Abstract
This study investigated the effects and safety of eplerenone or thiazide diuretics in patients with hypertension and albuminuria (pretreatment urinary albumin/creatinine ratio ≥10 mg/gCr) treated with an angiotensin II receptor blocker. The primary end point was the mean percent change in the urinary albumin/creatinine ratio from baseline to 48 weeks. An efficacy analysis was performed in 195 patients (98 in the eplerenone group and 97 in the thiazide group). Systolic and diastolic blood pressures at 48 weeks were similar in the two groups. The mean percent change in the urinary albumin/creatinine ratio from baseline to 48 weeks was similar in the two groups (P=.804). In the safety analysis, the withdrawal rates for adverse events were similar in both groups. The antialbuminuric effects and safety of eplerenone therapy were similar to those of thiazide diuretics when combined with an angiotensin II receptor blocker in patients with hypertension and albuminuria.
Keywords: antihypertensive therapy, clinical trials, combination therapy, proteinuria, renal disease
1. INTRODUCTION
Albuminuria is a potent risk factor of not only end‐stage renal disease but also cardiovascular disease.1, 2, 3, 4 The renin‐angiotensin system (RAS) plays an important role in the development of hypertension and albuminuria, and thus strategies targeting treatment of albuminuria in the combination of RAS inhibitors and other hypertensive drugs are of great interest.5, 6, 7
The efficacy of low‐dose thiazide diuretics established it as an additional drug to RAS inhibitors.8, 9 In contrast, close involvement of the aldosterone breakthrough has been reported in the development of refractory hypertension and organ damage in patients being treated with RAS inhibitors,10, 11, 12 and the efficacy of inhibition of this phenomenon by combination treatment with mineralocorticoid receptor antagonists has been reported.13, 14 A new mineralocorticoid receptor antagonist, eplerenone, has fewer adverse events compared with spironolactone,15 and thus is expected to play an important role in the treatment of hypertension.
The objective of this study was to investigate the effects and safety of the combination of eplerenone with angiotensin II receptor blockers (ARBs) by comparing them with those of thiazide diuretics in patients with hypertension. We hypothesized that eplerenone would have a higher antialbuminuric effect than thiazides in patients with hypertension and albuminuria when combined with an ARB.
2. MATERIALS AND METHODS
2.1. Study design
OWASE (the Optimal Hypertension Therapy With Aldosterone Blocker Selara study) was a multicenter, prospective, open‐label randomized controlled trial conducted between June 2011 and March 2014 in 12 hospitals in Mie Prefecture, Japan. The random assignment, data collection, and analyses were performed by the Clinical Research Support Center of Mie University. This study was registered with the University Hospital Medical Information Network‐Clinical Trials Registry (UMIN‐CTR No: 000005956). The ethics committee of Mie University Hospital approved the study protocol (No. 2157) in accordance with the Declaration of Helsinki, and all patients gave written informed consent to participate.
2.2. Participants
Patients with ARB‐treated hypertension and albuminuria were recruited into the present study if they satisfied the inclusion and exclusion criteria (Table 1). Inclusion criteria were age 20 years and older; pre‐enrolled urinary albumin/creatinine ratio (UACR) ≥10 mg/gCr in spot urine; seated office systolic blood pressure (BP) ≥130 mm Hg and/or diastolic BP ≥80 mm Hg in patients with urinary protein <1 g/d, or systolic BP ≥125 mm Hg and/or diastolic BP ≥75 mm Hg in those with urinary protein ≥1 g/d; and ARB treatment for at least 3 months. BP criteria for inclusion was determined according to the Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009).16 Exclusion criteria were uncontrolled hypertension (systolic BP ≥220 mm Hg and/or diastolic BP ≥110 mm Hg); unstable angina pectoris; myocardial infarction within 6 months; severe heart failure (New York Heart Association class III or higher); frequent ventricular and/or atrial extrasystoles, prolonged ventricular tachycardia, atrial tachyarrhythmia, sick sinus syndrome and/or atrioventricular block; estimated glomerular filtration rate (eGFR) <36 mL/min/1.73 m2; severe liver dysfunction with Child‐Pugh class C; active cancer; serum potassium ≥5.0 mEq/L; diabetic microalbuminuria (UACR ≥30 mg/gCr) or proteinuria; supplementation of potassium or administration of potassium‐sparing diuretics; administration of itraconazole, ritonavir, or nelfinavir; administration of steroids or immunosuppressants; possibility of pregnancy; patients already receiving the study drugs (eplerenone or thiazide diuretic); patients with allergies or contraindications to the study drugs.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| 1. Age ≥20 y |
| 2. Pre‐enrolled UACR ≥10 mg/gCr in spot urine |
| 3. Seated office systolic BP ≥130 mm Hg and/or diastolic BP ≥80 mm Hg in patients with urinary protein <1 g/d, or systolic BP ≥125 mm Hg and/or diastolic BP ≥75 mm Hg in those with urinary protein ≥1 g/d |
| 4. ARB treatment for at least 3 mo |
| Exclusion criteria |
| 1. Uncontrolled hypertension (systolic BP ≥220 mm Hg and/or diastolic BP ≥110 mm Hg) |
| 2. Unstable angina pectoris; myocardial infarction within 6 mo |
| 3. Severe heart failure with NYHA class ≥III |
| 4. Frequent ventricular and/or atrial extrasystoles, prolonged ventricular tachycardia, atrial tachyarrhythmia, sick sinus syndrome, and/or atrioventricular block |
| 5. eGFR <36 mL/min/1.73 m2 |
| 6. Severe liver dysfunction with Child‐Pugh class C |
| 7. Active cancer |
| 8. Serum potassium ≥5.0 mEq/L |
| 9. Diabetic microalbuminuria (UACR ≥30 mg/gCr) or proteinuria |
| 10. Supplementation of potassium or administration of potassium‐sparing diuretics |
| 11. Administration of itraconazole, ritonavir, or nelfinavir |
| 12. Administration of steroids or immunosuppressants |
| 13. Possibility of pregnancy |
| 14. Patients already receiving the study drugs (eplerenone or thiazide diuretic) |
| 15. Patients with allergies or contraindications to the study drugs |
Abbreviations: ARB, angiotensin II receptor blocker; BP, blood pressure; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; UACR, urinary albumin/creatinine ratio.
