Abstract
Background
The epidemiology of COVID-19 and its association with cardiometabolic disorders is poorly understood. This is a narrative review that investigates the effects of COVID-19 infection on insulin resistance in patients with diabetes.
Methods
An online search of all published literature was done via PubMed and Google Scholar using the MeSH terms “COVID-19,” “SARS-CoV-2,” “coronavirus,” “insulin resistance,” and “diabetes.” Only articles that were directly applicable to insulin resistance in COVID-19 and diabetes was reviewed.
Results
Current data shows an increased risk of mortality in patients with diabetes and COVID-19 compared to those without diabetes. COVID-19 triggers insulin resistance in patients, causing chronic metabolic disorders that were non-existent prior to infection.
Conclusion
Patients with diabetes are more susceptible to COVID-19 infection than those without diabetes. ACE2 expression decreases with infection, exaggerating Ang II activity with subsequent insulin resistance development, an exaggerated immune response and severe SARS-COV-2 infection.
Keywords: Angiotensin 2 converting enzyme, Diabetes mellitus, Insulin resistance, SARS-COV-2, Cardiometabolic disorders
1. Introduction
In December 2019, the World Health Organization (WHO) was notified of several rare cases of coronavirus related pneumonia in Wuhan, China. Since then the novel severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) has devastated the world with an emergence of the COVID-19 pandemic [1]. This pandemic is a colossal public health and economic challenge, since it has to-date resulted in more than 117 million infections and over 2.6 million deaths [2]. High infection and mortality rates highlight the interplay of poor healthcare, poverty, geographical disparity, economic and social risk factors [3]. The COVID-19 infected patient remains the main source of infection, exacerbated by close contact and transmission occurring via droplets/aerosol dissemination [4]. Whilst viral RNA has been identified on plastic, airborne specimens demonstrate negligible concentrations of RNA [5]. An estimated 44% of all transmission occurs prior to the onset of symptoms [6], despite the standard incubation period of approximately 3–9 days [4].
Four key demographic factors affect COVID-19 epidemiology, viz., advanced age (median age of death—75 yrs); gender (male sex), immunocompromisation and underlying co-morbidities such as cardiovascular disorders, diabetes mellitus, chronic respiratory disease and hypertension [[7], [8], [9]]. Organ failure, cancer, obesity, elevation in white blood cell count, high LDH and glucose also correlate with a greater risk of morbidity and mortality [10,11]. The mortality prediction score (Sequential Organ Failure Assessment: SOFA), which is centered on a dysfunctional grade of six organ systems, is higher in COVID-19 patients at hospital admittance combined with advanced age and hypercoagulation activity [9,12,13]. Hyper-coagulatory activity (d-dimer levels >1 μg/mL) strongly correlates with higher hospital deaths [9,12,13].
Acute respiratory distress syndrome (ARDS) characterized by accumulation of infiltrated leukocytes is the cause of mortality in COVID-19 patients [14,15]. Acute respiratory distress syndrome causes a cytokine storm, where an extreme inflammatory response persists potentially causing acute lung injury [16]. To-date diabetes has been shown to influence the severity and mortality of COVID-19 patients [9,17,18]. This is corroborated by several meta-analyses that demonstrates increased disease severity/mortality in diabetic patients that are comorbid with COVID-19 infection [[19], [20], [21], [22]]. Patients with a history of diabetes, hypertension, insulin resistance (IR), and respiratory disorders experience a deterioration of immune function with consequential endothelial and ventilation disruption [8,23]. Furthermore, hypercoagulation predisposes COVID-19 patients to a greater risk of thrombosis, ischemia and myocardial injury [14]. In light of the high predisposition of diabetic and insulin resistance COVID-19 patients to death, the primary focus of this review is to evaluate the duality of insulin resistance comorbid with COVID-19.
2. Insulin resistance and diabetes mellitus
Insulin resistance (IR) is due to a reduced tissue sensitivity to insulin and refers to the inability of the pancreas to secrete sufficient insulin for blood glucose regulation [24]. Insulin is a hormone secreted by the islets of Langerhans in the pancreas; it stimulates glucose transport to muscle, adipose tissue and the liver [25]. Failure of glucose regulation leads to diabetes and cardiovascular diseases [26]. Approximately 46.5% of the world’s adult population is affected by IR, with the highest prevalence reported in Lebanon, Asia (44.6%), the second highest occurring in Thailand (39.1%) and the lowest in European (15.5%) countries [24]. Data on the prevalence of IR in Africa is limited, however, a South African study reported a decrease in IR from 24.8% in 2009 to 16.9% in 2016 [25].
