualized cofactors to be included in ANCOVA. As seen in Table 2, cfPWV was positively associated with age (as expected), female sex, BMI, and BP (SBP, DBP, mean arterial pressure, and PP). It is noteworthy that females were older than males (Table S1), which could explain the positive association between cfPWV and female sex. Additionally, in this analysis, females showed positive associations with cfPWV, age, BMI, SBP, DBP, PP, and heart rate. Additionally, age was positively associated with cfPWV, sex, cholesterol, BMI, and BP (SBP, DBP, mean arterial pressure, and PP) (Table 2.
As seen in Table 3 (model 1) the study of sex‐related cfPWV differences should consider not only age and BP values, but also other variables as cofactors in ANCOVA (hematocrit, serum cholesterol, and BMI). ANCOVA included SBP, DBP, mean BP, and PP values (despite their collinearity), to consider the contribution of differences in BP to the sex‐related differences in cfPWV. Analysis of sex differences in terms of cfPWV (ANCOVA) showed significant differences after adjustments using model 1 and model 2. As seen in Table 3, cfPWV values obtained in males were higher than those found in females after adjustment by age and mean BP (P = .002). Similarly, when adjustment included age, BMI, total cholesterol, hematocrit, and mean BP, the significant and clinical difference was maintained (Table 3. These findings suggest that cfPWV RIs could be discriminated by sex. Furthermore, it is important to take into account this analysis, since, as seen in Table 1, cfPWV mean values were higher in females than in males, while opposite results were obtained after covariate analysis (including age, total cholesterol, hematocrit, BMI, and BP levels as cofactors) (Table 3.
Based on the statistical analysis detailed above, the resulting equations were as follows:
For all patients:
For females:
For males:
In the equations, cfPWV and age were expressed in m/s and years, respectively.
The resulting equations to determine height‐related cfPWV percentile were:
In the equations, cfPWV and body height were expressed in m/s and centimeters, respectively.
3.3. cfPWV––age RIs (percentile analysis)
As seen in Table 4, specific percentiles of cfPWV values for 5‐year age intervals were generated including the entire population according to sex. Moreover, a similar analysis was performed using values corresponding to each year of age (from 9 to 87 years), as shown in Table S2.
Figure 1A shows age‐specific cfPWV percentiles for the entire population (N = 1722). As expected, there was a positive correlation between age and cfPWV values, representing a gradual and continuous increase in terms of AS associated with age. Residual distribution (z scores) for the AS‐age analysis is illustrated in Figure 1B.
3.4. cfPWV RIs according to age and sex (percentile analysis)
According to results derived from data shown in Tables 2 and 3, we opted to perform cfPWV percentile analysis and age analyses, discriminating males from females.
As seen in Table 4, cfPWV percentiles corresponding to 5‐year age intervals were generated for females (top) and males (bottom). A similar analysis was performed for each year of age (from 9 to 87 years), as seen in Tables S3 and S4 for females and males, respectively.
Figures S3A and S4A show the cfPWV age percentiles for females and males, respectively. Residual distribution (z scores) are illustrated in Figures S3B and S4B.
3.5. cfPWV––height RIs in patients younger than 21 years (percentile analysis)
We used ANCOVA to analyze a young‐patient cohort (ie, patients younger than 21 years, n = 407). As seen in Table 5, before adjusting for body height, males showed higher cfPWV values than females (5.31 ± 0.04 m/s vs 5.14 ± 0.07 m/s, respectively; P < .05) and higher body height (170.32 ±0.57 cm vs 158.94 ±1.11 cm, respectively; P ˂ .001). After adjusting for age and body height, cfPWV values of females and males were compared. There were no statistically significant differences in cfPWV values between young females and males of similar age and body height (P = .836). Consequently, in the young patient cohort, the height‐related cfPWV percentile analyses did not require discrimination by sex.
The specific percentile analysis of cfPWV vs body height in the young cohort is shown in Figure S5A, where sex was not discriminately differentiated. Figure S5B shows the distribution of residuals for cfPWV according to body height of the analyzed population.
