Abstract
This retrospective cohort study compared blood pressure (BP) control (BP <140/90 mm Hg) and all‐cause mortality between US‐ and foreign‐born blacks. We used data from a clinical data warehouse of 41 868 patients with hypertension who received care in a New York City public healthcare system between 2004 and 2009, defining BP control as the last recorded BP measurement and mean BP control. Poisson regression demonstrated that Caribbean‐born blacks had lower BP control for the last BP measurement compared with US‐ and West African–born blacks, respectively (49% vs 54% and 57%; P<.001). This pattern was similar for mean BP control. Caribbean‐ and West African–born blacks showed reduced hazard ratios of mortality (0.46 [95% CI, 0.42–0.50] and 0.28 [95% CI, 0.18–0.41], respectively) compared with US‐born blacks, even after adjustment for BP. BP control rates and mortality were heterogeneous in this sample. Caribbean‐born blacks showed worse control than US‐born blacks. However, US‐born blacks experienced increased hazard of mortality. This suggests the need to account for the variations within blacks in hypertension management.
Keywords: clinical management of high blood pressure, hypertension, hypertension in blacks, vascular disease
1. INTRODUCTION
Hypertension disproportionately affects blacks in the United States and this disparity may account for a portion of the disparity in life expectancy noted in blacks compared with other ethnic/racial groups in the United States.1 With current hypertension prevalence rates at 43% for blacks compared with approximately 32% in whites,2, 3, 4, 5, 6, 7, 8 there is an urgency to identify black patients with hypertension who are at increased risk to better target preventative measures. This is important given the ethnic variations in hypertension prevalence and its outcomes among blacks,7, 9 with the various ethnic groups showing differential risk.10 Moreover, although foreign‐born blacks make up 13.2% of the nation's black population of African descent,11 most studies treat blacks as a monolithic group, ignoring the contribution of important behavioral and lifestyle variations influenced by ethnicity on hypertension and its management.1, 12, 13
Findings from the few available studies show significant differences in hypertension risk, prevalence, and control between US‐born blacks and foreign‐born blacks. Cooper and colleagues7 suggest a consistent increasing gradient of hypertension risk in populations among the African diaspora. Rural West African populations were noted to have significantly lower hypertension prevalence rates compared with urban US‐born black populations, with intermediate prevalence noted in the Caribbean.7 Moran and colleagues’ 10‐year Multiethnic Study of Atherosclerosis (MESA)14 found that foreign‐born blacks had a relatively low prevalence of hypertension; however, hypertension increased with years of acculturation.14 Hyman and colleagues’15 cross‐sectional survey of 87 African‐born and 95 US‐born black health professionals found a lower prevalence of hypertension in first‐generation Africans in the United States. Also, national data from 2005 to 2008 show that the blood pressure (BP) control rates for foreign‐born blacks was 24.9% vs 30.8% for US‐born blacks.16
Identifying the differential risk of hypertension among blacks will ensure early detection and timely treatment for these high‐risk ethnic groups. Findings from Fejerman and colleagues17 show fewer Nigerians reporting inconstant treatment for hypertension than US‐born blacks, although the rate of hypertension among Nigerians was more than twice that of US‐born blacks (71% vs 29%, respectively). Furthermore, there exists a nativity difference in all‐cause and disease‐specific mortality. Analysis of data from the national longitudinal mortality study from 1979 to 1989 found an 8% higher all‐cause mortality risk for US‐born blacks compared with US‐born whites.18 Moreover, the mortality risk for US‐born blacks is twice the risk for foreign‐born blacks.18 Clearly, there is a need to document the heterogeneous nature of hypertension within the US black population and evaluate hypertension control rates (defined as BP <140/90 mm Hg) and possible associated mortality outcomes.
The aims of this study are two‐fold. First, we examined the rates of BP control among Caribbean, West African, and US blacks among patients attending New York City's (NYC's) Health and Hospital Corporation's (HHC's) outpatient clinics. Second, we compared the all‐cause mortality rates between the various black ethnic groups.
2. METHODS
2.1. Study design, setting, and population
Using a retrospective cohort design, we extracted electronic health record data (BP measurements, weight, prescription refills, laboratory test results, clinical diagnoses, encounter diagnoses for outpatient visits, diagnostic imaging tests, and healthcare utilization) from NYC HHC's clinical data warehouse, using derived codes and fields in the electronic health record.3
NYC HHC is a municipal healthcare system with 15 data warehouses containing healthcare data from 11 acute care hospitals, six diagnostic and treatment centers, four long‐term care facilities, and over 80 community‐based ambulatory care practices.2 NYC HHC provides 20% to 30% of all general hospital, emergency department, and clinic visits in NYC. Approximately 35% of patients seen in the HHC system are black and 7% are white.
The study population was composed of patients who self‐identified as black or African American; were 18 years and older; received care within HHC between January 1, 2004, and December 31, 2009; and had a hypertension diagnosis (based on hypertension International Classification of Diseases, Ninth Revision codes) in their medical record. We excluded patients who were not self‐identified as black or African American. The study was approved by the institutional review boards of both the New York University School of Medicine and the NYC HHC.