Diabetes mellitus (DM) occurs when blood glucose levels are high due to a failure/inadequate regulation by insulin [27]. The two most common types of diabetes are type 1 and type 2 [28].
-
•
Type 1 diabetes (T1DM) occurs when the pancreas is unable to synthesize insulin, due to a genetic mutation in the genes responsible for pancreatic beta-cell activation or due to an autoimmune-mediated beta-cell destruction [29,30]. This may coincide with either endothelial dysfunction, a modification in lipid metabolism and/or hyperglycaemia-mediated oxidative stress and cell death [30,31].
-
•
Type 2 diabetes (T2DM) is described as glucose intolerance emanating from a dysregulation of carbohydrate and lipid metabolism [27]. This disorder is commonly associated with genetic or lifestyle/environmental factors which affect the physiological function of beta-cells and insulin sensitivity [27]. The commonest incidence of T2DM occurs in obese individuals, since adipose tissue releases leptin, resistin, adiponectin and tumour necrosis factor (TNF-α), all of which disrupts glucose metabolism by decreasing insulin sensitivity [32]. Tumour necrosis factor also down-regulates the expression of GLUT4, which is necessary for glucose translocation [33]. A decrease in insulin sensitivity leads to insulin resistance, a characteristic of T2DM whereas a deficiency in insulin secretion and function results in an exaggerated blood glucose level [27].
Exocrine and endocrine pancreatic expression of angiotensin-converting enzyme 2 (ACE2) is likely to be associated with an exaggerated manifestation of diabetes in subsets of severely ill SARS-CoV-2 infected patients [34]. The SARS-CoV-2 confers pancreatic islet injury and acute diabetes onset by binding to the ACE2 receptor [35], mirroring similar signal transduction pathways as SARS-CoV-1 [36]. The prevalence of T1DM cases increases amongst SARS-CoV-2 infected individuals who are genetically predisposed to diabetes [37], as highlighted by a 1.5% rate in a sample of hospitalized COVID-19 English patients compared to non infected patients [38]. Notably, SARS-CoV-2 also exacerbates diabetic ketoacidosis (DKA), thereby creating a proinflammatory milieu where IL-6, IL-beta and TNF levels are increased [39]. It is plausible that the proinflammatory milieus created by COVID-19 and T1DM coexist and exacerbates severity. However, individuals diagnosed with T1DM were less vulnerable to SARS-CoV-2 infections since the use of telemedicine combined with the disconnection/slowdown from routine activities (work, school, exercise dietary habits) had a positive effect on glucose regulation [34].
In contrast, the prevalence of T2DM in SARS-CoV-2 infected patients is based on patient age, gender and disease severity [34]. For example, a prevalence rate of 15% was noted in a hospitalized sample of Chinese children and adults infected with SARS-CoV-2 [40], whereas a 8.2% rate was highlighted in a hospitalised SARS-CoV-2 infected Chinese sample of 1590 participants (mean age 48.9 yrs), with much higher rates (34.6% vs. 14.3%) amongst those with severe illness i.e. admitted in intensive care units and in need of respiratory support [41].
3. Impact of COVID-19 on the renin-angiotensin-aldosterone-system and diabetes mellitus
The pathophysiology of COVID-19 in diabetic patients remains inconclusive; however, contentious debate remains focused on the interaction between the virion, the hyperinflammatory milieu, the renin-angiotensin aldosterone system (RAAS), as well as the hypercoagulation loop [42]. In the RAAS pathway, the protease renin cleaves angiotensinogen to produce angiotensin I (AngI), which is subsequently cleaved by angiotensin-converting enzyme (ACE) to generate angiotensin II (AngII) [14].