2.3. Intervention
At randomization, eligible study patients were randomly assigned to receive either eplerenone (50 mg daily) or a thiazide diuretic (hydrochlorothiazide 12.5 mg daily or trichlormethiazide 1 mg daily) once daily in the morning by a minimization method that took into account the following factors: office systolic BP (<140 mm Hg, ≥140 mm Hg), UACR (10≤UACR<30 mg/gCr, 30≤UACR<300 mg/gCr, UACR≥300 mg/gCr), dosage of ARB (low dose, usual dose, high dose), and study institution. The usual dose of ARB was determined as follows: losartan 50 mg/d, candesartan 8 mg/d, valsartan 80 mg/d, telmisartan 40 mg/d, olmesartan 20 mg/d, and irbesartan 100 mg/d. The study medications were initiated within 8 weeks after randomization.
At week 0, baseline BP and laboratory dates were measured in all patients. They were then scheduled to visit at weeks 8, 16, 24, 32, and 40, and 12 months or 48 weeks as the day of their final visit. The study protocol was originally designed to assess the primary end point at 12 months (365 days) ±2 weeks. However, the day of final visit was modified to 48 weeks (336 days) ±2 weeks in the middle of the study period to allow participating institutions to schedule patient office visits more conveniently. At each visit, office BPs were measured two times with an interval of 1 or 2 minutes, and were averaged in the morning after several minutes of rest. Patients were instructed to avoid intake of caffeine or alcohol‐containing beverages from early morning and to stop smoking at hospital visits. The target office BP was <130/80 mm Hg in patients with urinary protein <1 g/d and <125/75 mm Hg in those with urinary protein ≥1 g/d. These office BP targets used in the present study were determined by the Japanese guidelines (JSH 2009)16 clinically used in 2011 when the study protocol was finalized. These BP targets were lower than those recommended in our current guidelines. If patients failed to achieve target BP control at all 6 visits before their final visit, physicians were encouraged to add antihypertensive drugs including a calcium channel blocker, an α‐blocker, and/or a β‐blocker and to increase the dosages of these drugs until the target BP was reached. Dose modification of study‐related drugs (ARBs, eplerenone, and thiazide diuretics) was prohibited.
Renal function, uric acid, electrolytes, lipid profile, and blood glucose were measured by blood examination, and urine protein (urine protein/Cr ratio) and UACR were measured at each visit. The urinary albumin concentration was measured by immunoturbidimetry at each participant institution. eGFR was calculated using the Modified Diet in Renal Disease formula modified by the Japanese Society of Nephrology (for men, 194×serum Cr levels−1.094×age−0.287; for women, 194×serum Cr levels−1.094×age−0.287×0.739).17 Glycated hemoglobin, brain natriuretic peptide, plasma renin activity, aldosterone, and brachial‐ankle pulse wave velocity were also measured at week 0 and at the final visit. When serum potassium level was elevated above 5.5 mEq/L (but <6.0 mEq/L), patients were asked to take eplerenone every 2 days by their physicians with careful observation. When serum potassium level was elevated above 6.0 mEq/L, treatment with eplerenone was discontinued. If other study drug–related adverse events occurred, the drugs were reduced or stopped at the discretion of the physicians.
2.4. End points
The primary end point was the mean percent change in UACR from baseline to the final visit. The secondary end points were changes in office BP, eGFR, brain natriuretic peptide, brachial‐ankle pulse wave velocity, and adverse events that required withdrawal of eplerenone or the thiazide diuretic.
2.5. Sample size calculation
One previous study showed a 41% to 48% reduction in UACR by 50 to 100 mg of eplerenone,18 and another study showed a 52% reduction in UACR after 50 to 200 mg of eplerenone.19 Assuming that the mean percent change in UACR in the eplerenone and thiazide diuretic groups are −50% and −40% (SD: 25% in both groups),18, 19, 20, 21 it was calculated that 100 patients would be necessary in each group if the significance level and power were set at 5% and 80%, respectively.
2.6. Statistical analysis
For analyses of the primary and secondary end points, analyses involving the full analysis set were regarded as the main analyses. The full analysis set was defined as a population consisting of enrolled patients treated at least once with a study drug in whom the primary end point, the percent change in the UACR at the final visit, can be calculated. Data are expressed as mean±SD in the Tables and mean±SE in the Figures. Continuous variables at week 0 were compared using an unpaired t test or Mann‐Whitney U test after validation of normality, and nominal variables were analyzed using chi‐square test. The mean percent change in the UACR from week 0 to the final visit was compared between the two groups using an unpaired t test. For BPs and the UACR, two‐way repeated measures analysis of variance with post hoc analysis was applied to determine the main effects for group, time, and interaction, and to evaluate the differences between groups.