2.2. Independent and dependent variables
2.2.1. Region of birth
The black sample, consisting of 41 868 individuals from the HHC, was examined separately in terms of self‐reported region of birth. Among the black HHC population, the three most common regions of birth were the United States, the Caribbean, and West Africa. Of the analysis sample, 2058 participants reported being born in a West African country. Of the total sample, 18 476 reported being born in a Caribbean country (Antigua/Barbuda, Aruba, Barbados, British Virgin Islands, Cayman Islands, Cuba, Curacao, Dominica, Dominican Republic, Grenada, Haiti, Jamaica, Martinique, Montserrat, Puerto Rico, St Kitts/Nevis, St Lucia, St Maarten, St Vincent/Grenadines, Trinidad/Tobago, and US Virgin Islands), while 21 334 individuals reported being born in the United States.
2.2.2. Controlled hypertension
The dependent variable was controlled hypertension, defined as BP <140/90 mm Hg. BP data were collected a minimum of 4 months following the patient's diagnosis with hypertension for a 1‐year assessment window. This lag was used to allow all patients time to receive treatment for their hypertension. Patients were then evaluated in terms of BP control over this 1‐year period. BP control over the 1‐year assessment period was defined using two metrics based on previous observational research conducted in a managed care setting.19 These metrics included having the last BP measurements during the year controlled (last controlled BP reading) and having the average BP measurement over the year controlled (mean BP controlled). The majority of BP measurements (88.1%) were taken from outpatient facilities. The remaining 11.9% of measurements were taken from acute or ambulatory surgery facilities. In the sample, 78.2% of patients had all of their BP measurements in the clinic, while 21.8% had at least one BP measurement in an acute care setting.
For the secondary analysis, all‐cause mortality was confirmed based on medical records and New York State vital statistics data. All‐cause mortality included death caused by any illnesses including cardiovascular disease. Mortality rates were examined in the subset of the sample with follow‐up data following their BP assessment window.
2.2.3. Sample demographics, medications, and comorbidity
Important sample demographics included age, sex, and body mass index. We also measured the number of antihypertensive medications prescribed. If a patient was prescribed an antihypertensive medication prior to the end of their 12‐month BP assessment range, they were coded as having a history of taking that medication. Antihypertensive medications included aldosterone antagonists, combined α‐/β‐blockers, angiotensin‐converting enzyme inhibitors, peripherally acting α‐adrenergic receptor antagonists, angiotensin receptor blockers, β‐blockers, centrally acting adrenergic drugs, calcium channel blockers, diuretics, and centrally acting α‐agonists. The total number of antihypertensive medication classes was determined by summing the number of classes prescribed to the patient prior to the start of their assessment window.
Comorbidity was measured using the Charlson comorbidity index.20 We determined whether individuals had International Classification of Diseases, Ninth Revision codes relating to myocardial infarction, congestive heart failure (CHF), cerebrovascular disease, chronic kidney disease, and diabetes mellitus prior to the end of their 12‐month BP assessment window. If these diagnoses occurred prior to the end of that window, individuals were coded as having the conditions. Continuous values of the Charlson comorbidity index were then calculated based on prevalence of these conditions and were generated using the comorbidity equation.20 Insured individuals were classified as all individuals who had support for medical bills (including private insurance, Medicare, Medicaid, and government benefits).
2.3. Statistical analysis
All individuals were required to have three or more BP measurements during the course of their 1‐year assessment window. Thus, BP control for both metrics (last and mean controlled) were calculated using three distinct BP measurements taken from three distinct HHC visits. Rates of BP control for both metrics were compared between regions of birth using chi‐square tests. In sensitivity analyses, we examined two additional metrics of defining BP control (having 75% of measures controlled during the assessment window and having 50% of measures controlled) (Table S1). Kruskal‐Wallis analysis of variance was used to compare mean BP between regions of birth.
We used regression analyses to compare BP control between the three ethnic groups with covariate adjustment. Poisson regression with robust covariance estimation was used to predict prevalence of BP control using both metrics (last measure controlled and mean BP controlled). Model 1 was unadjusted while model 2 was fully adjusted for covariates including age, sex, body mass index, Charlson comorbidity index score, the total number of antihypertensive medications prescribed, insurance status, and number of BP measurements in the assessment window. Rates of mortality were compared between groups using a Cox proportional hazards regression with progressive covariate adjustment. The mortality analyses were also adjusted for average systolic BP and diastolic BP during the BP assessment window in model 3. All analyses were performed using SPSS software version 2221 and R version 3.2.4.22 Survival analyses and tests of the proportional hazards assumption were run using the R packages “survival” and “greg.”23, 24
3. RESULTS
3.1. Sample characteristics
Of the 434 646 individuals in the database from 2004 to 2009, there were 135 910 blacks diagnosed with hypertension who reported being born in the United States, Caribbean, or West Africa (Figure 1). Of this sample, 84 389 individuals had at least one BP reading within the 1‐year assessment window (Figure S1). The final analysis sample consisted of 41 868 individuals who had three or more BP measurements within the assessment window and were not missing any study variables. Of this sample, 51% of participants were born in the United States, 44.1% were born in the Caribbean, and 4.9% were born in West Africa. See Figure 1 for clarification on the final sample and exclusion criteria.
Figure 1.