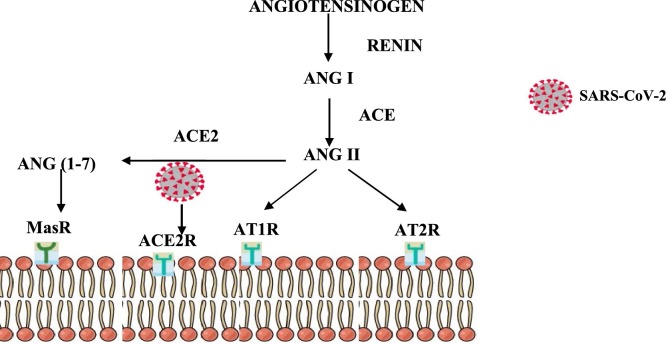
Angiotensin II signalling occurs via AngII receptor type 1 (AT1R) and receptor type 2 (AT2R). The activation of AT1R exerts pro-inflammatory, proliferative, profibrotic and vasoconstrictive effects via the activation of NF-ĸβ, NADH/NADPH oxidase and toll-like receptor 4 (Fig. 1 ) [14]. A homologue of ACE called ACE2, cleaves Ang II and generates Ang (1–7), thereby exerting an anti-inflammatory effect by binding to and activating G protein-coupled Mas receptor [43]. Notably, SARS-CoV-2 enters host cells via the ACE-II receptor where it rapidly undergoes replication, amplication and dissemination (Fig. 1) [14,44]. The ACE2/Ang (1–7)/Mas receptor pathway antagonises the biological effects of the ACE/AngII/ATR1 pathway, thereby exerting its anti-flammatory and antifibrotic effect.
Fig. 1.
The role of the Renin Angiotensin Aldosterone System (RAAS) pathway in COVID-19, Insulin resistance and diabetes infection.
Adapted from Huang et al., 2020. This diagram illustrates the RAAS pathway, highlighting the cleavage of angiotensinogen by renin to produce angiotensin I (AngI) and Ang II via angiotensin-converting enzyme (ACE). AngII receptors type 1 (AT1R) and type 2 (AT2R), initiates the biological functions of AngII. ACE2 (homologue of ACE) cleaves Ang II and generates Ang (1–7) which exerts its function via the Mas receptor. The SARS CoV-2 binds to ACE2 receptor and decreases its expression and subsequently increases AngII resulting in multi-systemic inflammatory responses
Viral binding to ACE2 receptors downregulate their expression on the alveolar epithelial cells and results in elevated Ang II expression [45], which perpetuates activation of the host adaptative immune response [42]. The consequence is an amplified inflammatory response via secretion of interleukin-1 βeta, (IL-1β), interleukin-4 (IL-4), and interleukin 10 (IL-10), and monocyte chemoattractant protein-1 (MCP-1), interferon gamma (IFNγ), and infrapatellar fat pad (IFP-10) [20], which is central to the ARDS of SARS-COV-2 infection outcome [46]. This cytokine storm triggers a hyperinflammatory and hypercoagulatory response, which disrupts endothelial cell integrity, thereby upsetting the alveolar-capillary barrier causing severe hypoxemia [47]. Also, in COVID-19 patients decreased ACE2 levels influence RAAS signalling [48].
Angiotensin II interaction with AT1 receptors up-regulate expression of the anti-angiogenic factor, sFlt-1 levels during hypoxia [49]. This lowers endothelial nitric oxide synthase phosphorylation, augmenting oxidative stress [50]. Moreover, patients with severe COVID 19 have high plasma levels of sFlt-1, indicative of an anti-angiogenic favored microenvironment that is already stressed by the ARDS, thus this may lead to organ failure [51]. The impact of SARS-COV-2 on the expression of ACE2 levels in diabetic patients is currently unknown, but ACE2 receptor expression has been confirmed in the islet cells of the pancreas [52]. Animal studies have shown that diabetes mellitus causes a remarkable increase of ACE2 expression in pancreatic cells, lungs and other tissues [53]. The increased ACE2 expression may increase the risk of COVID-19 infection in individuals with diabetes mellitus. A high degree of hyperglycaemia is noted in individuals with SARS-COV-1 infection, as this virus dysregulates effective functioning of the islet cells, with consequential IR, hyperglycaemia and new-onset diabetes mellitus [36].