Patients who were treated at least once with a study drug after enrollment were defined as the safety analysis set. For comparison of discontinuation because of adverse events between the two groups, analyses involving the safety analysis set were performed using chi‐square test. In addition, as a subanalysis, subgroups with a urinary albumin level at 0 weeks of “high normal” (10≤UACR<30 mg/gCr), “microalbuminuria” (30≤UACR<300 mg/gCr), and “macroalbuminuria” (UACR ≥300 mg/gCr) were analyzed. Statistical analyses were performed with SAS version 9.4 (SAS Institute). P<.05 was considered significant.
3. RESULTS
3.1. Participant flow
We enrolled 253 patients; 127 were assigned to receive eplerenone and 126 to thiazide diuretics (Figure 1). Of the allocated patients, 15 patients were excluded before administration of study drugs and thus 238 patients (eplerenone, n=117; thiazide, n=121) were included in the safety assessment. Ten of 117 patients in the eplerenone group and 12 of 121 patients in the thiazide group discontinued the study drugs mainly because of adverse events. The UACR was not measured at baseline or the last visit in 21 patients and thus the mean percent change in the UACR was assessed in 195 patients. The changes in office BP, eGFR, brain natriuretic peptide, and brachial‐ankle pulse wave velocity were assessed in 195 patients, and adverse events that required withdrawal of eplerenone or thiazide diuretic were assessed in 238 patients.
Figure 1.
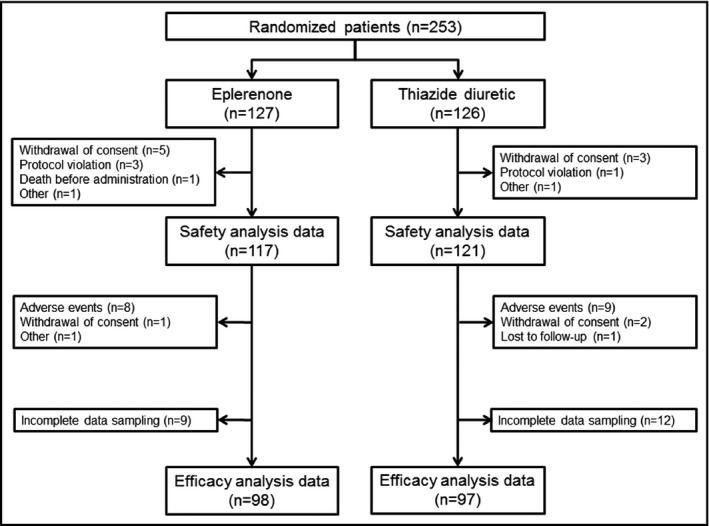
Participant flow. The efficacy analysis was performed in 195 patients with complete data sampling (98 in the eplerenone group and 97 in the thiazide diuretic group)
3.2. Baseline data
There was no significant difference between the two groups in systolic and diastolic BPs when randomized, but systolic BP was significantly higher in the thiazide group (eplerenone, 142.7±13.9 mm Hg; thiazide, 147.2±16.3 mm Hg [P=.040]) at week 0 (Figure 2A). Systolic BP and history of myocardial infarction were the only differences in baseline characteristics between the two groups (Table 2). Serum creatinine was the only difference between the two groups in the clinical laboratory parameters, while the UACR tended to be higher in the thiazide group, without statistical significance (eplerenone, 90.9±120.8 mg/gCr; thiazide, 179.5±584.2 mg/gCr; [P=.146]) (Table 3).
Figure 2.
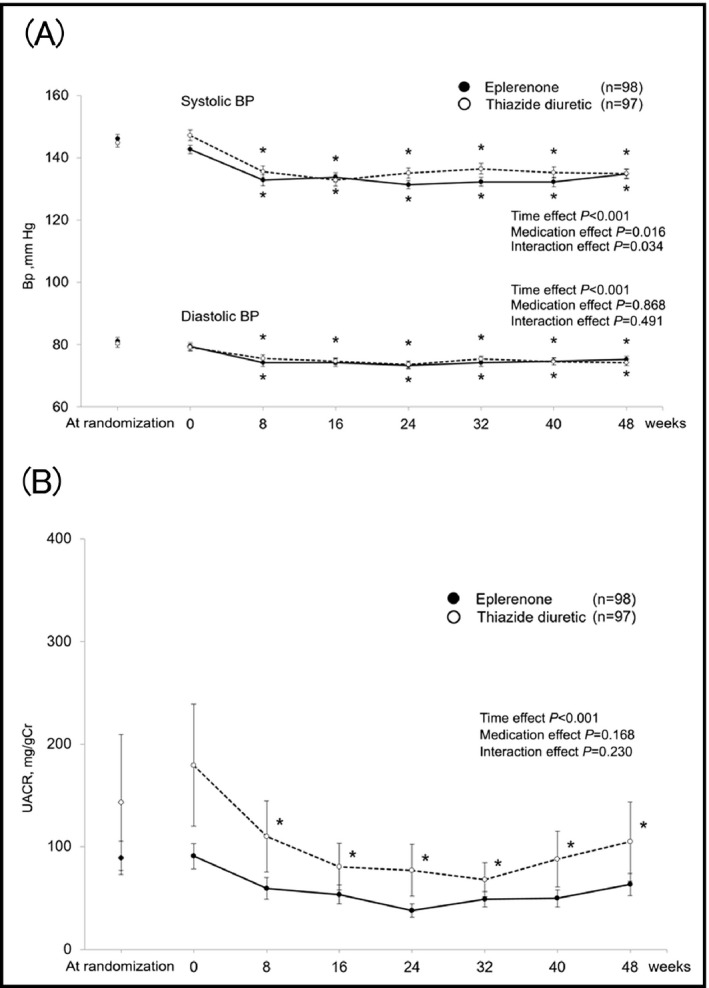
Time course of blood pressure (BP) (A) and urinary albumin/creatinine ratio (UACR) (B). Data are mean±SE. *P<.05 vs 0 wk
Table 2.