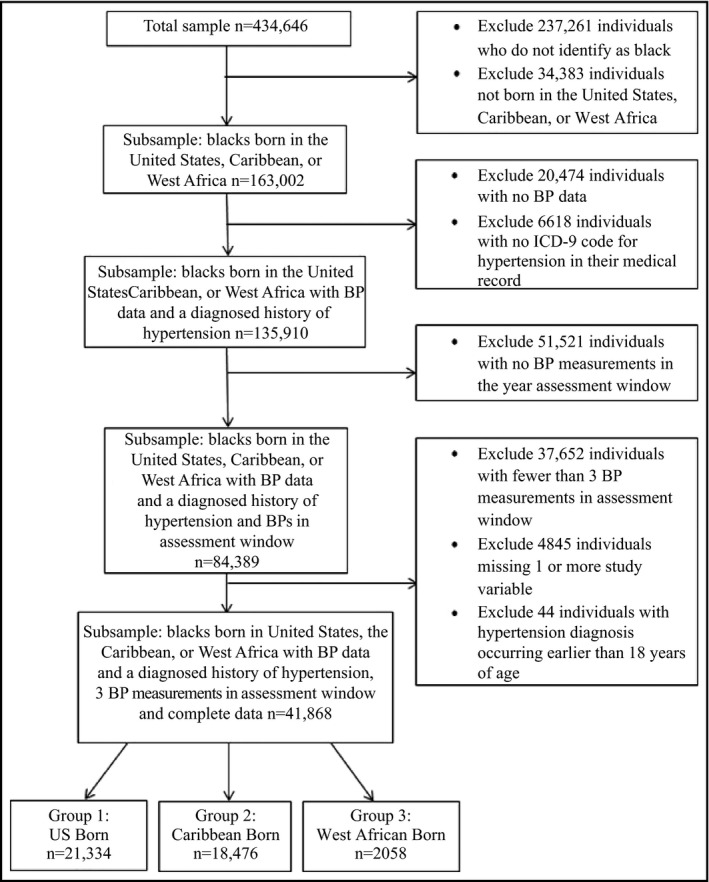
Study design illustration. BP indicates blood pressure; ICD‐9, International Classification of Diseases, Ninth Revision; US, United States
Patient characteristics of the final analysis sample show that the mean age was 52.7 years (SD, 14.2 years), 65.3% were women, and mean body mass index was 32.3 (SD, 10.8). Patients born in West Africa were the youngest (mean age 46.8 years), while those born in the Caribbean were the oldest (mean age 53.9 years). On average, patients received 1.85 classes of antihypertensive medications. West African blacks were prescribed more β‐blockers and calcium channel blockers than US‐born blacks. Caribbean‐born blacks were less likely to receive diuretics but were more likely to receive angiotensin‐converting enzyme inhibitors than US‐born blacks. The majority of US‐born blacks were insured (92.9%), whereas insurance was less common among Caribbean‐ and West African–born blacks (69.2% and 67.4%, respectively). Caribbean blacks had the highest occurrence of diabetes mellitus (31.4%), whereas US‐born blacks had greater prevalence of cerebrovascular disease, myocardial infarction, and CHF (5.0%, 0.9%, and 5.2%, respectively) (Table 2). More information about the sample characteristics can be found in Tables 1 and 2.
Table 2.
Comorbidity by region of birth
| Variables | Total sample (N=41 868) | United States (n=21 334) | Caribbean (n=18 476) | West Africa (n=2058) |
|---|---|---|---|---|
| Charlson comorbidity score (continuous), SD) | 0.47 (0.70) | 0.46 (0.71) | 0.50 (0.71) | 0.33 (0.59) |
| Charlson categorical, No. (%) | ||||
| 0 | 26 310 (62.8) | 13 700 (64.2) | 11 107 (60.1) | 1503 (73.0) |
| 1–3 | 15 468 (36.9) | 7577 (35.5) | 7337 (39.7) | 554 (26.9) |
| 4 | 90 (0.2) | 57 (0.3) | 32 (0.2) | 1 (0.0) |
| Cerebrovascular accident, No. (%) | 1912 (4.6) | 1064 (5.0) | 777 (4.2) | 71 (3.4) |
| Myocardial infarction, No. (%) | 277 (0.7) | 196 (0.9) | 78 (0.4) | 3 (0.1) |
| Diabetes, No. (%) | 11 597 (27.7) | 5385 (25.2) | 5796 (31.4) | 416 (20.2) |
| Chronic kidney disease, No. (%) | 45 (0.1) | 29 (0.1) | 11 (0.1) | 5 (0.2) |
| Congestive heart failure, No. (%) | 1706 (4.1) | 1119 (5.2) | 543 (2.9) | 44 (2.1) |
Table 1.