Notwithstanding that, ACE2 functions as a viral dock for entry into cells where it acts as an anti-inflammatory enzyme [54]. Therapeutic interventions with focus on stimulating the RAAS pathway has potential to enhance cardiovascular function and lower the severity of COVID-19. The association of the renin-angiotensin-system with diabetes mellitus has been established, and both T1DM and T2DM patients are treated with ACE inhibitors (ACEIs) and Ang II type-I receptor blockers (ARBs) [55]. In diabetics, ACEIs and ARBs up-regulate ACE2 levels and potentially elevate the risk and severity of COVID-19 infection [56]. Both ACEIs and ARBs are likely to decrease hyperinflammation and viral reproduction whereas soluble ACE2 has potential to boost Ang-(1–9) and Ang-(1–7) levels via acting as a decoy for circulating viral particles [54]. Others contend that diabetics receiving ARBs/ACEIs also express higher ACE2 levels, and are protected from COVID-19 infection compared to untreated diabetics [57,58]. Thus, it is unclear if the use of ACEIs elevates ACE2 receptors in the lung or whether diabetic patients not on ARBs/ACEIs are protected from a predisposition to COVID-19 infection [19].
The COVID-19 infection comorbid with diabetes mellitus, hypertension and cardiovascular diseases are prone to a more aggressive diagnosis/prognosis in contrast to either condition by itself [19,20]. Patients with T2DM are predisposed to an amplified inflammatory response prior to COVID-19 exposure and infection because of their advanced age and other cardiovascular related anomalies [46,59]. Inflammation and oxidative stress are also key regulators in the manifestation of diabetes mellitus, especially in individuals with increased body mass index [60]. Inflammation and oxidative stress impair insulin activity with consequent insulin resistance, a direct cause of hyperglycemia and secondary diabetes mellitus [60]. Increased inflammation also aggrevates the pathology COVID-19, leading to more critical and fatal outcomes [57]. Notably, interleukin-6 (IL-6) levels are higher in patients with COVID-19 infection comorbid with diabetes mellitus in contrast to non-diabetic and diabetic patients without COVID-19 [57]. Higher mortality rates and lengthier hospital stays are noted in COVID-19 infected diabetics with uncontrolled hyperglycaemia in contrast to non-diabetic patients [61]. Nonetheless, a 41.7% mortality rate was noted in COVID-19 infected patients with uncontrolled hyperglycaemia versus 14.8% in controlled diabetes mellitus [61].
During the first wave of the pandemic in Hong Kong, the first three deaths due to COVID-19 were associated with diabetes mellitus [62]. In another group of 52 ICU admitted COVID-19 positive patients, 32 died of which 22% were diabetic [20]. A meta-analysis study of 76,993 Chinese patients, revealed an association between increased mortality rates with complications such as hypertension (16.37%), cardiovascular disease (12.11%), history of smoking (7.63%), and diabetes mellitus (7.87%) [63]. Furthermore, of 1099 patients infected with COVID-19, 132 had severe pathological features of COVID-19, 16. 2% were diabetic, and 5.7% were non-diabetic [64], corroborating a definite correlation between increased morbidity and mortality in diabetic patients comorbid with COVID-19 infection.
4. Insulin resistance and increased COVID-19 infection susceptibility
There is a paucity of data regarding the association between IR and COVID-19. However, it is hypothesized that viral binding to ACE2 increases Ang II implicating it as the main culprit in the synergy of IR and cardiovascular disease [65,66]. In a healthy microenviroment, ACE2 regulates blood pressure by converting Ang II into Ang (1–7), thus decreasing IR, oxidative stress, and increasing the activity of GLUT4 [67]. However, during COVID-19 infection, ACE2 expression is decreased and this results in an exaggerated activity of Ang II with subsequent IR, oxidative stress, inflammation, hypertension and cardiac dysfunction [66]. Interestingly, obese and diabetic individuals demonstrate increased inflammation, which induces IR and vice versa [66]. Since COVID-19 infections are characterised by an exaggerated inflammatory response, its coexistence with obesity and diabetes can lead to hyperinflammation and severe/fatal outcomes due to the preexisting inflammation [68]. Increased IR leads to increased pacreatic expression of ACE2 receptors, creating more afinity for the spike protein to bind thereby predisposing those with IR to increased vulnerability COVID-19 infections [53]. Furthermore, patients with preexisting IR have other comorbidities including hypertension, hyperglycemia and diabetes mellitus, which lead to severe pathological symptoms during COVID-19 and consequent mortality [46] (Fig. 2 ). COVID-19 as a respiratory disease, also causes infiltrates in the lungs amongst diabetic and obese indivuals thereby increasing their vulnerability to lung injury due to IR [68]. Thus, IR in diabetes mellitus causes chronic inflammation and hyperinsulinemia and consequent lung dysfuntion [69].