Baseline characteristics
| Eplerenone (n=98) | Thiazide (n=97) | P value | |
|---|---|---|---|
| Age, y | 70.9±10.1 | 71.9±9.7 | .500 |
| Men | 49 (50.0) | 49 (50.5) | >.999 |
| Office blood pressure, mm Hg | |||
| Systolic | 142.7±13.9 | 147.2±16.3 | .040 |
| Diastolic | 79.4±11.8 | 79.1±10.4 | .851 |
| Pulse rate, beats per min | 72.3±12.2 | 72.3±12.6 | .962 |
| Body mass index, kg/m2 | 24.7±3.5 | 24.8±4.4 | .914 |
| Smoking | 9 (9.2) | 2 (2.1) | .058 |
| Diabetes mellitus | 14 (14.3) | 22 (22.7) | .144 |
| Dyslipidemia | 54 (55.1) | 63 (64.9) | .189 |
| Cardiovascular diseases | |||
| Myocardial infarction | 4 (4.1) | 12 (12.4) | .040 |
| Stroke | 10 (10.2) | 16 (16.5) | .213 |
| Heart failure | 5 (5.1) | 3 (3.1) | .721 |
| Medications | |||
| Angiotensin receptor blocker | |||
| Candesartan | 30 (30.6) | 34 (35.1) | .409 |
| Olmesartan | 20 (20.4) | 24 (24.7) | |
| Valsartan | 24 (24.5) | 19 (19.6) | |
| Telmisartan | 17 (17.3) | 11 (11.3) | |
| Losartan | 3 (3.1) | 6 (6.2) | |
| Irbesartan | 4 (4.1) | 1 (1.0) | |
| Angiotensin receptor blocker dose | |||
| High | 17 (17.3) | 17 (17.5) | >.999 |
| Medium | 67 (68.4) | 66 (68.0) | |
| Low | 14 (14.3) | 14 (14.4) | |
| Concomitant antihypertensive drugs | |||
| Calcium channel blocker | 66 (67.3) | 62 (63.9) | .653 |
| Alpha blocker | 7 (7.1) | 4 (4.1) | .537 |
| Beta blocker | 25 (25.5) | 20 (20.6) | .497 |
| Others | |||
| Statin | 47 (48.0) | 60 (61.9) | .062 |
| Insulin | 2 (2.0) | 2 (2.1) | >.999 |
| Antihyperuricemic drug | 9 (9.2) | 9 (9.3) | >.999 |
Data are number (percentage) or mean±SD.
Table 3.
Baseline laboratory data
| Eplerenone (n=98) | Thiazide (n=97) | P value | |
|---|---|---|---|
| Serum creatinine, mg/dL | 0.82±0.24 | 0.76±0.21 | .044 |
| eGFR, mL/min per 1.73 m2 | 67.0±18.2 | 76.2±47.8 | .078 |
| Uric acid, mg/dL | 5.7±1.6 | 5.4±1.4 | .205 |
| Serum potassium, mEq/L | 4.10±0.42 | 4.17±0.45 | .268 |
| Total cholesterol, mg/dL | 182±28 | 183±30 | .837 |
| Triglycerides, mg/dL | 117±62 | 128±120 | .432 |
| HDL cholesterol, mg/dL | 57±17 | 60±16 | .295 |
| Blood glucose, mg/dL | 105±21 | 107±33 | .580 |
| Glycated hemoglobin, % | 5.8±0.5 | 5.9±0.5 | .250 |
| Plasma renin activity, ng/mL per h | 3.2±4.5 | 2.5±3.7 | .251 |
| Plasma aldosterone, pg/mL | 76.5±48.3 | 68.4±32.1 | .177 |
| BNP, pg/mL | 45.9±60.9 | 44.0±53.8 | .839 |
| UACR, mg/gCr | 90.9±120.8 | 179.5±584.2 | .146 |
| (mg/mmolCr) | 9.09±12.1 | 18.0±58.4 | |
| Urine protein, g/gCr | 0.19±0.21 | 0.27±0.48 | .252 |
| baPWV, cm/s | 1896±502 | 1892±432 | .966 |
Data are mean±SD. Abbreviations: baPWV, brachial‐ankle pulse wave velocity; BNP, brain natriuretic peptide; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; UACR, urinary albumin/creatinine ratio.
3.3. Time courses in BP
Systolic and diastolic BPs reduced after the initiation of study drug administration in both groups (time effect P<.001) with a significant interaction effect in the changes in systolic BP (interaction effect P=.034). The BPs were similar in the two groups at the final visit (systolic BP: 134.8±15.2 mm Hg in the eplerenone group and 134.9±14.4 mm Hg in the thiazide group, diastolic pressure: 75.3±10.3 mm Hg in the eplerenone group and 74.3±10.2 mm Hg in the thiazide group [P=.940 and 0.479, respectively]) (Figure 2A). The achievement rates of target BP at the final visit were similar between the two groups (eplerenone, 33.0%; thiazide, 34.0% [P=.879]). Only 19.4% of patients in the eplerenone group and 15.5% in the thiazide group were prescribed additional antihypertensive drugs during study drug administration.
3.4. Time courses in albuminuria and primary end point
There was no statistical difference in UACR between the two groups at week 0, but several patients in the thiazide group had extremely high UACR, resulting in high standard errors. UACR decreased after 8 weeks only in the thiazide group, while there was no significant group×time interaction in the effects of the two treatments on UACR (Figure 2B). The mean percent change in the UACR at the final visit was not significantly different between the two groups (eplerenone, 1.7%; thiazide, −1.4%, [P=.804]) (Figure 3).