Participant characteristics and medications prescribed
| Total sample (N=41 868) | United States born (n=21 334) | Caribbean born (n=18 476) | West African born (n=2058) | |
|---|---|---|---|---|
| Age (SD), y | 52.7 (14.2) | 52.2 (14.8) | 53.9 (13.4) | 46.8 (12.2) |
| Women, No. (%) | 27 327 (65.3) | 12 869 (60.3) | 13 294 (72.0) | 1164 (56.6) |
| BMI (SD) | 32.3 (10.8) | 32.9 (11.4) | 31.6 (9.7) | 33.0 (12.2) |
| Blood pressure measurements, No. (SD) | 6.47 (5.23) | 6.79 (5.73) | 6.11 (4.41) | 6.49 (6.26) |
| Insured, No. (%) | 33 989 (81.2) | 19 814 (92.9) | 12 787 (69.2) | 1388 (67.4) |
| Medication classes (continuous), No. (SD) | 1.85 (1.57) | 1.93 (1.64) | 1.76 (1.49) | 1.84 (1.53) |
| Medications, No. (%) | ||||
| 0 | 8915 (21.3) | 4346 (20.4) | 4137 (22.4) | 432 (21.0) |
| 1 | 11 662 (27.9) | 5859 (27.5) | 5236 (28.3) | 567 (27.6) |
| 2 | 8714 (20.8) | 4367 (20.5) | 3902 (21.1) | 445 (21.6) |
| 3 | 12 577 (30.0) | 6762 (31.7) | 5201 (28.2) | 614 (29.8) |
| ACEI, No. (%) | 11 865 (28.3) | 5933 (27.8) | 5466 (29.6) | 466 (22.6) |
| β‐Blocker, No. (%) | 7135 (17.0) | 3748 (17.6%) | 2993 (16.2%) | 394 (19.1%) |
| Calcium channel blocker, No. (%) | 6103 (14.6) | 3027 (14.2) | 2737 (14.8) | 339 (16.5) |
| Diuretic, No. (%) | 7478 (17.9) | 5001 (23.4) | 1963 (10.6) | 514 (25.0) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; BMI, body mass index. Bold values indicate significance.
3.2. BP control
BP control rates were similar across both metrics used in the study (Table 3). Rates of BP control ranged between 48% and 60% for last and mean metrics of control, with Caribbean‐born blacks showing significantly lower control rates (P<.001, Table 3). Caribbean‐born blacks also displayed higher average systolic BP levels than US‐born blacks (141.3 mm Hg vs 137.6 mm Hg). Sensitivity analyses conducted with different metrics (75% of values controlled and 50% of values controlled) confirmed that Caribbean‐born blacks have the lowest rates of BP control among all metrics (Table 3, Fig. S2).
Table 3.
Blood pressure control by region of birth
| Variable | Total sample | United States | Caribbean | West Africa | P value |
|---|---|---|---|---|---|
| Last BP controlled, No. (%) | 21 615 (51.6) | 11 465 (53.7) | 8984 (48.6) | 1166 (56.7) | <.001 |
| Mean BP controlled, No. (%) | 22 705 (54.2) | 12 448 (58.3) | 9055 (49.0) | 1202 (58.4) | <.001 |
| Average SBP, mean (SD) | 139.25 (15.44) | 137.6 (15.1) | 141.3 (15.7) | 137.5 (14.6) | <.001 |
| Average DBP, mean (SD) | 79.2 (8.9) | 79.0 (9.2) | 79.4 (8.7) | 79.7 (8.5) | <.001 |
Abbreviations BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
P values reported from Kruskal‐Wallis one‐way analysis of variance on ranks.
Poisson regression showed that Caribbean‐born blacks had significantly reduced prevalence of BP control compared with US‐born blacks in unadjusted (prevalence rate, 0.89 and 0.84; P<.001) and fully adjusted (prevalence rate, 0.92 and 0.88; P<.001) analyses (Table 4). West African–born blacks did not differ significantly in adjusted analyses from US‐born blacks in terms of their BP control on either metric.
Table 4.
Predicting SBP control based on region of birth
| Variables | Model 1 (Unadjusted) | Model 2 (Fully adjusted) | ||||
|---|---|---|---|---|---|---|
| United States | Caribbean | West Africa | United States | Caribbean | West Africa | |
| Last BP measure controlled | Reference | 0.89 (0.87–0.92) a | 1.08 (1.02–1.14) b | Reference | 0.92 (0.90–0.95) a | 1.04 (0.98–1.10) |
| Mean BP controlled | Reference | 0.84 (0.81–0.86) a | 1.01 (0.95–1.07) | Reference | 0.88 (0.85–0.90) a | 0.98 (0.92–1.04) |
Abbreviation: BP, blood pressure. Sample size: United States born (21 334); Caribbean born (18 476); West African born (2058). Model 2 adjusted for age, sex, body mass index, Charlson comorbidity score, total classes of antihypertensive medication, insurance status, and number of systolic blood pressure (SBP) measurements in assessment window. Bold values indicate significance.
a P<.001; b P<.01.
3.3. Mortality
The secondary analysis consisted of 34 358 (82.1%) individuals of the total sample who had mortality data following their BP assessment window. Of this sample, 50.7% were born in the United States, 45.0% of individuals were born in the Caribbean, and 4.4% of individuals were born in West Africa. This subsample demonstrated similar patterns of BP control compared with the full analysis sample (Table S2). Of this group, 2616 (7.6%) died after the BP window but prior to when measurement was stopped and were included in the analysis. The remaining 31 742 participants died after measurement was stopped. The 2616 cases of all‐cause mortality identified included 10.6% US‐born, 4.8% Caribbean‐born, and 1.6% West African–born blacks (Table 5). Compared with US‐born individuals, Caribbean‐born blacks had a 56% reduced hazard of mortality in unadjusted analyses (hazard ratio, 0.44; 95% CI, 0.41–0.48) and a 53% reduced hazard of mortality in adjusted analyses (hazard ratio, 0.46; 95% CI, 0.42–0.50). West African–born blacks showed a 79% reduced hazard of mortality in unadjusted analyses (hazard ratio, 0.21; 95% CI, 0.14–0.30) and a 72% reduced hazard in adjusted analyses (hazard ratio, 0.28; 95% CI, 0.18–0.41). Both Caribbean and West African–born blacks displayed significantly reduced hazard of mortality compared with US‐born blacks at all levels of adjustment (Table 5, Figure 2).