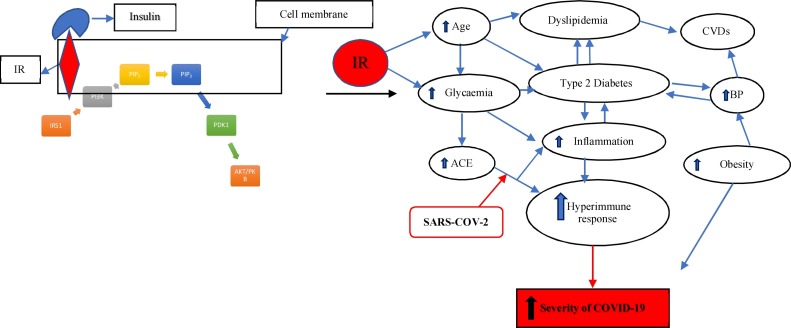
Fig. 2.
The Insulin signalling pathway and Insulin resistance in COVID-19 and Diabetes infection.
Adapted from Jung & Choi (2014), Finucane and Davenport (2020), Rajpal et al. (2020) and Santos et al. (2021). Insulin binds to the insulin receptor (IR) to produce the activated insulin receptor substrate (IRS1). This leads activates phosphatidylinositol-3 kinase (PI3K), which binds to its receptor phosphatidylinositol-4,5-biphosphate (PIP2) to form a second messenger, phosphatidylinositol-3, 4, 5-triphosphate (PIP3). This activates Protein kinase B (AKT/PBK) which binds to the membrane, and is phosporylated by protein kinase-1(PDK1). Protein kinase B is essential for glucose transport activation, glycogenesis and lipogenesis. A dysregulation of the insulin signalling pathway due to viral infection disrupts the function of AKT/PKB and may lead to commodities such as hyperglycemia, dyslipidemia, Type 2 diabetes, obesity, chronic cardiovascular disease, and hypertension. The binding of the spike protein of SARS-CoV-2 in patients with commobidities and advanced age results in a hyperactive immune response and increases the severity of the virus after infection. Insulin resistance together with the hyperinflammation exacerbates the onset of all commodities and increase the pancreatic ACE2 expression. Increased pancreatic ACE2 expression creates a higher binding affinity for SARS-CoV-2. This predisposes patients with diabetes mellitus to suffer from a more aggressive pathology of COVID-19 infection.
5. Reccommendations: clinical management for the primary care physician
Diabetes mellitus together with hypertension, obesity, lung disease, kidney disease, and chronic heart disease has been associated with more severe pathology of SARS-CoV-2. Older individuals with type 2 diabetes mellitus are more susceptible to infections such as influenza, and pneumonia, since chronically raised blood glucose levels supress the immune system resulting in increased bacterial and viral invasion. Moreover, an infection induces a stress response which can further increase blood glucose levels and exacerbate the infection. Notably, patients become particularly resistant to insulin during the course of the COVID-19 infection, since SARS-CoV-2 virus is believed to cause direct damage to the insulin secreting pancreatic beta-cells. Hence, some patients may require insulin for the first time while others may need their insulin doses increased significantly. Vigilant monitoring of blood glucose levels is essential during the disease process to mitigate COVID-19.
Severe inflammation characteristic of COVID-19 causes tissue damage throughout the human body, which is likely to increase blood clotting and blood vessel damage, conditions that are already compromised by chronic hypertension and diabetes. Most patients with diabetes who develop COVID-19 demonstrate mild symptoms, hence it is important to review their chronic prescriptions and “sick day management” since some diabetic medications may increase the risk of dehydration and acidosis. Such medication maybe temporarily halted. Patients on insulin will have to increase their doses particulary during episodes of fever, hence hydration levels require monitoring as well. Telehealth communication is also essential for diabetic patients with COVID-19 to ensure ongoing contact with their primary care physician. Other recommendations include being up-to-date with seasonal flu vaccines and pneumococcal vaccines, in addition to the general public health measures of social/physical distancing, face masksing and good hygiene practices.
6. Conclusion
This review demonstrates that patients with diabetes mellitus have a high susceptibility to COVID-19 disease advancement and severe outcomes. Also, during COVID-19 infection, ACE2 expression is decreased and this results in an exaggerated activity of Ang II with subsequent IR development. Insulin resistance is the main factor that initiates the activation of an inflammatory response that pre-empts cytokine storms. This affect the alveolar epithelium causing acute respiratory distress symptoms. Essentially, glycaemic control is imperative during this pandemic for prognosis to improve, thus preventative approaches are vital to reduce morbidity and mortality. It is possible that insulin resistance may be a key facilitator between diabetes mellitus and COVID-19 infection. However, more studies are required to confirm this, as insulin resistance may potentially be used as predictor for both COVID-19 and diabetes mellitus. In addition, the expression of ACE2, and its role in the occurrence of IR needs further investigation.
