Abstract
According to the National Health and Nutrition Examination Survey 2012, one third of antihypertensive prescriptions in the United States in the past decade were for angiotensin‐converting enzyme inhibitors (ACEIs). An important and serious side effect of ACEIs is angioedema caused by a reduction in bradykinin degradation. In a national medical chart abstraction study conducted at the US Veterans Affairs Health Care System in 2008, 0.20% of ACEI initiators developed angioedema while on the medication. The angiotensin‐converting enzyme is a part of the renin‐angiotensin system that converts angiotensin I to angiotensin II. It is additionally responsible for the degradation of bradykinin, which is generated from high molecular weight kininogen by kallikrein. Via bradykinin 2 receptors, bradykinin affects vascular permeability and stimulates the release of substance P, which is a peptide that causes vasodilation and fluid extravasation into tissues. Inhibition of the angiotensin‐converting enzyme and subsequent blockade of bradykinin degradation is thought to be a likely explanation for ACEI‐induced angioedema. Studies have shown that blacks, women, and smokers are at an increased risk for ACEI‐induced angioedema. A 2005 study identified black race, history of drug rash, age older than 65 years, and seasonal allergies as independent risk factors for angioedema related to enalapril. Angioedema may occur at any time during treatment with ACEIs and may continue after the medication is discontinued. The degree of ACEI‐angiotensin receptor blocker angioedema cross‐reactivity is difficult to determine from the literature. However, multiple studies have reported relatively low rates of native angioedema with angiotensin receptor blockers (approximately half that of ACEIs, or 0.1%) and a low incidence of cross‐reactivity (<10%). Common treatments for angioedema, such as antihistamines and glucocorticoids, have not been shown to be effective in ACEI‐induced angioedema. However, medications that have been used for acute treatment of hereditary angioedema and target the factors that cause ACEI‐mediated angioedema are being explored.
Keywords: angioedema, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, bradykinin, risk factors
1. INTRODUCTION
Angioedema is a localized swelling of deep dermis, subcutaneous, or submucosal tissue caused by the vascular extravasation of fluid into the interstitium. Angioedema may result from a deficiency of the C1 inhibitor, which causes a condition known as hereditary angioedema and is mediated by bradykinin. Other causes include allergic angioedema, which is mediated by histamine, and idiopathic angioedema, which is of unclear etiology. In addition, angioedema may result from the use of angiotensin‐converting enzyme inhibitors (ACEIs), caused by a reduction in bradykinin degradation. In a national medical chart abstraction study conducted at the US Veterans Affairs Health Care System, 0.20% of ACEI initiators developed angioedema while taking the medication.1 A retrospective study using the French national pharmacovigilance database (1994–2013) reported 112 cases of bradykinin‐mediated angioedema caused by medications. Of the 112 patients, 71 were treated with an ACEI alone.2 Likewise, a meta‐analysis of 16 randomized trials of ACEIs' safety and efficacy in patients 65 years and older documented a 2.8‐fold increase in risk of angioedema by ACEIs compared with active controls.3 Data from a National Health and Nutrition Examination Survey show that in the past decade, one third of antihypertensive prescriptions in the United States were for ACEIs.4 Therefore, it is critical to recognize the presentation, natural history, and prognosis of ACEI‐induced angioedema, to understand the association of angioedema with angiotensin receptor blocker (ARB) therapy, and to be aware of new medications proposed for the treatment of this potentially fatal condition.
2. MECHANISM OF ACTION
Via the renin‐angiotensin system, renin is secreted by the juxtaglomerular cells in the kidney in response to decreases in blood pressure. The angiotensin‐converting enzyme (ACE) is integral to this system as it converts angiotensin I to angiotensin II, which then exerts multiple physiologic effects, leading to an increase systemic blood pressure. ACEIs inhibit ACE and therefore block the conversion of angiotensin I to angiotensin II, causing a decrease in systemic blood pressure (Figure 1).5
Figure 1.