Table 5.
Participant mortality based on region of birth
| Variables | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| United States | Caribbean | West Africa | United States | Caribbean | West Africa | United States | Caribbean | West Africa | |
| Hazard of mortality | Reference | 0.44 a (0.41–0.48) | 0.21 a (0.14–0.30) | Reference | 0.47 a (0.43–0.51) | 0.28 a (0.19–0.42) | Reference | 0.46 a (0.42–0.50) | 0.28 a (0.18–0.41) |
| Cases of all‐cause mortality, No. (%) | 1852 (10.6) | 740 (4.8) | 24 (1.6) | N/A | N/A | N/A | N/A | N/A | N/A |
Abbreviation: N/A, not available. Sample size: United States born (17 409); Caribbean born (15 446); West African born (1503).
Model 1: unadjusted.
Model 2: adjusted for age, sex, body mass index, Charlson comorbidity score, total classes of antihypertensive medication, insurance status, and number of systolic blood pressure measurements in assessment window.
Model 3: adjusted for all previous covariates as well as mean systolic blood pressure during assessment window. Bold values indicate significance.
P<.001.
Figure 2.
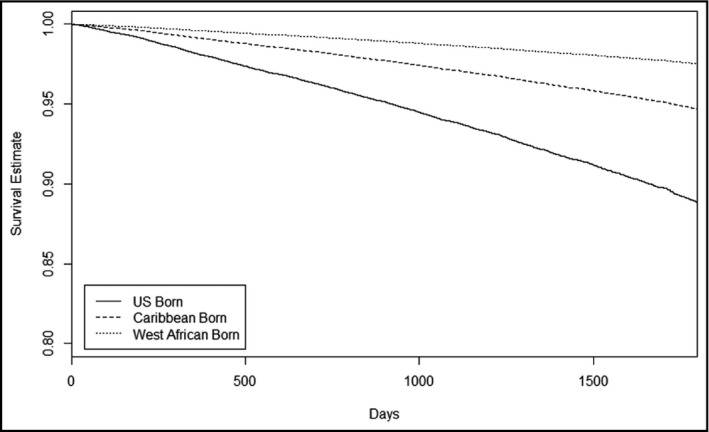
Participant survival estimate by region of birth
3.4. Analysis of excluded sample
A large number of patients were excluded from the analysis because of missing data or having an insufficient number of BP measurements (fewer than 3 readings) during their 1‐year assessment window. We decided to examine the characteristics of this excluded sample and determine whether they were significantly different from the main analysis sample. We first compared the analysis sample with the sample of individuals who were excluded because of having 2 or fewer BP measurements in the assessment window, having missing data, or having an improbable early diagnosis of hypertension (earlier than age 18). Comparing our analysis sample (N=41 868) with the excluded sample (N=42 521) using Kruskal‐Wallis for continuous variables and chi‐square testing for categorical variables shows that individuals who were excluded were younger, less likely to be female, were prescribed fewer medications, had fewer comorbidities, and were less likely to be insured (Table S3). Looking at BP in this sample showed that individuals in the excluded sample had lower rates of control among both metrics (last and mean control) as well as the supplementary metrics (75% and 50% control).
We also compared our analysis sample with the full sample of black patients in the HHC system with a diagnosis of hypertension and some BP measurements (N=135 910). Comparing our analysis sample (N=41 868) with the excluded sample (94 042) in this analysis showed that the excluded sample was younger, less likely to be female, had more recent hypertension diagnoses, were taking fewer medications, had fewer comorbidities, and were less likely to be insured. BP was not assessed in this sample because not all individuals had BP measurements in the same time frame.
4. DISCUSSION
In this study, we evaluated the BP control and all‐cause mortality among US‐born and foreign‐born blacks. We used two metrics for evaluating BP control (last measure BP controlled and mean BP controlled) during the study period. Further, a sensitivity analysis was conducted evaluating control at 75% and 50% of measures. During the assessment period, Caribbean‐born patients had lower rates of BP control for the last measurement when compared with US‐born and West African–born blacks. This pattern was similar for the mean BP control metric. West African–born patients had similar BP control rates as US‐born patients. Caribbean‐born patients had consistently less controlled BP at all control metrics. Caribbean‐ and West African–born patients showed reduced hazard of mortality when compared with US‐born patients, even after adjustment for BP levels. Rates of BP control ranged between 48% and 60%. This is consistent with the 49.5% control rate found among black patients with hypertension in the United States.24
Furthermore, our findings suggest that US‐born blacks are the group most associated with hypertension‐related cardiovascular comorbidities including cerebrovascular disease, myocardial infarction, and CHF. Caribbean‐born blacks experience a higher burden of diabetes mellitus and are less likely to have controlled BP regardless of the metric used (last vs mean) over a 1‐year period. The Caribbean‐born blacks were the oldest of all three groups, which is consistent with findings by other researchers that older individuals are more likely to have uncontrolled BP.25 All of the metrics produced similar frequencies of control. This is similar to what was found by Alexander and colleagues19 in 1999. Rates of BP control were high (>50%) in all metrics, indicating that most black patients with hypertension seen in HHC have controlled BP. Regressions predicting BP control status by region of birth showed that Caribbeans have lower rates of controlled BP in all metrics. Sensitivity analysis revealed that our sample is somewhat different than the total sample of blacks with hypertension in the HHC system. There is a trade‐off between our internal validity (estimating control accurately) and external validity (showing our results are representative of blacks in the HHC).