Figure 3.
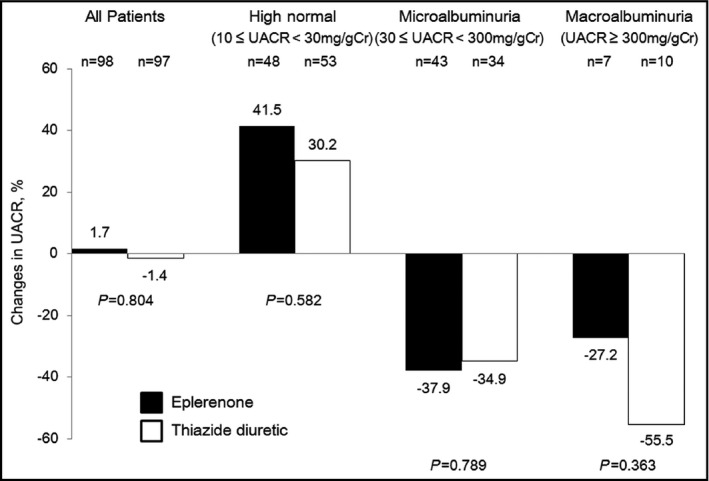
Changes from baseline in urinary albumin/creatinine ratio (UACR) at 48 weeks. All patients and subanalysis
3.5. Secondary end points
Table 4A shows the results of the secondary end points. No significant differences were noted in the change of eGFR, brain natriuretic peptide, or brachial‐ankle pulse wave velocity between the two groups. Table 4B shows adverse events that required discontinuation of study drug administration in the two groups (eplerenone, n=117; thiazide, n=121). There was no significant difference in the incidence of adverse events between the two groups (eplerenone, 6.8%; thiazide: 7.4% [P=.857]). The most frequent causes of discontinuation were hyperkalemia (2 cases, 1.7%) in the eplerenone group and skin eruption (4 cases, 3.3%) in the thiazide group. Average baseline serum potassium level in the eplerenone group (n=117) was 4.11±0.40 mEq/L and that in the hydrochlorothiazide group (n=121) was 4.18±0.45 mEq/L in patients analyzed by safety analysis set analysis. At the end of the intervention, the serum potassium level in the eplerenone group and hydrochlorothiazide group were 4.24±0.46 and 4.13±0.41 mEq/L, respectively. The percent change in serum potassium was 3.49% in the eplerenone group and −0.74% in the hydrochlorothiazide group (P=.001).
Table 4.
Secondary end points and adverse events that required withdrawal of a study drug
| (A) Secondary end points | |||
|---|---|---|---|
| Eplerenone (n=98) | Thiazide (n=97) | P value | |
| Change in systolic BP, mm Hg | −8.0±13.5 | −12.4±15.5 | .037 |
| Change in diastolic BP, mm Hg | −4.4±8.9 | −5.1±11.0 | .629 |
| Change in eGFR, mL/min per 1.73 m2 | −4.2±10.1 | −11.0±42.0 | .084 |
| Change in BNP, pg/mL | −1.2±44.9 | 3.0±40.5 | .872 |
| Change in baPWV | −102±341 | −65±243 | .281 |
| (B) Adverse events that required withdrawal of study drug | |||
|---|---|---|---|
| Eplerenone (n=117) | Thiazide (n=121) | P value | |
| 8 (6.8) | 9 (7.4) | .857 | |
| Hyperkalemia | 2 | 0 | |
| Hypokalemia | 0 | 1 | |
| Heart failure | 1 | 0 | |
| Edema | 1 | 0 | |
| Headache | 1 | 0 | |
| Memory disorder | 1 | 0 | |
| Thirstiness | 1 | 0 | |
| Skin eruption | 0 | 4 | |
| Hypotension | 0 | 2 | |
| Polyuria | 0 | 1 | |
| Renal dysfunction | 0 | 1 | |
| Other | 1 | 0 | |
3.6. Subanalysis
To minimize the confounding effect of baseline UACR values on their reduction after the administration of study drugs, subanalyses were performed in subgroups with baseline UACR (high normal, microalbuminuria, and macroalbuminuria). Figures [Link], [Link], [Link] show time‐course changes in BP and the UACR for subanalyses. Systolic and diastolic BPs at final visit were not significantly different between the two groups in all subgroups. The mean percent change in the UACR at final visit was not significantly different between the two groups in all subgroups (Figure 3).
4. DISCUSSION
The effects and safety of eplerenone, administered additionally to ARBs, were compared with those of thiazide diuretics in patients with hypertension who had albuminuria, and the mean percent change in UACR at about 48 weeks was similar in the two treatment groups, although the thiazide diuretic may have had a greater BP‐lowering effect in the present study. In addition, there was no significant difference in the incidence of adverse events.