7. Summary
The epidemiology of COVID-19 and its association with cardiometabolic disorders is poorly understood. Current data shows an increased risk of mortality in patients with diabetes mellitus and COVID-19 compared to those without diabetes mellitus. COVID-19 triggers insulin resistance in patients, causing chronic metabolic disorders that were non-existent prior to infection. Patients with diabetes mellitus are more susceptible to COVID-19 infection than those without diabetes mellitus. ACE2 expression decreases with infection, exaggerating Ang II activity with subsequent insulin resistance development, an exaggerated immune response and severe SARS-COV-2 infection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
None.
Acknowledgements
The authors wish to thank Sapna Ramdin for her administrative assistance.
References
- 1.Liang H., Acharya G. Novel corona virus disease (COVID‐19) in pregnancy: what clinical recommendations to follow? Acta Obstet. Gynecol. Scand. 2020;99(4):439–442. doi: 10.1111/aogs.13836. [DOI] [PubMed] [Google Scholar]
- 2.Medical News Today . 2021. COVID-19 Live Updates: Total Number of Cases Passes 117 Million. Available from: https://www.medicalnewstoday.com/articles/live-updates-coronavirus-covid-19#1. (Accessed 10 March 2021) [Google Scholar]
- 3.Egede L.E., Walker R.J. Structural racism, social risk factors, and Covid-19—a dangerous convergence for black Americans. N. Engl. J. Med. 2020;383(12):e77. doi: 10.1056/NEJMp2023616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . 2020. Coronavirus Disease 2019 (COVID-19): Situation Report, 82. [Google Scholar]
- 5.Ben-Shmuel A., Brosh-Nissimov T., Glinert I., Bar-David E., Sittner A., Poni R., Cohen R., Achdout H., Tamir H., Yahalom-Ronen Y., Politi B. Detection and infectivity potential of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) environmental contamination in isolation units and quarantine facilities. Clin. Microbiol. Infect. 2020;26(12):1658–1662. doi: 10.1016/j.cmi.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoehl S., Rabenau H., Berger A., Kortenbusch M., Cinatl J., Bojkova D., et al. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N. Engl. J. Med. 2020;382(13):1278–1280. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wenham C., Smith J., Morgan R. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395(10227):846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020:369. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonsick M., Ferrucci L., Resnick S. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin. Infect. Dis. 2020;10 doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira F.L., Bota D.P., Bross A., Mélot C., Vincent J.-L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 13.Milbrandt E.B., Reade M.C., Lee M., Shook S.L., Angus D.C., Kong L., et al. Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol. Med. 2009;15(11–12):438–445. doi: 10.2119/molmed.2009.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J. Med. Virol. 2020;92(9):1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He L., Ding Y., Zhang Q., Che X., He Y., Shen H., et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006;210(3):288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill M.A., Mantzoros C., Sowers J.R. Commentary: COVID-19 in patients with diabetes. Metabolism. 2020;107 doi: 10.1016/j.metabol.2020.154217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klonoff D.C., Umpierrez G.E. Letter to the Editor: COVID-19 in patients with diabetes: risk factors that increase morbidity. Metabolism. 2020;108:154224. doi: 10.1016/j.metabol.2020.154224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azar W.S., Njeim R., Fares A.H., Azar N.S., Azar S.T., El Sayed M., et al. COVID-19 and diabetes mellitus: how one pandemic worsens the other. Rev. Endocr. Metab. Disord. 2020;21(4):451–463. doi: 10.1007/s11154-020-09573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12(7):6049. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Lorenzo A., Escobar S., Tibiriçá E. Systemic endothelial dysfunction: a common pathway for COVID-19, cardiovascular and metabolic diseases. Nutr. Metab. Cardiovasc. Dis. 2020;30(8):1401–1402. doi: 10.1016/j.numecd.