The finding that Caribbean blacks have poorer BP control compared with US‐born blacks refutes the theory that immigrants are more “hardy” than US‐born blacks. Studies suggest that those who make it to the United States have the best health outcomes, and thus may potentially maintain healthier lifestyles until they are fully acculturated.14, 26 Moreover, among our sample, West African–born had similar BP control rates as US‐born blacks and had better health outcomes than Caribbean‐ and US‐born blacks groups. The explanations for the West African–born health advantageous position are unclear but may be related to their more favorable socioeconomic position relative to Caribbean‐ and US‐born blacks.28 Furthermore, the degree of acculturation may contribute to the differences seen between US‐born blacks and foreign‐born blacks. Immigrants who have not fully immersed themselves into the society may retain their cultural norms, perhaps including healthy eating habits, thus reducing their risk of worsening hypertension.14 In addition, findings from previous studies suggest that among foreign‐born minorities, longer length of stay in the United States and degree of assimilation improves hypertension diagnosis and care.28, 29 Unfortunately, we could not evaluate socioeconomic position because of lack of data in the available clinical data set.
Mortality data among the cohort described in this investigation appears to have been well assessed. The additional use of the New York State vital statistics data to account for deaths in our population resulted in a mortality rate that is consistent with mortality rates documented in NYC.30 Our study found foreign‐born blacks to have lower all‐cause mortality than US‐born blacks. This is consistent with findings from a historically prospective cohort study conducted in Denmark that compared Danish‐born patients with immigrants and found that all‐cause and cause‐specific survival after cardiovascular disease, acute myocardial infarction, and stroke was significantly better for immigrants, regardless of country of origin.31 Nevertheless, a limitation in our secondary outcome analysis is that we cannot account for domestic/international migration out of New York State. Estimates of net domestic and international migration out of New York State are lacking.32 However, the difference in mortality noted between US‐born and foreign‐born blacks is consistent with the known mortality difference in these two groups.33 While BP control is usually related to improvement in mortality in all ethnic/racial groups with hypertension,34 the incremental improvement in mortality from BP control is different in different groups based on comorbidities and baseline BP levels.35 Therefore, BP control cannot be expected to improve mortality for all populations.
Despite the relatively good control of hypertension in US‐born blacks, the high mortality rate indicates a need to direct focus on other comorbidities. Public health resources should be focused on intervening on those comorbidities that would have more of an incremental effect on mortality than BP control. These comorbidities may include cancer and infectious disease–related diseases. We were not able to explore this theory because of lack of access to cause‐specific mortality data. An example of disparate mortality findings within the black diaspora is in the Netherlands, where ethnic minority groups experienced higher mortality attributed to cardiovascular disease compared with those born in the Netherlands.36
Overall, our findings are consistent with other investigations in that ethnic minority groups have a higher risk of cardiovascular diseases and are complicated by their lack of awareness of their condition in most cases.7, 38, 39 An analysis of participant responses from the 2003–2008 US National Health and Nutritional Survey found that 47% of foreign‐born English speakers and 70% of non‐English speakers with hypertension were more likely than those born in the United States to be unaware of their condition.40 Foreign‐born minorities with hypertension, perhaps because of lack of access to health care as a result of being uninsured, are often undiagnosed.41 Findings from Stewart and colleagues42 show that US‐born blacks were less likely to be uninsured (1.7%) compared with Latin American and Caribbean‐born (8.4%) minority groups, and the rate of insurance was even less in African‐born individuals (23.2%). We found that Caribbean‐born and West African–born blacks were less likely to be insured compared with those born in the United States.
Mortality differences between foreign‐ and US‐born blacks may be partially explained by differences in Charlson comorbidity and CHF prevalence.42 These comorbidities may have contributed to the differences shown in mortality, even after controlling for BP. A limitation of using the CHF diagnosis from the HHC data set is that we do not know how it was determined. CHF has at times been considered a suspect outcome in clinical trials when the use of biomarkers or other objective signs are not used to make the diagnosis. The significance of the disparate diagnosis of CHF in this data set is of questionable significance. Of all the comorbidities assessed in this data set, it is perhaps the most subjective of them all and therefore its relevance to our mortality assessment is uncertain.
STUDY STRENGTHS AND LIMITATIONS
To our knowledge this is the first study to examine ethnic variations in hypertension, associated outcomes, and mortality using clinical data from the NYC HHC system. Nonetheless, although the majority of the clinic population included US‐born blacks (over 50%), our sample of foreign‐born persons was somewhat limited, especially the African‐born population. This can be attributed to either underrepresentation of this group as a result of access to care issues or misclassification, as the country of origin was self‐reported and manually entered into the system by clinic staff, thus unfamiliar countries may have been misspelled or completely left out. In addition, foreign‐born blacks have a more favorable all‐cause mortality profile than US‐born blacks (ie, Caribbean‐ and West African–born blacks had a lower mortality rate than US‐born blacks).