Finally, Tables S5 and S6 show body height–specific cfPWV percentiles for healthy patients younger than 21 years for 1‐cm and 5‐cm intervals from 131 to 194 cm.
Supplementary Material Tables S7 and S8 show body height–specific cfPWV patients younger than 21 years for 1‐cm intervals for males and females, respectively.
4. DISCUSSION
AS assessed through the measurement of cfPWV is currently the gold standard for noninvasive AS because of its accuracy, reproducibility, and predictive value. Since it is a well‐known and easy‐to‐use technique, cfPWV determinations are widely used, but using a single reference value of cfPWV to discriminate normal from abnormal AS values.38 The first cutoff value was set to 12 m/s, and then reduced to 10 m/s, considering standardized AS measurements using the direct carotid‐femoral distance and the intersecting tangent algorithm to determine the pulse transit time.33 The cutoff value was recommended as a biomarker of end organ damage in patients with aortic stiffening. However, there are some aspects to consider with respect to this assumption. First, this cutoff value is the result of analyzing cfPWV values from several specialized medical centers. Second, these data were acquired using different technologies and mathematical conversion factors.17, 33, 38 Third, linked to this limitation, there are unsolved questions, such as the influence of sex on cfPWV values and methodological differences approaching data analysis. Fourth, the cutoff value of 10 m/s is used only in adult or elderly patients, and not children or adolescents, in which even abnormal values would be below that threshold.
The assessment of AS in young healthy individuals was only recently reported. In 2009, the European Society of Hypertension mentioned that increases in AS were found more frequently in children with hypertension than in those with normotension; however, normal ranges of AS in children should only be determined after obtaining undoubted conclusions regarding the value and relevance of the arterial parameter.46 Evidently, conclusions are only possible if RIs are established for any population. In 2016, the Guidelines for the Management of High Blood Pressure in Children and Adolescents recommended that cfPWV measurements should be considered as “criteria to define hypertension‐induced organ damage” in children and adolescents. Moreover, the identification of cfPWV values ˃95th percentile (cutoff) for age and sex were considered abnormal.32 This contributed to the growing interest in the definition of RIs or cutoff levels according to age and sex, including children and adolescents.
In addition to the above‐described considerations, there are reported differences among European, Hispanic, Chinese, African, subcontinental Indian, and Amerindian populations from Brazil,11, 12, 13, 14, 15 in terms of AS and exposure to CRFs. These discrepancies encouraged authors to characterize an Argentinean population, not exposed to traditional CRFs, in terms of AS. In our study, AS was evaluated using a single device and considering the following criteria: (1) the distance between pulse sensors was directly measured and referred to as “direct distance,” (2) values were considered by data processing (nonmathematical conversion factors were used), (3) data from children and adult patients were included, (4) the arterial pulse wave foot was identified using a validated algorithm. Moreover, our data were analyzed following accepted, recommended, and standardized methods, considering the reference values for Arterial Stiffness’ Collaboration group data.18, 35, 36 This strategy allowed us to encourage other researchers who, using our data, could reproduce results described in this work using equations shown in Material and Methods.
As previously described, arterial cfPWV has been measured since 1893; however, RIs obtained in large samples are not as frequent as would be desirable. In recent years, the reference values from the Arterial Stiffness’ Collaboration group has made significant contributions to clinical and epidemiological research by providing the reference values of the regional AS (PWV for the femoral‐carotid pathway)18 and reference values of local stiffness for the femoral35 and carotid artery.36 It is known that the contribution of risk factors other than age and BP to cfPWV is small or insignificant.47 Nevertheless, in our study, we included asymptomatic patients not exposed to CRFs. As in the European group, the values of cholesterol, triglycerides, and glycemia were within the physiological ranges18 and according to the thresholds of the European Society of Hypertension/European Society of Cardiology hypertension guidelines.38 This consideration is important when determining the normal values or IRs to minimize the possible effects of risk factor clustering.
