Abstract
Angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have become standards of care for diabetic nephropathy. The authors assessed the association between treatment adherence to ACEIs and ARBs and the development of end‐stage renal disease (ESRD). The cohort comprised the 9895 members of the Central District of Clalit Health Services aged 40 to 70 years, diagnosed with diabetes before 2002, who filled at least four ACEI or ARB monthly prescriptions during 2002–2011. Forty‐six percent of patients made 10 or more purchases a year. Hazard ratios for ESRD development and death decreased as adherence increased, with no evidence of a cutoff threshold or plateau. For both outcomes, hazard ratios were significantly lower among patients who purchased at least 10 monthly prescriptions (83% adherence), after adjusting for age, sex, and a number of clinically relevant factors. While ACEIs/ARBs have become standards of care in diabetes, treatment adherence is essential to achieve full benefit.
1. BACKGROUND
Diabetic nephropathy is the leading cause of end‐stage renal disease (ESRD) in Israel.1 Diabetes mellitus caused 18.1% of ESRD cases in 1990, and 44.7% in 2010.1 According to the Centers for Disease Control and Prevention, seven of 10 new cases of ESRD in the United States in 2006 were caused by diabetes or hypertension.2
More than 2 decades ago, angiotensin‐converting enzyme inhibitors (ACEIs) were found to be effective in preventing diabetic nephropathy progression,3 and captopril specifically was found to be cost‐effective in diabetic patients with proteinuria. Among nondiabetic patients with advanced renal disease, ACEI treatment was found to be highly cost‐effective.4 ACEI and angiotensin receptor blockers (ARBs) have become standards of care for diabetic nephropathy and an integral component of guidelines for diabetes treatment.5
Medication adherence is a major challenge in clinical practice. The effect of adherence on outcomes differs among drugs. We assessed the association between treatment adherence to ACEIs and ARBs and the development of ESRD. We chose ESRD as an end point since it is the actual complication to be prevented, and its presence signifies a great reduction in patient prognosis and quality of life.
2. METHODS
Israel has a national health insurance program with universal access to medical care. Health care is delivered by four health maintenance organizations throughout the country. Every Israeli resident receives medical coverage from the health maintenance organization of their choice. Clalit Health Services (CHS) is the largest health maintenance organization in Israel. It serves 54% of the population. Patient records in CHS have been completely computerized for over a decade and an extensive healthcare database has been created. Patient records include demographic data, working diagnoses, medications, laboratory results, hospitalizations, referrals, and administrative data. The demographic data are updated directly from the population registry of the Interior Ministry. All laboratory tests are sent to a central laboratory, and laboratory results are sent directly to primary care physicians and to patients' files. The working diagnosis of “diabetes mellitus” detects over 90% of known diabetes patients.6
This retrospective cohort study was approved by the local ethical committee.
The cohort comprised all members of the Central District of CHS during the inclusive period 2002–2011, aged 40–70 years, diagnosed with diabetes before 2002, who filled at least four ACEI and/or ARB monthly prescriptions during the years 2002–2011. Individuals who had ESRD before 2002 were excluded. We used 2002 as a washout period. Patients who were diagnosed with ESRD or who died during 2002 were also excluded from the analysis.
End points were ESRD or death from any cause. ESRD was defined as stage 5 chronic kidney disease (estimated glomerular filtration rate <15 mL/min, using the Modification of Diet in Renal Disease Study equation) for more than 3 months, initiation of dialysis, or having a kidney transplant. Adherence was calculated as the number of prescriptions that were filled during the entire study period divided by the number of treatment years (counting from the first filled prescription) during the study period. We defined a low compliance baseline group as the purchase of a mean of four to six prescriptions annually.
