Abstract
We report the case of a young woman treated with selective renal embolization for renovascular hypertension caused by intrarenal artery stenosis and show follow‐up imaging of the treated kidney. An 18‐year‐old woman had renin‐dependent hypertension with intrarenal artery stenosis caused by fibromuscular dysplasia. A middle branch artery was nearly occluded, resulting in segmental renal ischemia with excessive renin secretion. Because our angioplasty attempt for revascularization failed as a result of technical difficulty, we performed selective embolization of the diseased vessel by anhydrous ethanol. The embolization promptly ameliorated hyperreninemia and resistant hypertension without deterioration of renal function. Findings from magnetic resonance imaging showed disappearance of the blood flow in the embolized area corresponding to the ischemic lesion that had been revealed by diffusion‐weighted imaging. Thus, selective embolization can be effective in treating renovascular hypertension by intrarenal stenosis for which angioplasty is not feasible. Additionally, renal magnetic resonance imaging is useful for evaluating the causative ischemic lesion and embolized area.
Keywords: angioplasty, embolization therapy, fibromuscular dysplasia, renal artery stenosis, renovascular hypertension
1. INTRODUCTION
Renal artery stenosis causes renovascular hypertension (RVH) through excessive renin secretion from the ischemic kidney. Percutaneous transluminal renal angioplasty (PTRA) is recommended for curative treatment of RVH caused by fibromuscular dysplasia,1 which is a leading cause of curable hypertension in adolescents.2
In patients who do not undergo PTRA, antihypertensive medication with renin‐angiotensin system (RAS) inhibitors including angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers, is the mainstay for controlling blood pressure.3 However, treatment with RAS inhibitors is unsuitable for women planning to get pregnant because of the associated teratogenicity.4 Thus, when the lesion is not amenable to PTRA, the management of RVH in a young woman presenting with resistant hypertension is a difficult clinical issue. Here, we report a case of a young woman treated with selective renal embolization for RVH caused by intrarenal artery stenosis and show the follow‐up imaging of the treated kidney.
2. CASE REPORT
An 18‐year‐old woman was referred for hypertension (170/100 mm Hg) with hypokalemia (3.0 mEq/L), hyperreninemia (plasma renin activity, 15.7 ng/mL/h; reference range, 0.3–2.9 ng/mL/h), and secondary aldosteronsim (aldosterone, 56.5 ng/dL; reference range, 3–16 ng/dL). We diagnosed RVH caused by intrarenal artery stenosis based on branch‐type fibromuscular dysplasia by examinations including renal angiography (Figure 1A). The clinical course of the case until diagnosis was previously reported.5 A middle branch artery of the right kidney was nearly occluded, resulting in segmental renal ischemia in the mid portion with collateral flow (Figure 1A). Our PTRA attempt for revascularization failed because of technical difficulty. Afterward, treatment with high doses of olmesartan, nifedipine, and carvedilol was required to maintain the patient's blood pressure at 140/90 mm Hg. However, RAS inhibitors are considered unsuitable for patients planning to become pregnant. Thus, we attempted selective embolization of the diseased vessel that caused segmental renal ischemia and RVH. To decide the treatment area, reassessment by venous renin sampling and diffusion‐weighted magnetic resonance imaging (MRI) was performed. Catheters were introduced via the femoral vein and guided to the renal veins. Blood samples were collected from the left renal vein, inferior vena cava, and multiple points in the right kidney including the drainage vein of the lesion affecting the stenosis (Figure 2A). As a result, the renin activity level was the highest (50.7 ng/mL/h) at the draining vein of the middle portion of the right kidney, indicating excessive renin secretion in the limited area. Consistently, findings from diffusion‐weighted MRI confirmed that the middle portion was functionally hypoperfused (Figure 2B). Therefore, we planned to selectively embolize the lesion.
Figure 1.
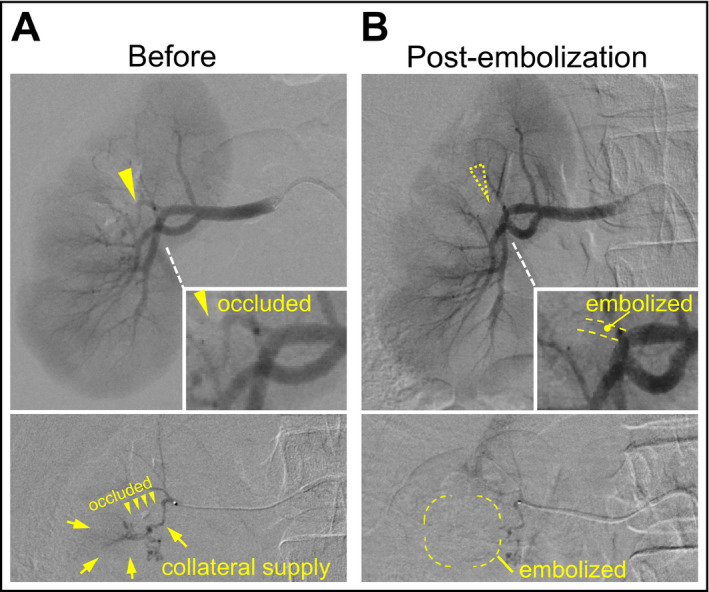
Selective renal embolization for intrarenal branch stenosis and collaterals. Right renal angiography (upper) and selective branch angiography (lower) before (A) and 5 minutes after embolization (B). The insets show enlarged images of the target branch artery. The arrowheads indicate the nearly occluded stenotic lesion. The arrows indicate the collateral supply that disappeared after embolization
Figure 2.