RAS inhibitors (angiotensin‐converting enzyme inhibitor or ARB) are recommended as first‐line drugs for patients with hypertension who have chronic kidney disease, and ARBs are more frequently used than angiotensin‐converting enzyme inhibitors because of their strong antihypertensive effect and lower incidence of adverse events in Japan. However, to achieve target BP control, ARBs alone are sometimes insufficient, and multidrug combination therapy is necessary.22 Low‐dose thiazide diuretics effectively reduce BP in combination with ARBs.23, 24 Several previous studies reported that they have not only an antihypertensive but also a urinary protein–decreasing effect.25, 26, 27 Separately, add‐on therapy of spironolactone to RAS inhibitors inhibits the aldosterone breakthrough phenomenon and may lead to renal protection in patients with chronic kidney disease. Sato and colleagues13, 28 demonstrated that add‐on therapy of spironolactone to ARB treatment reduced urinary protein in patients with diabetes who experienced the aldosterone breakthrough phenomenon. In patients with resistant hypertension, some countries advocate low‐dose spironolactone, however, spironolactone has not been widely used in the treatment of hypertension because of a high incidence of sex hormone–related adverse events including gynecomastia. Eplerenone has been available since 2007 in Japan, and it causes fewer sex hormone–related adverse events as a result of its negligible affinity for androgen or progesterone receptors than spironolactone. Previous clinical studies reported that eplerenone significantly reduced urinary albumin compared with amlodipine or enalapril,19 but only a few studies have investigated its effect on albuminuria by comparison with thiazide diuretics.29 The lack of clinical data on eplerenone may have been because of restriction of its administration for patients with diabetic nephropathy with overt proteinuria and patients with chronic kidney disease with moderate or severe renal dysfunction.
Contrary to our expectations, UACR did not decrease from baseline and the mean percent change was 1.7% after about 48 weeks of eplerenone treatment. In the subgroup analyses in patients with 30≤UACR<300 mg/gCr, the mean percent change in UACR after about 48 weeks was more than 30% in both groups. In several previous studies, the urinary albumin reduction rate achieved by a mineralocorticoid receptor antagonist was −20% to −50%,18, 19 and we determined the necessary number of patients based on these reports. However, the reduction in UACR was smaller in the present study compared with previous studies. Many previous studies selected patients with diabetes with a very high baseline urinary albumin level,30 or patients without administration of RAS inhibitors.19 In the present study, since patients with 10≤UACR<30 mg/gCr, who had exhibited no decrease in UACR accounted for about half of the enrolled patients, the antialbuminuric effect of the BP reduction may not have readily appeared. These differences in baseline urine albumin levels and study protocols may partly explain the discrepancy.
There was no significant difference in the incidence of adverse events requiring discontinuation of study drug administration between the two groups. Combination therapy of RAS inhibitors such as ARBs and angiotensin‐converting enzyme inhibitors effectively decreases urinary protein,31 but it is not recommended for the treatment of hypertension because of a high incidence of adverse events including hyperkalemia.32, 33 However, the incidence of hyperkalemia in the combination of an ARB and eplerenone was within the acceptable range in the present study. For patients with relatively mild renal disease, the combination of an ARB and eplerenone is as safe as the combination of an ARB and thiazide diuretics.
In the present study, only about 30% of patients achieved the target BP by additional administration of eplerenone or thiazide diuretics according to JSH 2009.16 About 65% of the enrolled patients had already been treated with a calcium channel blocker in the present study, which might narrow the choices of additional antihypertensive agent after study drug administration even when BP control was suboptimal. Indeed, only 19.4% in the eplerenone group and 15.5% in the thiazide group were prescribed additional antihypertensive drugs during study drug administration.
4.1. Limitations
There were several limitations to this study. Baseline systolic BP at week 0 was significantly higher in the thiazide group despite being one of the allocation factors for randomization. Urinary albumin level at baseline, measured at each participant institution not at a central laboratory, tended to be higher in the thiazide group. The present study protocol was originally designed to assess the primary end point at 12 months (365 days) ±2 weeks. However, the day of final visit was modified to 48 weeks (336 days) ±2 weeks in the middle of the study period to allow the participating institution to schedule patient office visits more conveniently. As a result, the final visit ranged between 322 and 379 days, which may have influenced the study results. In addition, the number of excluded patients from the efficacy analysis was not small, partly because of incomplete bladder emptying for urine sampling. Twenty‐four‐hour urine collections were not included in the present study. Salt intake and the effects of the study drug were not investigated in the enrolled patients.34 We hypothesized that 50 mg of eplerenone and 12.5 mg of hydrochlorothiazide would lower BP to a similar extent. However, the dose of hydrochlorothiazide prescribed in the present study appeared to be low, and our dose selection may have affected the results. The standard dose and maximum dose of ARBs prescribed in Japan are smaller than those in Western countries mainly because of smaller body size, which can lead to underestimation of adverse effects including renal impairment and hyperkalemia. We were unable to completely exclude patients with secondary hypertension such as hyperaldosteronism, who may show the beneficial effects of eplerenone on BP. As no measurements of urinary drug metabolites were performed, we were unable to ensure compliance with the study protocol. Finally, renal and overall prognoses were not evaluated. A large‐scale clinical study with long‐term follow‐up is warranted.
5. CONCLUSIONS
The antialbuminuric effects and safety of additional administration of eplerenone to patients under antihypertensive therapy with ARBs are equivalent to those of thiazide diuretics.
6. AUTHORS' CONTRIBUTIONS
All of the authors met the criteria for authorship. Toshiki Sawai contributed to conception and design of the work, acquisition of data, and wrote the first draft of the paper. Setsuya Okubo, Naoki Isaka, Katsutoshi Makino, Shinya Okamoto, Sukenari Koyabu, and Tetsuya Kitamura contributed to the acquisition of data. Naoki Fujimoto and Kaoru Dohi contributed to the interpretation of data and helped revise the manuscript. Toru Ogura contributed to the analysis of data. Tomomi Yamada contributed to the design of the work and analysis of data. Satoshi Tamaru and Masakatsu Nishikawa contributed to the design of the work. Mashio Nakamura contributed to the conception of the work and revised the manuscript critically for important intellectual content. Masaaki Ito contributed to the conception of the work and provided final approval of the version to be published. All authors approved the final draft of the manuscript.