All community pharmacies used by CHS are computerized and report to one central repository. We documented all prescriptions of ACEI and ARB medications that were filled by the study group during 2002–2011. CHS issues medications and requires only nominal copayments (the copayment is equal to US $3–$4 per month). This system ensures that all prescriptions are documented.7
2.1. Statistical analysis
We compared demographic and clinical characteristics of diabetic patients with low and high adherence for ACEI/ARB medications. We calculated unadjusted and adjusted hazard ratios for high adherence (increasing degrees of adherence, from seven to 12 mean monthly purchases), compared with low adherence (four to six mean monthly purchases). Adjustments were for age, sex, socioeconomic status, glycated hemoglobin, systolic blood pressure (BP), estimated glomerular filtration rate, hypertension, smoking, and insulin use at the start of the study period (January 2002).
STATA 8.0 statistical software (StataCorp, College Station, TX, USA) was used for statistical analysis.
3. RESULTS
The study group comprised 9895 diabetic patients who filled at least four ACEI and/or ARB monthly prescriptions during the years 2002–2011. Age, sex distribution, BMI, and most of the clinical characteristics assessed were similar between the low and high adherence groups (Table 1). The proportions of patients with hypertension and ischemic heart disease were greater for the high adherence group, 64% vs 46% and 23% vs 18%, respectively (Table 1). Of the diabetic patients who purchased an ACEI/ARB at least once monthly (total purchasing during the study period divided by the number of the relevant treatment years), 94% made at least four purchases each year since treatment was started, and 46% made 10 or more purchases, equivalent to 83% adherence (Figure 1).
Table 1.
Demographic and clinical characteristics of the study patients according to monthly purchases of ACEIs/ARBs
| All Patients | Purchased 4–6 Monthly Prescriptions per Year (n=1278) | Purchased ≥7 Monthly Prescriptions per Year (n=7816) | P Value | |
|---|---|---|---|---|
| Age, y | 59.7±7.4 | 58.6 ±8.0 | 59.9±7.2 | <.001 |
| Men, % | 51.1 | 52.4 | 50.9 | .22 |
| Low socioeconomic status, % | 36.6 | 40.9 | 35.9 | <.001 |
| BMI | 30.5±5.8 | 29.9±5.8 | 30.6±5.7 | <.001 |
| Systolic blood pressure | 141.3±20.1 | 139.5±20.5 | 141.6±20.0 | <.001 |
| Glycated hemoglobin at start | 8.5±2.0 | 8.9±2.3 | 8.4±2.0 | <.001 |
| eGFR at start | 67.9±16.8 | 70.3±17.1 | 67.5±16.7 | <.001 |
| Hypertension, % | 61.6 | 45.7 | 64.1 | <.001 |
| Ischemic heart disease, % | 22.3 | 17.7 | 23.1 | <.001 |
| Past cerebrovascular accident, % | 6.6 | 7.3 | 6.5 | .29 |
| Smoking, % | 39.6 | 41.2 | 39.3 | .20 |
| Insulin use, % | 16.3 | 14.0 | 16.7 | .01 |
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Figure 1.
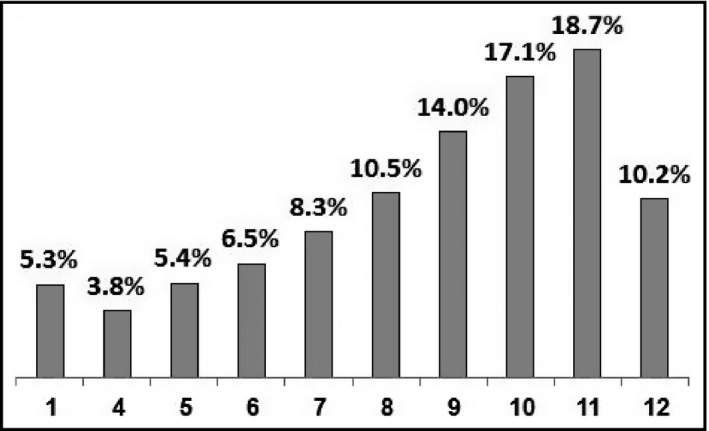
Average monthly prescription purchasing of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (percentage of patients)
During the study period, there were 2063 (20.8%) deaths and 346 (3.5%) cases of ESRD. For ESRD development, death, and the combined outcome, hazard ratios decreased as adherence increased, with no evidence of a cutoff threshold or plateau (Table 2) in a linear fashion (Figure 2). For both single outcomes and the combined outcome, hazard ratios were significantly lower among patients who purchased at least 10 monthly prescriptions (83% adherence), after adjusting for age, sex, socioeconomic status, glycated hemoglobin, systolic BP, estimated glomerular filtration rate, hypertension, ischemic heart disease, cardiovascular accident, smoking, and insulin use at the start of the study period.