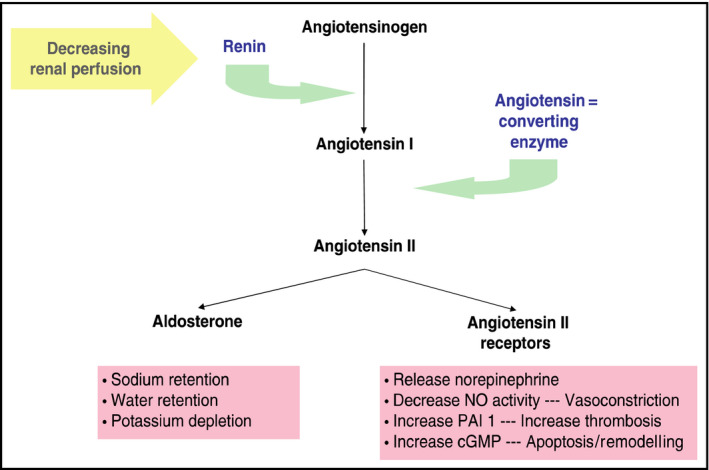
Overview of the renin‐angiotensin‐aldosterone system. cGMP indicates cyclic guanosine monophosphate; NO, nitric oxide; PAI 1, plasminogen activator inhibitor‐1
ACE, also known as kininase II, is additionally responsible for the degradation of bradykinin. Bradykinin is generated from high‐molecular‐weight kininogen by kallikrein. Via bradykinin 2 receptors, bradykinin affects vascular permeability and stimulates the release of substance P, which is a peptide that causes vasodilation and fluid extravasation into tissues. Blockade of bradykinin degradation is thought to be a likely explanation for ACEI‐induced angioedema. Patients who develop angioedema while taking an ACEI have been shown to have a slower rate of bradykinin degradation than those taking an ACEI who do not develop angioedema.5 Secondary enzymes, such as aminopeptidase P, may also regulate bradykinin degradation when ACE is inhibited. Decreased levels or impaired function of any of these enzymes have also been associated with a higher incidence of the development of ACEI‐induced angioedema.6
3. RISK FACTORS
Studies have determined that blacks are at increased risk for ACEI‐induced angioedema.1, 7, 8 Other studies have shown increased risk associated with female sex, chronic heart failure or coronary artery disease,1 and a history of smoking.7 In the OCTAVE (Omapatrilat Cardiovascular Treatment vs. Enalapril) trial, 12 634 patients were randomized to enalapril and followed for a 24‐week, double‐blind treatment period. This study identified black race, history of drug rash, age older than 65 years, and seasonal allergies as independent risk factors for angioedema related to enalapril.8 Conversely, the risk of angioedema was significantly decreased in patients with diabetes mellitus.1, 8 Finally, several medications have been shown to increase the risk of angioedema in patients taking ACEIs. Dipeptidyl peptidase 4 inhibitors, which degrade substance P when ACE is inhibited, have been demonstrated to cause a ninefold increased risk of angioedema in individuals using an ACEI.9 In addition, renal transplant recipients treated with mammalian target of rapamycin inhibitors while also receiving ACEIs have been shown to have an increased incidence of angioedema caused by interference with the bradykinin pathway.10
4. TIMING
It is critical to recognize that angioedema can develop at any time during the treatment course in patients taking ACEI therapy. The incidence of angioedema within the first week of initiating treatment is approximately one in 1000 cases.11 While angioedema that occurs soon after starting ACEI use is most common, one study showed that up to 20% of cases occurred more than 6 weeks after initiating treatment.12 A literature search using PubMed and the keywords “angiotensin‐converting enzyme inhibitor” and “angioedema” revealed multiple cases of ACEI‐induced angioedema that developed more than 1 year after the initiation of the drug. One case described the development of visceral angioedema after 9 years of enalapril therapy,13 while another described two episodes of angioedema occurring 11 years after the initiation of therapy with lisinopril.14 A separate case report noted the development of ACEI‐induced angioedema 23 years after the initiation of enalapril15 in a patient without any known risk factors, which, per our review, is the longest reported time from the initiation of ACEI therapy to the development of angioedema in the literature. The Table summarizes the cases of late‐onset angioedema. In addition, an interruption in ACEI therapy may be a risk factor for the development of late‐onset angioedema. A woman taking enalapril for 7 months with no complications interrupted therapy for 72 hours as a result of a delay in her medication refill. Upon resuming the medication, she developed angioedema.16
Table 1.