Figure 2 shows a comparison of data obtained in our Argentinean population with those reported for healthy Europeans patients (n = 1455 patients) by the reference values from the Arterial Stiffness’ Collaboration group.18 For the 10th and 50th percentiles, similar AS values were observed in each age category, since the Argentinean population exhibited lower values. However, the 90th percentile for patients aged 60 to 69 years and for those older than 70 years, differences in cfPWV values were observed (P < .05). In fact, cfPWV values of the Argentine cohort were particularly lower (−2.7 and −2.59 m/s) than those of the European population in the subgroup of patients older than 60 years (Figure 2). Unfortunately, we are not able to determine the origin of these differences, but some methodological aspects could explain those findings. First, the software used to measure the transit time between two pressure waves used the point of maximal upstroke. This method has been shown to provide cfPWV values lower than those calculated using the intersecting tangent algorithm (which was used for the European measurements). This difference is particularly evident when the rise time of the pressure waveform is low, which is frequently found in aged patients.18 Second, as mentioned in the work reported by the Reference Values for Arterial Stiffness’ Collaboration group,18 outliers were more frequent towards older ages. This could be the origin (at least partially) of the observed differences, particularly in the higher percentiles. Third, there is a curious and remarkable increase of cfPWV observed in the European research, specifically in the 90th percentile in patients aged 60 and older.18
Figure 2.
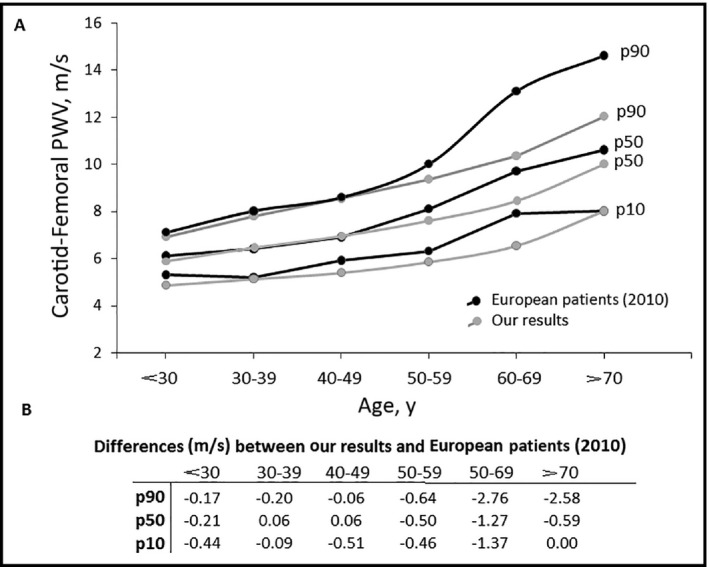
Differences in carotid‐femoral pulse wave velocity (PWV) (m/s) between data obtained in the Argentinean population and the European population from the reference values for Arterial Stiffness’ Collaboration18
In 2012, Fischer et al48 demonstrated that sex, age, and mean arterial pressure were significant independent predictors of cfPWV values. In 2015, Thurn et al49 reported that sex differences in terms of cfPWV were found starting at age 9 years, and they were significantly higher in boys than in girls. Interestingly, this research includes patients from Turkey and Germany. However, when analyzing the specialized literature, this is not the rule, and in cases where sex‐related differences were detected, they were deemed not to be relevant. For example, the European Arterial Stiffness’ Collaboration group reported significant differences in terms of cfPWV (P < .05) after adjustments for age and BP; however, no sex discrimination was exhibited when the European RIs were reported.18 In our research, in agreement with the investigation of the European group, we decided to analyze sex differences in cfPWV, adjusting for age and BP (model 1) and adding other CRFs (model 2). After a clear confirmation, we report the percentile study separating males from females. This was not the case for patients younger than 21 years, when RIs considering body height values were defined. After adjusting for age and body height, we did not find sex‐related differences in cfPWV levels.