Table 2.
HRs for ESRD, death, and the combined outcome of ESRD and death according to the monthly purchase of ACEIs/ARBsa
| Mean Months of Purchase | Unadjusted HR (CI) | P Value | Adjusted HRb (CI) | P Value |
|---|---|---|---|---|
| ESRD | ||||
| 7 | 1.41 (0.94–2.11) | .095 | 1.27 (0.84–1.93) | .547 |
| 8 | 1.08 (0.72–1.61) | .719 | 0.99 (0.64–1.52) | .970 |
| 9 | 0.81 (0.54–1.22) | .327 | 0.70 (0.45–1.07) | .103 |
| 10 | 0.80 (0.54–1.17) | .261 | 0.70 (0.46–0.87) | .087 |
| 11 | 0.44 (0.28–0.68) | <.001 | 0.39 (0.25–0.51) | <.001 |
| 12 | 0.18 (0.08–0.38) | <.001 | 0.14 (0.06–0.31) | <.001 |
| Death | ||||
| 7 | 1.00 (0.91–1.09) | .969 | 0.98 (0.89–1.08) | .703 |
| 8 | 0.97 (0.90–1.05) | .507 | 0.97 (0.89–1.05) | .456 |
| 9 | 0.97 (0.90–1.04) | .461 | 0.94 (0.87–1.02) | .164 |
| 10 | 0.94 (0.88–1.01) | .109 | 0.92 (0.85–0.99) | .034 |
| 11 | 0.85 (0.79–0.91) | <.001 | 0.82 (0.76–0.88) | <.001 |
| 12 | 0.81 (0.75–0.88) | <.001 | 0.77 (0.71–0.84) | <.001 |
| Combined ESRD and death | ||||
| 7 | 0.99 (0.84–1.16) | .890 | 0.93 (0.78–1.11) | .426 |
| 8 | 0.88 (0.75–1.03) | .115 | 0.85 (0.72–1.01) | .075 |
| 9 | 0.86 (0.75–1.00) | .054 | 0.75 (0.64–0.88) | <.001 |
| 10 | 0.75 (0.65–0.87) | <.001 | 0.69 (0.59–0.80) | <.001 |
| 11 | 0.43 (0.37–0.51) | <.001 | 0.37 (0.32–0.44) | <.001 |
| 12 | 0.28 (0.22–0.35) | <.001 | 0.23 (0.18–0.30) | <.001 |
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CI, confidence interval; ESRD, end‐stage renal disease; HR, hazard ratio.
The baseline group purchased 4 to 6 monthly prescriptions per year.
Adjusted for age, sex, socioeconomic status, glycated hemoglobin, systolic blood pressure, estimated glomerular filtration rate, hypertension, ischemic heart disease, cerebrovascular accident, smoking, and insulin use at the start of the study period (January 2002).
Figure 2.
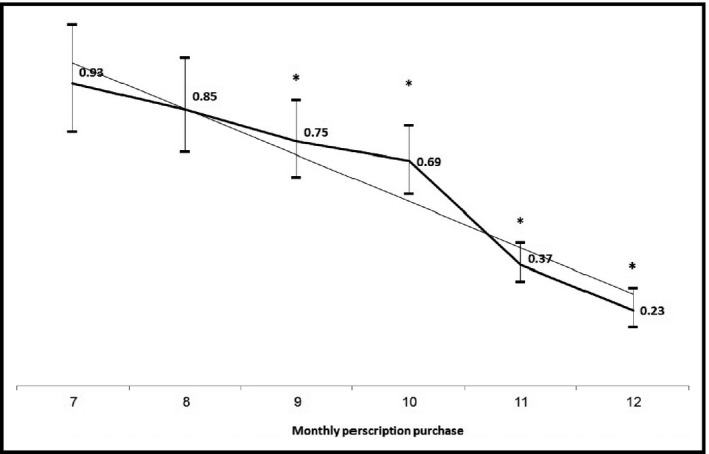
Adjusted hazard ratios for the combined outcome of end‐stage renal disease and death according to the monthly purchase of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers. *Statistically significant
4. DISCUSSION
This study found a linear relationship between ACEI and ARB treatment adherence and the development of ESRD and death. As treatment adherence increased, the risk reduction of ESRD decreased.