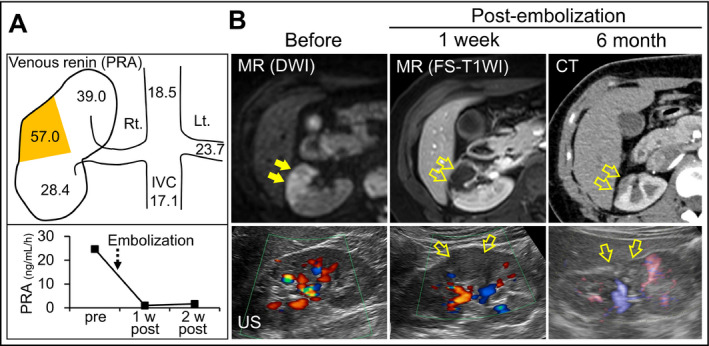
Evaluation of plasma renin secretion and follow‐up renal imaging. (A) Plasma renin activity (PRA; ng/mL/h) levels with selective venous sampling (upper panel). The level was markedly high in the draining vein of the mid portion in the right kidney (indicated in yellow). Change in PRA before and after the embolization (lower panel). (B) Diffusion‐weighted magnetic resonance (MR) imaging (DWI) before embolization showing segmental high intensity in the right kidney (arrows). Gadolinium‐enhanced fat‐suppressed T1‐weighted MR image (FS‐T1WI) at 1 week after embolization. Contrast‐enhanced computed tomographic image at 6 months after embolization. The kidney was also evaluated by Doppler ultrasonography. The open arrows indicate the embolized parenchyma. IVC indicates inferior vena cava
After a 2.8‐F balloon catheter was selectively wedged into the stenotic branch artery, the diseased vessel and tortuous collaterals were embolized by injection of 1.5 mL of anhydrous ethanol under balloon occlusion (Figure 1A). Five minutes later, findings from angiography confirmed the successful occlusion of the branch and collateral supply (Figure 1B). One week later, her blood pressure was controllable without RAS inhibitors. Findings from contrast‐enhanced MRI and Doppler ultrasonography showed the disappearance of the blood flow in the embolized area corresponding to the ischemic lesion that had been revealed by diffusion‐weighted MRI (Figure 2B). Plasma renin activity markedly decreased from 24.7 ng/mL/h to 1.0 ng/mL/h before and 1 week after embolization, respectively (Figure 2A). Additionally, serum creatinine level was not increased after embolization (0.74 mg/dL and 0.69 mg/dL before and 1 week later, respectively). During the following 1 year, her blood pressure was maintained at 120/70 mm Hg with only low‐dose nifedipine. Findings from computed tomography and ultrasonography demonstrated segmental scarring of the embolized parenchyma (Figure 2B).
3. DISCUSSION
This case demonstrates that selective embolization can be effective in treating RVH caused by an intrarenal artery stenosis for which PTRA is technically not feasible. Especially in young women, selective embolization is an important option to eliminate the need for RAS inhibitors for control of blood pressure. Additionally, renal MRI was useful for evaluating the causative ischemic lesion and embolized area before and after the selective embolization, respectively.
Selective embolization of an intrarenal stenotic vessel and its collaterals feeding ischemic parenchyma resulted in amelioration of RVH, as in previous reports including a pediatric case.6, 7 Segmental renal ischemia, even in a limited area, increases focal renin secretion resulting in hypertension.8 In our case, embolization of the causative ischemic lesion efficiently normalized the renin secretion and lowered blood pressure. In addition, selective embolization achieved maximal preservation of renal function with minimal loss of the renal parenchyma.
Fibromuscular dysplasia–associated RVH occurs most frequently in women of childbearing age.2 In most cases of RVH, blood pressure is controllable by medication including RAS inhibitors. However, the condition required RAS inhibitors to control blood pressure is inappropriate for woman planning to get pregnant, because exposure to RAS inhibitors during pregnancy, especially in the second and third trimesters, is associated with neonatal renal complications and extrarenal involvement.9 In addition, fibromuscular dysplasia occasionally leads to intrarenal stenosis and branch occlusion for which PTRA is technically difficult.10 Thus, in cases such as young women with RVH, the clinician should consider performing curative treatment including selective embolization.