In the Argentinean population, the 50th percentile of boys aged between 9 and 15 years shows slightly higher values of cfPWV than those found by Fischer in the German population48 and by Reusz in Hungarian, Italian, and Algerian populations.50 However, in boys aged 16 to 17 years, these differences tend to disappear. AS measured in Argentinean girls were always halfway between values found by Reusz and Fischer for patients aged 9 to 13 years, the highest observed at 14 years and older.48, 50 Those observations were also found when the 90th and 95th percentiles were analyzed by comparing our young cohort with those reported by Fischer and Reusz (patients aged 9–17). Our data regarding the 90th and 95th percentiles found in boys and girls were in an intermediate position between those reported by Reusz and Fischer.48, 50
5. LIMITATIONS
This research used a cross‐sectional design. Consequently, the increase of AS with age should be interpreted with caution, since it may misestimate the real age‐related change of cfPWV of the patients included in this study. Finally, it should be noted that this population has certain characteristics that may not be fully applicable to other races, ethnicities, or regions.
6. CONCLUSIONS
Our study provides the largest database to date concerning cfPWV in healthy people in Argentina. cfPWV RIs and percentiles for an adult healthy Argentinian population are now available, taking into account the age and sex of the patients. Additionally, specific height‐related cfPWV percentiles are reported for a young (younger than 21 years) and healthy population. The cfPWV percentiles can assist in the definition of AS in an Argentinian population and contribute to the correct interpretation of stiffness data obtained in both research and clinical settings.
CONFLICT OF INTEREST
None.
Supporting information
Diaz A, Zócalo Y, Bia D, Wray S, Fischer EC. Reference intervals and percentiles for carotid‐femoral pulse wave velocity in a healthy population aged between 9 and 87 years. J Clin Hypertens. 2018;20:659–671. 10.1111/jch.13251
REFERENCES
- 1. Von Frey M. Die Bewengung des Blutes und der Lymphe. In: von Julius V, ed. Physiologie, vierteTeil, ZweiteAuflage. Berlin: Springer; 1911:66‐96. [Google Scholar]
- 2. Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50‐58. [DOI] [PubMed] [Google Scholar]
- 3. Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alecu C, Labat C, Kearney‐Schwartz A, et al. Reference values of aortic pulse wave velocity in the elderly. J Hypertens. 2008;26:2207‐2212. [DOI] [PubMed] [Google Scholar]
- 5. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318‐1327. [DOI] [PubMed] [Google Scholar]
- 6. van Popele NM, Grobbee DE, Bots ML, et al. Association Between Arterial Stiffness and Atherosclerosis The Rotterdam Study. Stroke. 2001;32:454‐460. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236‐1241. [DOI] [PubMed] [Google Scholar]
- 9. Mattace‐Raso FU. Gender differences in arterial structure and function. Are men really from Mars and women from Venus? Artery Res. 2009;3:148‐150. [Google Scholar]
- 10. Khoshdel AR, Carney SL, Nair BR, Gillies A. Better management of cardiovascular diseases by pulse wave velocity: combining clinical practice with clinical research using evidence‐based medicine. Clin Med Res. 2007;5:45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duprez DA, Jacobs DR Jr, Lutsey PL, et al. Race/ethnic and sex differences in large and small artery elasticity–results of the multi‐ethnic study of atherosclerosis (MESA). Ethn Dis. 2009;19:243‐250. [PMC free article] [PubMed] [Google Scholar]
- 12. Markert MS, Della‐Morte D, Cabral D, et al. Ethnic differences in carotid artery diameter and stiffness: the Northern Manhattan Study. Atherosclerosis. 2011;219:827‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Lima Santos PC, Alvim RO, Ferreira NE, de Sá Cunha R, Mill JG. Ethnicity and arterial stiffness in Brazil. Am J Hypertens. 2011;24:278‐284. [DOI] [PubMed] [Google Scholar]