A medication adherence rate of 80% of the total amount is generally considered good adherence.8, 9 In the current study, 46% met this criterion; however, outcomes continued to improve with even greater adherence. Such a pattern was not found in a study that investigated adherence to statins.10 In that study, better statin adherence was shown to be related to reduction in all‐cause mortality, yet risk reduction did not change dramatically above 60% adherence.
The beneficial effect of renin‐angiotensin system blockade involves a number of mechanisms. Reduction in BP is an important mechanism in the prevention of diabetic nephropathy.11 Yet, this phenomenon cannot explain all the beneficial effects of ACEI and ARBs on diabetic nephropathy since a similar reduction in BP by other antihypertensive medication groups was not associated with the same renoprotective effect.12 In a meta‐analysis comparing BP‐lowering agents in adults with diabetic kidney disease, no BP‐lowering strategy prolonged survival in adults with diabetes and kidney disease. ACEIs and ARBs, alone or in combination, were the most effective strategies against end‐stage kidney disease.13 The reduction in ESRD risk according to increasing ACEI and ARB adherence is probably related to local activities of angiotensin II blockade in the kidney, which reduce arteriolar resistance and intraglomerular pressure. This is a local phenomenon and lasts as long as angiotensin II activity is blocked. Better ACEI/ARB adherence yields a longer effect.
In our study, nearly 80% of the patients filled more than half the prescriptions per year during the course of 9 years, although only 10% filled 12 prescriptions per year. Adherence to ACEIs/ARBs was shown to be similar to adherence to β‐blocker and lipid‐lowering medications at 3 months after hospitalization for acute coronary syndrome; the drug discontinuation rate was 26%.14 This compares with a discontinuation rate of 14% within 6 months among patients prescribed an ACEI in Hong Kong.15
We report more favorable outcomes among diabetes patients with high adherence to ACEIs/ARBs despite their increased prevalence of hypertension and ischemic heart disease, compared with diabetes patients with low adherence. The poorer clinical profile in the high adherence group might explain the better adherence rate, as the health threat seems greater. On the other hand, patients with poorer clinical profile with good adherence might benefit from greater risk reduction.
4.1. Study limitations and strengths
A major limitation of our study is that we have no information about actual medication use, only purchasing. We have no information about side effects or other medical conditions that would lead to drug discontinuation. On the other hand, since all community pharmacies used by CHS are computerized and report to one central repository, and since medications have only nominal copayments in CHS pharmacies, we presume that medications were not bought without documentation. Another caveat of our study is possible misclassification bias in distinguishing nonadherence from medication discontinuation by physicians. Unfortunately, the limitation of our database does not allow us to prove nor refute the concern. Still, we believe that this caveat of our database may not obligatory impact our results. In both cases (nonadherence and medication discontinuation by physician), the direction of possible bias is the same: less treatment days covered.
We have no information regarding the reasons why physicians decided to prescribe ACEIs/ARBs at a certain point. Moreover, we have no information regarding individuals who would have benefitted from ACEIs/ARBs and did not receive them. To avoid the inclusion of totally nonadherent patients, who represent a different group than those analyzed here, we included in the risk analysis only patients who purchased at least four monthly prescriptions of ACEIs/ARBs per year. This also ensures that the study patients would represent patients with diabetes who require ACEIs/ARBs.