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tam C.S., Xie W., Johnson W.D., Cefalu W.T., Redman L.M., Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35(7):1605–1610. doi: 10.2337/dc11-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davids S.F.G., Matsha T.E., Peer N., Erasmus R.T., Kengne A.P. The 7-year change in the prevalence of insulin resistance, inflammatory biomarkers, and their determinants in an urban south african population. J. Diabetes Res. 2020;2020 doi: 10.1155/2020/3781214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauterbach M.A., Wunderlich F.T. Macrophage function in obesity-induced inflammation and insulin resistance. Pflügers Arch. 2017;469(3–4):385–396. doi: 10.1007/s00424-017-1955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheen A. Pathophysiology of type 2 diabetes. Acta Clin. Belg. 2003;58(6):335–341. doi: 10.1179/acb.2003.58.6.001. [DOI] [PubMed] [Google Scholar]
- 28.Ndisang J.F., Vannacci A., Rastogi S. Insulin resistance, type 1 and type 2 diabetes, and related complications 2017. J. Diabetes Res. 2017:1–3. doi: 10.1155/2017/1478294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra M., Fomusi Ndisang J. A critical and comprehensive insight on heme oxygenase and related products including carbon monoxide, bilirubin, biliverdin and ferritin in type-1 and type-2 diabetes. Curr. Pharm. Des. 2014;20(9):1370–1391. doi: 10.2174/13816128113199990559. [DOI] [PubMed] [Google Scholar]
- 30.Petersen M.C., Vatner D.F., Shulman G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017;13(10):572–587. doi: 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ndisang J.F., Jadhav A. Hemin therapy improves kidney function in male streptozotocin-induced diabetic rats: role of the heme oxygenase/atrial natriuretic peptide/adiponectin axis. Endocrinology. 2014;155(1):215–229. doi: 10.1210/en.2013-1050. [DOI] [PubMed] [Google Scholar]
- 32.Duvnjak L., Duvnjak M. The metabolic syndrome: an ongoing story. J. Physiol. Pharmacol. 2009;60(S7):19–24. [PubMed] [Google Scholar]
- 33.Olson A.L. Regulation of GLUT4 and insulin-dependent glucose flux. Int. Sch. Res. Notices. 2012;2012 doi: 10.5402/2012/856987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drucker D.J. Coronavirus infections and type 2 diabetes—shared pathways with therapeutic implications. Endocr. Rev. 2020;41(3):457–470. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L., Han Y., Nilsson-Payant B.E., Gupta V., Wang P., Duan X., et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(1) doi: 10.1016/j.stem.2020.06.015. 125–136.e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchand L., Pecquet M., Luyton C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020;57(10):1265–1266. doi: 10.1007/s00592-020-01570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barron E., Bakhai C., Kar P., Weaver A., Bradley D., Ismail H., et al. 2020. Type 1 and Type 2 Diabetes and COVID-19 Related Mortality in England: A Whole Population Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trevisani V., Bruzzi P., Madeo S.F., Cattini U., Lucaccioni L., Predieri B., et al. COVID-19 and type 1 diabetes: concerns and challenges. Acta Biomed. 2020;91(3) doi: 10.23750/abm.v91i3.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan W., Liang W., Zhao Y., Liang H., Chen Z., Li Y., et al. 2020. China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on. 2020;1590:13993003.13900547-13992020. [Google Scholar]
- 42.Domingo P., Mur I., Pomar V., Corominas H., Casademont J., de Benito N. The four horsemen of a viral Apocalypse: the pathogenesis of SARS-CoV-2 infection (COVID-19) EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan W.S.D., Liao W., Zhou S., Mei D., Wong W.-S.F. Targeting the renin–angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr. Opin. Pharmacol. 2018;40:9–17. doi: 10.1016/j.coph.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Varga Z., Flammer A., Steiger P., Haberecker M., Andermatt R., Zinkernagel A. Infecção de células endoteliais e endotelite em COVID-19. Lanceta. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010;84(2):1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajpal A., Rahimi L., Ismail‐Beigi F. Factors leading to high morbidity and mortality of COVID‐19 in patients with type 2 diabetes. J. Diabetes. 2020;12(12):895–908. doi: 10.1111/1753-0407.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L., Huang Q., Wang D.C., Ingbar D.H., Wang X. Acute lung injury in patients with COVID‐19 infection. Clin. Transl. Med. 2020;10(1):20–27. doi: 10.1002/ctm2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valencia I., Peiró C., Lorenzo Ó., Sánchez-Ferrer C.F., Eckel J., Romacho T. DPP4 and ACE2 in diabetes and COVID-19: therapeutic targets for cardiovascular complications? Front. Pharmacol. 