Renal artery embolization has played an important role in the management of multimodal renal pathologies such as arteriovenous fistulas and tumors in addition to renal artery stenosis.11 Anhydrous ethanol is an embolic agent that has direct toxic effects on the endothelium, leading to irreversible occlusion of the vascular lumen. In our case, the embolized portion that had been ischemic showed no vascular flow, resulting in segmental scarring that prevents the production of renin. Although the present therapy achieved an optimal outcome, embolization treatment has possible complications. When the embolized portion is large, it can cause abdominal pain from swelling of the embolized parenchyma during the acute phase. In our case, since the embolized area was only segmental, the pain was manageable by administering oral nonsteroidal anti‐inflammatory drugs for several days. Another possible complication is embolization of an unexpected area of the renal parenchyma or other organs such as adrenal glands. Thus, careful, selective intervention is required in embolization.
Our case also illustrates that MRI is useful for evaluation of renal vascular flow before and after embolization. Unlike contrast‐enhanced computed tomography, MRI does not require radiation exposure or the use of nephrotoxic iodine contrasts, and is therefore beneficial for young patients and patients with renal dysfunction, respectively. In addition to gadolinium‐enhanced MRI, which is well established for assessing renal vasculature, the utility of diffusion‐weighted MRI for evaluating ischemia in renal parenchyma has been recently reported.5, 12
4. CONCLUSIONS
Selective embolization is a useful treatment for RVH caused by intrarenal artery stenosis for which revascularization is technically not feasible. In addition, this report shows the utility of MRI for the assessment of renal vascular flow including detection of ischemia and evaluation of embolization.
CONFLICT OF INTEREST
The authors report no conflicts of interest to disclose.
ACKNOWLEDGMENT
A portion of the clinical course until the diagnosis was previously published5 but the subject of the present manuscript substantially differs from that of the prior publication. Part of this report was presented at the 39th Annual Scientific Meeting of the Japanese Society of Hypertension, Sendai, Japan, October 2016.
Mishima E, Suzuki T, Seiji K, et al. Selective embolization therapy for intrarenal artery stenosis causing renovascular hypertension: Efficacy and follow‐up renal imaging. J Clin Hypertens. 2017;19:1028–1031. 10.1111/jch.13040
REFERENCES
- 1. Chrysant SG, Chrysant GS. Treatment of hypertension in patients with renal artery stenosis due to fibromuscular dysplasia of the renal arteries. Cardiovasc Diagn Ther. 2014;4:36‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862‐1871. [DOI] [PubMed] [Google Scholar]
- 3. Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation. 2014;129:1048‐1078. [DOI] [PubMed] [Google Scholar]
- 4. Nadeem S, Hashmat S, Defreitas MJ, et al. Renin angiotensin system blocker fetopathy: a Midwest Pediatric Nephrology Consortium report. J Pediatr. 2015;167:881‐885. [DOI] [PubMed] [Google Scholar]
- 5. Mishima E, Kikuchi K, Ota H, et al. Detection of segmental renal ischemia by diffusion‐weighted magnetic resonance imaging: clinical utility for diagnosis of renovascular hypertension. J Clin Hypertens (Greenwich). 2016;18:364‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teigen CL, Mitchell SE, Venbrux AC, Christenson MJ, McLean RH. Segmental renal artery embolization for treatment of pediatric renovascular hypertension. J Vasc Interv Radiol. 1992;3:111‐117. [DOI] [PubMed] [Google Scholar]
- 7. Reuter SR, Pomeroy PR, Chuang VP, Cho KJ. Embolic control of hypertension caused by segmental renal artery stenosis. AJR Am J Roentgenol. 1976;127:389‐392. [DOI] [PubMed] [Google Scholar]
- 8. Mishima E, Hashimoto J, Akiyama Y, et al. Impact of small renal ischemia in hypertension development: renovascular hypertension caused by small branch artery stenosis. J Clin Hypertens (Greenwich). 2016;18:248‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bullo M, Tschumi S, Bucher BS, Bianchetti MG, Simonetti GD. Pregnancy outcome following exposure to angiotensin‐converting enzyme inhibitors or angiotensin receptor antagonists: a systematic review. Hypertension. 2012;60:444‐450. [DOI] [PubMed] [Google Scholar]
- 10. Bookstein JJ. Segmental renal artery stenosis in renovascular hypertension. Morphologic and hemodynamic considerations. Radiology. 1968;90:1073‐1083. [DOI] [PubMed] [Google Scholar]
- 11. Muller A, Rouviere O. Renal artery embolization‐indications, technical approaches and outcomes. Nat Rev Nephrol. 2015;11:288‐301. [DOI] [PubMed] [Google Scholar]
- 12. Grenier N, Merville P, Combe C. Radiologic imaging of the renal parenchyma structure and function. Nat Rev Nephrol. 2016;12:348‐359. [DOI] [PubMed] [Google Scholar]