We did not consider in the current study adherence to medications other than ACEIs/ARBs. A nationwide study of persons with diabetes based on the same CHS database reported an 80% adherence rate to oral antiglycemic medication among 46% of patients with diabetes treated with such.16 This is strikingly the same proportion that had >80% drug adherence in the current study. This suggests that better adherence to other medications may also have contributed to the favorable outcomes in the high adherence group in this study.
The strength of this study is that it was performed in a real‐life setting. The large number of patients, the long follow‐up period, and the completeness of follow‐up and data support the validity of the results.
CONCLUSIONS
While ACEIs/ARBs have become standards of care in diabetes, treatment adherence is essential to achieve full benefit.
CONFLICT OF INTEREST
None of the authors have any conflicts of interest to disclose.
Shani M, Vinker S, Feldman L. End‐stage renal disease and adherence to angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers among patients with diabetes. J Clin Hypertens. 2017;19:627–631. 10.1111/jch.12976
REFERENCES
- 1. Renal Replacement Therapy in Israel 1990–2010. www.health.gov.il/publicationsfiles/RRTI1990_2010.pdf. Accessed January 30, 2017.
- 2. Centers for Disease Control and Prevention . https://www.cdc.gov/diabetes/library/factsheets.html. Accessed January 30, 2017.
- 3. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. [DOI] [PubMed] [Google Scholar]
- 4. Adarkwah CC, Gandjour A, Akkerman M, Evers S. To treat or not to treat? Cost‐effectiveness of ACE inhibitors in non‐diabetic advanced renal disease—a Dutch perspective. Kidney Blood Press Res. 2013;37:168–180. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Standards of medical care in diabetes–2013. Diabetes Care. 2013;36(suppl 1):S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rennert G, Peterburg Y. Prevalence of selected chronic diseases in Israel. Isr Med Assoc J. 2001;3:404–408. [PubMed] [Google Scholar]
- 7. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. [DOI] [PubMed] [Google Scholar]
- 8. Vinker S, Shani M, Baevsky T, Elhayany A. Adherence with statins over 8 years in a usual care setting. Am J Manag Care. 2008;14:388–392. [PubMed] [Google Scholar]
- 9. Halpern R, Agarwal S, Dembek C, Borton L, Lopez‐Bresnahan M. Comparison of adherence and persistence among multiple sclerosis patients treated with disease‐modifying therapies: a retrospective administrative claims analysis. Patient Prefer Adherence. 2011;5:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shalev V, Chodick G, Silber H, Kokia E, Jan J, Heymann AD. Continuation of statin treatment and all‐cause mortality: a population‐based cohort study. Arch Intern Med. 2009;169:260–268. [DOI] [PubMed] [Google Scholar]
- 11. de Galan BE, Perkovic V, Ninomiya T, et al. Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol. 2009;20:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vejakama P, Thakkinstian A, Lertrattananon D, Ingsathit A, Ngarmukos C, Attia J. Reno‐protective effects of renin‐angiotensin systemblockade in type 2 diabetic patients: a systematic review and network meta‐analysis. Diabetologia. 2012;55:566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure‐lowering agents in adults with diabetes and kidney disease: a network meta‐analysis. Lancet. 2015;385:2047–2056. [DOI] [PubMed] [Google Scholar]
- 14. Allen LaPointe NM, Ou FS, Calvert SB, et al. Association between patient beliefs and medication adherence following hospitalization for acute coronary syndrome. Am Heart J. 2011;161:855–863. [DOI] [PubMed] [Google Scholar]
- 15. Wong MC, Lau RK, Jiang JY, Griffiths SM. Discontinuation of angiotensin‐converting enzyme inhibitors: a cohort study. J Clin Pharm Ther. 2012;37:335–341. [DOI] [PubMed] [Google Scholar]
- 16. Feldman BS, Cohen‐Stavi CJ, Leibowitz M, et al. Defining the role of medication adherence in poor glycemic control among a general adult population with diabetes. PLoS One. 2014;9:e108145. [DOI] [PMC free article] [PubMed] [Google Scholar]