- 14. Magalhães P, Capingana DP, Silva AB, et al. Age‐ and gender‐specific reference values of pulse wave velocity for African adults: preliminary results. Age (Dordr). 2013;35:2345‐2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Avolio AP, Deng FQ, Li WQ, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71:202‐210. [DOI] [PubMed] [Google Scholar]
- 16. Farro I, Bia D, Zocalo Y, et al. Pulse wave velocity as marker of preclinical arterial disease: reference levels in a Uruguayan population considering wave detection algorithms, path lengths, aging, and blood pressure”. Int J Hypertens. 2012;2012:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Díaz A, Galli CN, Tringler M, Ramírez A, Cabrera Fischer EI. Reference values of pulse wave velocity in healthy people from an urban and rural Argentinean population. Int J Hypertens. 2014:2014;1‐7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reference Values for Arterial Stiffness' Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values”. Eur Heart J. 2010;31:2338‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cunha PG, Cotter J, Oliveira P, et al. Pulse wave velocity distribution in a cohort study: from arterial stiffness to early vascular aging. J Hypertens. 2015;33:1438‐1445. [DOI] [PubMed] [Google Scholar]
- 20. Vermeersch SJ, Rietzschel ER, De Buyzere ML, et al. Age and gender related patterns in carotid‐femoral PWV and carotid and femoral stiffness in a large healthy, middle‐aged population. J Hypertens. 2008;26:1411‐1419. [DOI] [PubMed] [Google Scholar]
- 21. Whincup PH, Gilg JA, Donald AE, et al. Arterial distensibility in adolescents: the influence of adiposity, the metabolic syndrome, and classic risk factors. Circulation. 2005;112:1789‐1797. [DOI] [PubMed] [Google Scholar]
- 22. Urbina EM, Khoury PR, McCoy CE, Dolan LM, Daniels SR, Kimball TR. Triglyceride to HDL‐C ratio and increased arterial stiffness in children, adolescents, and young adults. Pediatrics. 2013;131:e1082‐e1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Urbina EM, Khoury PR, McCoy C, Daniels SR, Kimball TR, Dolan LM. Cardiac and vascular consequences of pre‐hypertension in youth. J Clin Hypertens. (Greenwich). 2011;13:332‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lurbe E, Torro I, Garcia‐Vicent C, Alvarez J, Fernandez‐Fornoso JA, Redon J. Blood pressure and obesity exert independent influences on pulse wave velocity in youth. Hypertension. 2012;60:550‐555. [DOI] [PubMed] [Google Scholar]
- 25. Zhu H, Yan W, Ge D, et al. Cardiovascular characteristics in American youth with prehypertension. Am J Hypertens. 2007;20:1051‐1057. [DOI] [PubMed] [Google Scholar]
- 26. Lee JW, Lee DC, Im JA, Shim JY, Kim SM, Lee HR. Insulin resistance is associated with arterial stiffness independent of obesity in male adolescents. Hypertens Res. 2007;30:5‐11. [DOI] [PubMed] [Google Scholar]
- 27. Urbina EM, Gao Z, Khoury PR, Martin LJ, Dolan LM. Insulin resistance and arterial stiffness in healthy adolescents and young adults. Diabetologia. 2012;55:625‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wadwa RP, Urbina EM, Anderson AM, et al. Measures of arterial stiffness in youth with type 1 and type 2 diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2010;33:881‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity‐related type 2 diabetes mellitus. J Hypertens. 2010;28:1692‐1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Urbina EM, Williams RV, Alpert BS, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents recommendations for standard assessment for clinical research a scientific statement from the American Heart Association. Hypertension. 2009;54:919‐950. [DOI] [PubMed] [Google Scholar]