2020;11:1161. doi: 10.3389/fphar.2020.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou C.C., Ahmad S., Mi T., Xia L., Abbasi S., Hewett P.W., et al. Angiotensin II induces soluble fms-Like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ. Res. 2007;100(1):88–95. doi: 10.1161/01.RES.0000254703.11154.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burke S.D., Zsengellér Z.K., Khankin E.V., Lo A.S., Rajakumar A., DuPont J.J., et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J. Clin. Invest. 2016;126(7):2561–2574. doi: 10.1172/JCI83918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dupont V., Kanagaratnam L., Goury A., Poitevin G., Bard M., Julien G., et al. Excess soluble fms-like tyrosine kinase 1 correlates with endothelial dysfunction and organ failure in critically ill COVID -19 patients. Clin. Infect. Dis. 2020:1–4. doi: 10.1093/cid/ciaa1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Somasundaram N.P., Ranathunga I., Ratnasamy V., Wijewickrama P.S.A., Dissanayake H.A., Yogendranathan N., et al. The impact of SARS-Cov-2 virus infection on the endocrine system. J. Endocr. Soc. 2020;4(8) doi: 10.1210/jendso/bvaa082. bvaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roca-Ho H., Riera M., Palau V., Pascual J., Soler M.J. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int. J. Mol. Sci. 2017;18(3):563. doi: 10.3390/ijms18030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lumpuy-Castillo J., Lorenzo-Almorós A., Pello-Lázaro A.M., Sánchez-Ferrer C., Egido J., Tuñón J., et al. Cardiovascular damage in COVID-19: therapeutic approaches targeting the renin-angiotensin-aldosterone system. Int. J. Mol. Sci. 2020;21(18):6471. doi: 10.3390/ijms21186471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 56.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaduganathan M., Vardeny O., Michel T., McMurray J.J., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saiepour D., Sehlin J., Oldenborg P.-A. Hyperglycemia-induced protein kinase C activation inhibits phagocytosis of c3b-and immunoglobulin g–opsonized yeast particles in normal human neutrophils. Exp. Diabesity Res. 2003;4 doi: 10.1155/EDR.2003.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vikram A., Tripathi D.N., Kumar A., Singh S. Hindawi; 2014. Oxidative Stress and Inflammation in Diabetic Complications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 2020 doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lui G.C.-Y., Yip T.C.-F., Wong V.W.-S., Chow V.C.-Y., Ho T.H.-Y., Li T.C.-M., et al. Significantly lower case-fatality ratio of coronavirus disease 2019 (COVID -19) than severe acute respiratory syndrome (SARS) in Hong Kong—a territory-wide cohort study. Clin. Infect. Dis. 2020:1–10. doi: 10.1093/cid/ciaa1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch. Acad. Emerg. Med. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 64.Guan W.-j., Ni Z.-y., Hu Y., Liang W.-h., Ou C.-q., He J.-x, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Underwood P.C., Adler G.K. The renin angiotensin aldosterone system and insulin resistance in humans. Curr. Hypertens. Rep. 2013;15(1):59–70. doi: 10.1007/s11906-012-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finucane F.M., Davenport C. Coronavirus and obesity: could insulin resistance mediate the severity of Covid-19 infection? Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeda M., Yamamoto K., Takemura Y., Takeshita H., Hongyo K., Kawai T., et al. Loss of ACE2 exaggerates high-calorie diet–induced insulin resistance by reduction of GLUT4 in mice. Diabetes. 2013;62(1):223–233. doi: 10.2337/db12-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santos A., Magro D.O., Evangelista-Poderoso R., Saad M.J.A. Diabetes, obesity, and insulin resistance in COVID-19: molecular interrelationship and therapeutic implications. Diabetol. Metab. Syndr. 2021;13(1):1–14. doi: 10.1186/s13098-021-00639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leiria L.O., Arantes-Costa F.M., Calixto M.C., Alexandre E.C., Moura R.F., Folli F., et al. Increased airway reactivity and hyperinsulinemia in obese mice are linked by ERK signaling in brain stem cholinergic neurons. Cell Rep. 2015;11(6):934–943. doi: 10.1016/j.celrep.2015.04.012. [DOI] [PubMed] [Google Scholar]