7. CONFLICT OF INTEREST
Toshiki Sawai, Setsuya Okubo, Naoki Isaka, Takehiko Ichikawa, Katsutoshi Makino, Shinya Okamoto, Sukenari Koyabu, Naoki Fujimoto, Toru Ogura, Tomomi Yamada, and Satoshi Tamaru have no conflicts of interest to declare.
Supporting information
ACKNOWLEDGMENTS
The authors gratefully acknowledge all medical doctors, nurses, and clinical research coordinators who cooperated with the OWASE study.
APPENDIX 1.
1.1.
The complete list of OWASE Study Investigators is as follows: Toshiki Sawai, MD; Setsuya Okubo, MD; Naoki Isaka, MD; Takehiko Ichikawa, MD; Katsutoshi Makino, MD; Shinya Okamoto, MD; Sukenari Koyabu, MD; Tetsuya Kitamura, MD; Naoki Fujimoto, MD; Kaoru Dohi, MD; Toru Ogura, PhD; Tomomi Yamada, PhD; Satoshi Tamaru, MD; Masakatsu Nishikawa, MD; Mashio Nakamura, MD; Masaaki Ito, MD; Yuki Nishimura, PhD; Eitaro Fujii, MD; Takashi Omura, MD; Takuya Mori, MD; Tetsushiro Takeuchi, MD; Ryuji Okamoto, MD; Tomohiro Murata, MD; Michiharu Senga, MD; Eiji Ishikawa, MD; Kazuki Oosugi, MD; Muneyoshi Tanimura, MD; Hiroo Itoh, MD; Satoshi Fujita, MD; Hiroshi Nakajima, MD; Satoko Uraki, MD; Keiichiro Nojiri, MD; Hideo Mizutani, MD; Norikazu Yamada, MD; Katsuhisa Konishi, MD; Sonoko Matsuyama, MD; Hitoshi Iwasaki, MD; Takashi Tanigawa, MD; Masaya Taniguchi, MD; Kiyotaka Watanabe, MD; Satoshi Ota, MD; Katsuya Shiraki, MD; Yasuhiro Hotta, MD; Daisuke Izumi, MD; Akihiro Tsuji, MD; Kentaro Kakuta, MD; Emiyo Sugiura, MD; Akimasa Matsuda, MD; Tadafumi Sugimoto, MD; Tetsuya Seko, MD, Yoshimi Hamanaka, Yukako Tsujimoto, Mayumi Kotera, Chisato Minamide, Rie Kurimoto, Sanae Muramatsu.
Sawai T, Dohi K, Fujimoto N, et al. Antialbuminuric effect of eplerenone in comparison to thiazide diuretics in patients with hypertension. J Clin Hypertens. 2017;19:990–998. 10.1111/jch.13054
Funding information
This study was an academic research project supported by a research grant from Pfizer Japan Inc., Tokyo, Japan. The study sponsor was not involved in the study design, collection, analysis, or interpretation of the data. The Department of Cardiology and Nephrology, Mie University Graduate School of Medicine, received research grants of equal to or more than 1 000 000 ¥ from Pfizer Japan Inc., Bristol‐Myers Squibb, Astellas Pharma Inc., Biotronik Japan. Inc., Genzyme Japan, Shionogi & Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., AstraZeneca K.K., MSD K.K., and Nippon Boehringer Ingelheim Co., Ltd. Masaaki Ito received lecture fees of more than 500 000 ¥ from Daiichi Sankyo Co., Ltd. and Mochida Pharmaceutical Co., Ltd. in 2015. Tetsuya Kitamura received lecture fees from Goodman Co., Ltd., Kaneka Medix Corp., Orbusneich Medical K.K., Shionogi & Co., Ltd., MSD K.K., and Biosensors Japan Co., Ltd., in 2015. Kaoru Dohi received lecture fees of more than 500 000 ¥ from Otsuka Pharmaceutical Co., Ltd. in 2015. Masakatsu Nishikawa received lecture fees of more than 500 000 ¥ from Daiichi Sankyo Co., Ltd. in 2015. Mashio Nakamura received lecture fees of more than 500 000 ¥ from Daiichi Sankyo Co., Ltd., Bayer Yakuhin, Ltd., and Pfizer Japan Inc. in 2015.
OWASE Study Investigators are listed in the Appendix.
Contributor Information
Masaaki Ito, Email: mitoka@clin.medic.mie-u.ac.jp.