- 31. Skrzypczyk P, Pańczyk‐Tomaszewska M. Methods to evaluate arterial structure and function in children––State‐of‐the art knowledge. Adv Med Sci. 2017;62:280‐294. [DOI] [PubMed] [Google Scholar]
- 32. Lurbe E, Agabiti‐Rosei E, Criuckshank JK, et al. European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887‐1920. [DOI] [PubMed] [Google Scholar]
- 33. Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30:445‐448. [DOI] [PubMed] [Google Scholar]
- 34. Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S. Reference intervals for commoncarotid intima‐media thickness measured with echotracking: relation with risk factors. Eur Heart J. 2013;34:2368‐2380. [DOI] [PubMed] [Google Scholar]
- 35. Bossuyt J, Engelen L, Ferreira I, et al. Reference values for local arterial stiffness. Part B: femoral artery. J Hypertens. 2015;33:1997‐2009. [DOI] [PubMed] [Google Scholar]
- 36. Engelen L, Bossuyt J, Ferreira E, et al. Reference values for local arterial stiffness. Part A: carotid artery. J Hypertens. 2015;33:1981‐1986. [DOI] [PubMed] [Google Scholar]
- 37. Diaz A, Tringler M, Wray S, Ramirez AJ, Cabrera Fischer EI. The effects of age on pulse wave velocity in untreated hypertension. J Clin Hypertens (Greenwich). 2017;00:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension”. The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281‐1357. [DOI] [PubMed] [Google Scholar]
- 39. Srinivasan SR, Frontini MG, Xu J, Berenson GS. Utility of childhood non‐high‐density lipoprotein cholesterol levels in predicting adult dyslipidemia and other cardiovascular risks: the Bogalusa Heart Study. Pediatrics. 2006;118:201‐206. [DOI] [PubMed] [Google Scholar]
- 40. Cabrera Fischer EI, Bia D, Valtuille R, Galli CN, Armentano RL. Vascular access localization determines regional changes in arterial stiffness. J Vasc Access. 2009;10:192‐198. [DOI] [PubMed] [Google Scholar]
- 41. Bia D, Cabrera‐Fischer EI, Zócalo Y, et al. Vascular accesses for haemodialysis in the upper arm cause greater reduction in the carotid‐brachial stiffness than those in the forearm: study of gender differences. Int J Nephrol. 2012;2012:598512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Royston P, Wright E. A method for estimating age‐specific reference intervals (‘normal ranges’) based on fractional polynomials and exponential transformation. J R Statist Soc. A. 1998;161:79‐101. [Google Scholar]
- 43. Ahimastos AA, Formosa M, Dart AM, Kingwell BA. Gender differences in large artery stiffness pre‐ and post puberty. J Clin Endocrinol Metab. 2003;88:5375‐5380. [DOI] [PubMed] [Google Scholar]
- 44. Stützle W, Gasser T, Molinari L, Largo RH, Prader A, Huber PJ. Shape‐invariant modelling of human growth. Ann Hum Biol. 1980;7:507‐528. [DOI] [PubMed] [Google Scholar]
- 45. Bellera CA, Hanley JA. A method is presented to plan the required sample size when estimating regression‐based reference limits. J Clin Epidemiol. 2007;60:610‐615. [DOI] [PubMed] [Google Scholar]
- 46. Lurbe E, Cifkova R, Cruickshank JK, et al. Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J Hypertens. 2009;27:1719‐1742. [DOI] [PubMed] [Google Scholar]
- 47. Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328‐1336. [DOI] [PubMed] [Google Scholar]
- 48. Fischer DC, Schreiver C, Heimhalt M, Noerenberg A, Haffner D. Pediatric reference values of carotid‐femoral pulse wave velocity determined with an oscillometric device. J Hypertens. 2012;30:2159‐2167. [DOI] [PubMed] [Google Scholar]
- 49. Thurn D, Doyon A, Sözeri B, et al. Aortic pulse wave velocity in healthy children and adolescents: reference values for the Vicorder device and modifying factors. Am J Hypertens. 2015;28:1480‐1488. [DOI] [PubMed] [Google Scholar]
- 50. Reusz GS, Cseprekal O, Temmar M, et al. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension. 2010;56:217‐224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