US‐born blacks paradoxically have better BP profiles and hypertension control rates than their foreign‐born counterparts, which could partially be explained by the “salmon bias,” where foreign‐born populations return home to be with family at a time immediately preceding death; thus, we did not capture all of the deaths occurring outside the country and in the HHC system. However, recent evidence seems to suggest that return to the country of birth immediately before death is unlikely.43 Since our study consists of a retrospective data analysis of a clinical database, we were not able to ascertain the length of stay in the United States for the various black ethnicities, which can be used as a measure of acculturation14 and an indication of health‐seeking behavior. Also, the specific cause of death information was not available in the data set. Additionally, we were unable to measure patients’ prior awareness of their hypertension status and risk factors, which may influence them to make better health decisions and/or modifications.
CONCLUSIONS
This study found that ethnic differences in hypertension control and mortality exist among blacks with hypertension served by an urban NYC clinic system. In our study sample, US‐born blacks experienced higher mortality rates despite lower BP and better BP control than Caribbean‐ and West African–born blacks. Future studies in blacks with hypertension should take ethnic variations within this population into account when trying to deliver hypertension management and control strategies. It is clear that effective interventions in the US black community must be ethnically/culturally tailored to reflect the diversity of this seemingly homogeneous group. In addition, caution must be taken in medical research when referring to the seemingly homogeneous group of blacks with hypertension.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Supporting information
ACKNOWLEDGMENT
Special thanks to Christopher Torgersen, MBA, Yu Guo, MA, and Dr Louis Capponi and his team at HHC for all their work on the data retrieval process.
Gyamfi J, Butler M, Williams SK, et al. Blood pressure control and mortality in US‐ and foreign‐born blacks in New York City. J Clin Hypertens. 2017;19:956–964. 10.1111/jch.13045
Funding information
This research was supported by funding from the Agency for Healthcare Research and Quality (R01HS018589; principal investigators: Drs Shah, Ogedegbe, and Bangalore). The Agency for Healthcare Research and Quality did not have any role in the design or conduct of the study; in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of this article.
The copyright line for this article was changed on December 12, 2017, after original online publication.
REFERENCES
- 1. Balfour PC Jr, Rodriguez CJ, Ferdinand KC. The role of hypertension in race‐ethnic disparities in cardiovascular disease. Curr Cardiovasc Risk Rep. 2015;9:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharma S, Malarcher AM, Giles WH, Myers G. Racial, ethnic and socioeconomic disparities in the clustering of cardiovascular disease risk factors. Ethn Dis. 2004;14:43‐48. [PubMed] [Google Scholar]
- 3. Ogedegbe G, Shah NR, Phillips C, et al. Comparative effectiveness of angiotensin‐converting enzyme inhibitor‐based treatment on cardiovascular outcomes in hypertensive blacks versus whites. J Am Coll Cardiol. 2015;66:1224‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38‐e360. [DOI] [PubMed] [Google Scholar]
- 5. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17:143‐152. [PubMed] [Google Scholar]
- 6. Ferdinand KC, Saunders E. Hypertension‐related morbidity and mortality in African Americans–why we need to do better. J Clin Hypertens (Greenwich). 2006;8(1 suppl 1):21‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cooper R, Rotimi C. Hypertension in blacks. Am J Hypertens. 1997;10(7 pt 1):804‐812. [DOI] [PubMed] [Google Scholar]
- 8. Bangalore S, Ogedegbe G, Gyamfi J, et al. Outcomes with angiotensin‐converting enzyme inhibitors vs other antihypertensive agents in hypertensive blacks. Am J Med. 2015;128:1195‐1203. [DOI] [PubMed] [Google Scholar]
- 9. Hicks LS, Fairchild DG, Cook EF, Ayanian JZ. Association of region of residence and immigrant status with hypertension, renal failure, cardiovascular disease, and stroke, among African‐American participants in the third National Health and Nutrition Examination Survey (NHANES III). Ethn Dis. 2003;13:316‐323. [PubMed] [Google Scholar]
- 10. Kaplan NM. Ethnic aspects of hypertension. Lancet. 1994;344:450‐452. [DOI] [PubMed] [Google Scholar]
- 11. Sowers JR, Ferdinand KC, Bakris GL, Douglas JG. Hypertension‐related disease in African Americans. Factors underlying disparities in illness and its outcome. Postgrad Med. 2002;112:24‐26,. 29–30, 33–34 passim. [DOI] [PubMed] [Google Scholar]
- 12. Borrell LN, Menendez BS, Joseph SP. Racial/ethnic disparities on self‐reported hypertension in New York City: examining disparities among Hispanic subgroups. Ethn Dis. 2011;21:429‐436. [PubMed] [Google Scholar]
- 13. Sewali B, Harcourt N, Everson‐Rose SA, et al. Prevalence of cardiovascular risk factors across six African Immigrant Groups in Minnesota. BMC Public Health. 2015;15:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moran A, Diez Roux AV, Jackson SA, et al. Acculturation is associated with hypertension in a multiethnic sample. Am J Hypertens. 2007;20:354‐363. [DOI] [PubMed] [Google Scholar]
- 15. Hyman DJ, Ogbonnaya K, Pavlik VN, Poston WS, Ho K. Lower hypertension prevalence in first‐generation African immigrants compared to US‐born African Americans. Ethn Dis. 2000;10:343‐349. [PubMed] [Google Scholar]
- 16. Keenan NL, Rosendorf KA. Prevalence of hypertension and controlled hypertension—United States, 2005–2008. MMWR Suppl. 2011;60:94‐97. [PubMed] [Google Scholar]
- 17. Fejerman L, Wu X, Adeyemo A, et al. The effect of genetic variation in angiotensinogen on serum levels and blood pressure: a comparison of Nigerians and US blacks. J Hum Hypertens. 2006;20:882‐887. [DOI] [PubMed] [Google Scholar]
- 18. Singh GK, Siahpush M. Ethnic‐immigrant differentials in health behaviors, morbidity, and cause‐specific mortality in the United States: an analysis of two national data bases. Hum Biol. 2002;74:83‐109. [DOI] [PubMed] [Google Scholar]
- 19. Alexander M, Tekawa I, Hunkeler E, et al. Evaluating hypertension control in a managed care setting. Arch Intern Med. 1999;159:2673‐2677. [DOI] [PubMed] [Google Scholar]
- 20. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245‐1251. [DOI] [PubMed] [Google Scholar]
- 21. IBM SPSS Statistics for Windows, Version 22.0. IBM Corp; 2013.