the OWASE Study Investigators:
Masaaki Ito, Yuki Nishimura, Eitaro Fujii, Takashi Omura, Takuya Mori, Tetsushiro Takeuchi, Ryuji Okamoto, Tomohiro Murata, Michiharu Senga, Eiji Ishikawa, Kazuki Osugi, Muneyoshi Tanimura, Hiroo Ito, Satoshi Fujita, Hiroshi Nakajima, Satoko Uraki, Keiichiro Nojiri, Hideo Mizutani, Norikazu Yamada, Katsuhisa Konishi, Sonoko Matsuyama, Hitoshi Iwasaki, Takashi Tanigawa, Masaya Taniguchi, Kiyotaka Watanabe, Satoshi Ota, Katsuya Shiraki, Yasuhiro Hotta, Daisuke Izumi, Akihiro Tsuji, Kentaro Kakuta, Emiyo Sugiura, Akimasa Matsuda, Tadafumi Sugimoto, Tetsuya Seko, Yoshimi Hamanaka, Yukako Tsujimoto, Mayumi Kotera, Chisato Minamide, Rie Kurimoto, and Sanae Muramatsu
REFERENCES
- 1. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154‐2169. [DOI] [PubMed] [Google Scholar]
- 2. Nakamura K, Okamura T, Hayakawa T, et al. Chronic kidney disease is a risk factor for cardiovascular death in a community‐based population in Japan: NIPPON DATA90. Circ J. 2006;70:954‐959. [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296‐1305. [DOI] [PubMed] [Google Scholar]
- 4. Irie F, Iso H, Sairenchi T, et al. The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int. 2006;69:1264‐1271. [DOI] [PubMed] [Google Scholar]
- 5. Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin‐angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta‐analysis. Lancet. 2005;366:2026‐2033. [DOI] [PubMed] [Google Scholar]
- 6. Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin‐converting enzyme inhibition: a patient‐level meta‐analysis. Ann Intern Med. 2003;139:244‐252. [DOI] [PubMed] [Google Scholar]
- 7. Makino H, Haneda M, Babazono T, et al. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care. 2007;30:1577‐1578. [DOI] [PubMed] [Google Scholar]
- 8. Bakris GL, Toto RD, McCullough PA, et al. Effects of different ACE inhibitor combinations on albuminuria: results of the GUARD study. Kidney Int. 2008;73:1303‐1309. [DOI] [PubMed] [Google Scholar]
- 9. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359:2417‐2428. [DOI] [PubMed] [Google Scholar]
- 10. Staessen J, Lijnen P, Fagard R, et al. Rise in plasma concentration of aldosterone during long‐term angiotensin II suppression. J Endocrinol. 1981;91:457‐465. [DOI] [PubMed] [Google Scholar]
- 11. Schjoedt KJ, Andersen S, Rossing P, et al. Aldosterone escape during blockade of the renin‐angiotensin‐aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia. 2004;47:1936‐1939. [DOI] [PubMed] [Google Scholar]
- 12. Chapman N, Dobson J, Wilson S, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839‐845. [DOI] [PubMed] [Google Scholar]
- 13. Sato A, Hayashi K, Naruse M, et al. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003;41:64‐68. [DOI] [PubMed] [Google Scholar]
- 14. Pitt B, Reichek N, Willenbrock R, et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E‐left ventricular hypertrophy study. Circulation. 2003;108:1831‐1838. [DOI] [PubMed] [Google Scholar]
- 15. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309‐1321. [DOI] [PubMed] [Google Scholar]
- 16. Ogihara T, Kikuchi K, Matsuoka H, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2009). Hypertens Res. 2009;32:3‐107. [PubMed] [Google Scholar]
- 17. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982‐992. [DOI] [PubMed] [Google Scholar]
- 18. Epstein M, Williams GH, Weinberger M, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1:940‐951. [DOI] [PubMed] [Google Scholar]
- 19. White WB, Duprez D, St Hillaire R, et al. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension. 2003;41:1021‐1026. [DOI] [PubMed] [Google Scholar]
- 20. Joffe HV, Kwong RY, Gerhard‐Herman MD, et al. Beneficial effects of eplerenone versus hydrochlorothiazide on coronary circulatory function in patients with diabetes mellitus. J Clin Endocrinol Metab. 2007;92:2552‐2558. [DOI] [PubMed] [Google Scholar]
- 21. Ekinci EI, Thomas G, Thomas D, et al. Effects of salt supplementation on the albuminuric response to telmisartan with or without hydrochlorothiazide therapy in hypertensive patients with type 2 diabetes are modulated by habitual dietary salt intake. Diabetes Care. 2009;32:1398‐1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646‐661. [DOI] [PubMed] [Google Scholar]
- 23. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995‐1003. [DOI] [PubMed] [Google Scholar]
- 24. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022‐2031. [DOI] [PubMed] [Google Scholar]
- 25. Hasegawa H, Tayama Y, Takayanagi K, et al. Release from glomerular overload by the addition of low‐dose thiazide in patients with angiotensin receptor blocker‐resistant hypertension. Kidney Blood Press Res. 2013;37:521‐530. [DOI] [PubMed] [Google Scholar]
- 26. Vogt L, Waanders F, Boomsma F, et al. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol. 2008;19:999‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ueda T, Kai H, Imaizumi T, et al. Losartan/hydrochlorothiazide combination vs. high‐dose losartan in patients with morning hypertension–a prospective, randomized, open‐labeled, parallel‐group, multicenter trial. Hypertens Res. 2012;35:708‐714. [DOI] [PubMed] [Google Scholar]
- 28. Sato A, Hayashi K, Naruse M, et al. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2002;41:64‐68. [DOI] [PubMed] [Google Scholar]
- 29. Ohta Y, Ishizuka A, Hayashi S, et al. Effects of a selective aldosterone blocker and thiazide‐type diuretic on blood pressure and organ damage in hypertensive patients. Clin Exp Hypertens. 2015;37:569‐573. [DOI] [PubMed] [Google Scholar]
- 30. Navaneethan SD, Nigwekar SU, Sehgal AR, et al. Aldosterone antagonists for preventing the progression of chronic kidney disease: a systematic review and meta‐analysis. Clin J Am Soc Nephrol. 2009;4:542‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kunz R, Friedrich C, Wolbers M, et al. Meta‐analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30‐48. [DOI] [PubMed] [Google Scholar]
- 32. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547‐1559. [DOI] [PubMed] [Google Scholar]
- 33. Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double‐blind, controlled trial. Lancet. 2008;372:547‐553. [DOI] [PubMed] [Google Scholar]
- 34. Ando K, Ohtsu H, Uchida S, et al. Anti‐albuminuric effect of the aldosterone blocker eplerenone in non‐diabetic hypertensive patients with albuminuria: a double‐blind, randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2014;2:944‐953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