- 22. R Development Core Team . 2011, R: a language and environment for statistical computing. Vienna, Austria: the R Foundation for Statistical Computing. ISBN: 3‐900051‐07‐0. Available online at http://www.r-project.org/. [Google Scholar]
- 23. Therneau T. A Package for Survival Analysis in S. version 2.38. 2015.
- 24. Nwankwo T, Yoon S, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief. 2013;133:1‐8. [PubMed] [Google Scholar]
- 25. Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345:479‐486. [DOI] [PubMed] [Google Scholar]
- 26. Singh GK, Miller BA. Health, life expectancy, and mortality patterns among immigrant populations in the United States. Can J Public Health. 2004;95:I14‐I21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Agyemang C, Bhopal R. Is the blood pressure of people from African origin adults in the UK higher or lower than that in European origin white people? A review of cross‐sectional data. J Hum Hypertens. 2003;17:523‐534. [DOI] [PubMed] [Google Scholar]
- 28. Borrell LN, Crawford ND, Barrington DS, Maglo KN. Black/white disparity in self‐reported hypertension: the role of nativity status. J Health Care Poor Underserved. 2008;19:1148‐1162. [DOI] [PubMed] [Google Scholar]
- 29. Yi S, Elfassy T, Gupta L, Myers C, Kerker B. Nativity, language spoken at home, length of time in the United States, and race/ethnicity: associations with self‐reported hypertension. Am J Hypertens. 2014;27:237‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li W, Huynh M, Lee E, et al. Summary of Vital Statistics, 2014. The City of New York. New York, NY: New York City Department of Health and Mental Hygiene, Office of Vital Statistics; 2016. [Google Scholar]
- 31. Byberg S, Agyemang C, Zwisler AD, Krasnik A, Norredam M. Cardiovascular disease incidence and survival: are migrants always worse off? Eur J Epidemiol. 2016;31:667‐677. [DOI] [PubMed] [Google Scholar]
- 32. NYC Department of City Planning. http://www1.nyc.gov/site/planning/data-maps/nyc-population/current-future-populations.page.
- 33. Singh GK, Hiatt RA. Trends and disparities in socioeconomic and behavioural characteristics, life expectancy, and cause‐specific mortality of native‐born and foreign‐born populations in the United States, 1979–2003. Int J Epidemiol. 2006;35:903‐919. [DOI] [PubMed] [Google Scholar]
- 34. Law M, Morris J, Wald N. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lonn EM, Bosch J, López‐Jaramillo P, et al. Blood‐pressure lowering in intermediate‐risk persons without cardiovascular disease. N Engl J Med. 2016;374:2009‐2020. [DOI] [PubMed] [Google Scholar]
- 36. Agyemang C, Vaartjes I, Bots ML, et al. Risk of death after first admission for cardiovascular diseases by country of birth in the Netherlands: a nationwide record‐linked retrospective cohort study. Heart. 2009;95:747‐753. [DOI] [PubMed] [Google Scholar]
- 37. Hennis A, Wu SY, Nemesure B, Leske MC; Barbados Eye Studies G . Hypertension prevalence, control and survivorship in an Afro‐Caribbean population. J Hypertens. 2002;20:2363‐2369. [DOI] [PubMed] [Google Scholar]
- 38. Agyemang C, Snijder MB, Adjei DN, et al. Ethnic disparities in CKD in the Netherlands: the healthy life in an Urban setting (HELIUS) study. Am J Kidney Dis. 2016;67:391‐399. [DOI] [PubMed] [Google Scholar]
- 39. Brewster LM, Seedat YK. Why do hypertensive patients of African ancestry respond better to calcium blockers and diuretics than to ACE inhibitors and beta‐adrenergic blockers? A systematic review. BMC Med. 2013;11:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Langellier BA, Garza JR, Glik D, et al. Immigration disparities in cardiovascular disease risk factor awareness. J Immigr Minor Health. 2012;14:918‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stewart KA, London AS. Falling through the cracks: lack of health insurance among elderly foreign‐and native‐born blacks. J Immigr Minor Health. 2015;17:1391‐1400. [DOI] [PubMed] [Google Scholar]
- 42. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult—summary article. Circulation. 2005;112:1825‐1852. [Google Scholar]
- 43. Norredam M, Hansen OH, Petersen JH, et al. Remigration of migrants with severe disease: myth or reality?–a register‐based cohort study. Eur J Pub Health. 2015;25:84‐89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