Time of onset of delayed angioedema (>1 wk on an angiotensin‐converting enzyme inhibitor)
Of important clinical relevance is the recognition that angioedema that occurs while taking ACEI therapy can recur even after the ACEI has been discontinued. A retrospective study of 111 patients who developed angioedema on ACEI therapy found that 46% of patients had further recurrences of angioedema after discontinuation of ACEIs. In 16% of these patients, the frequency of angioedema after cessation of ACEIs remained unchanged. A total of 88% experienced their first recurrence of angioedema within the first month, while a first episode of angioedema occurring more than 3 months after the cessation of ACEI therapy was found in only one case (2%). In total, 92 patients had angioedema that lasted for at least 6 months after discontinuing ACEIs.17
5. CROSS‐REACTIVITY BETWEEN ACEIs AND ARBs
The exact incidence of angioedema cross‐reactivity between ACEIs and ARBs has been a contested value in the literature, primarily because of the low overall risk of this event, trials not powered to detect this adverse effect, and limitations in study design. Unlike ACEIs, ARBs do not inhibit the degradation of bradykinin. They lower blood pressure by blocking angiotensin II from binding to angiotensin type 1 receptors. The pathophysiology of ARB‐induced angioedema may involve upregulation of angiotensin type 2 receptors by the increased level of angiotensin II.18 Other hypotheses include the involvement of other vasoactive agents such as prostaglandins, or the deficiency of complement cascade mediators.19
The native incidence of ARB‐induced angioedema appears extremely low from numerous meta‐analyses and chart reviews of patients exposed to ARBs. A meta‐analysis of pooled data from 40 randomized controlled trials involving ACEIs, ARBs, and direct renin inhibitors included a total of 206 596 patients with a mean follow‐up of 123 weeks. The weighted incidence rates of angioedema with ACEIs was 0.30% (95% confidence interval [CI], 0.28%–0.32%), with ARBs was 0.11% (95% CI, 0.09%–0.13%), and with direct renin inhibitors was 0.13% (95% CI, 0.07%–0.19%).20 Similarly, a propensity score–adjusted examination of newly initiated monotherapy with ACEIs and ARBs using the MarketScan Commercial Claims and Encounters and Medicare Supplementary and Coordination of Benefit (Truven Healthcare, Inc.) claims databases estimated a hazard ratio of 1.91 (95% CI, 1.67–2.19) between ACEIs and ARBs and an overall rate of 0.1% of ARB‐induced angioedema. This database analysis also revealed a 91% greater incidence of angioedema with ACEIs relative to ARBs (hazard ratio, 1.91; 95% CI, 1.67–2.19).21
Case reports have documented ARB‐induced angioedema with numerous members of the ARB class, implicating angioedema as a class effect rather than an agent‐specific issue. Most of these cases involved either losartan or irbesartan, although the exact incidence rate could not be determined from postmarketing surveillance reporting (eg, ADRAC in Australia).22 One such review of 13 case reports revealed that ARB‐induced angioedema has occurred within 24 hours and up to 16 months following exposure to losartan.23
An early report reviewed one case series and six case reports for a total of 19 patients with ARB‐induced angioedema between January 1966 and August 1999. A retrospective review of their medical histories revealed that 32% had experienced previous ACEI‐induced angioedema.24
Haymore and colleagues25 conducted a meta‐analysis with a subsequent revision that compiled data from two large retrospective cohorts and two large randomized controlled trials of patients who had ACEI‐induced angioedema and were subsequently given an ARB. The randomized trials included the CHARM‐Alternative (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity) trial (comparing candesartan with placebo) and the TRANSCEND (Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease) trial (a component of the ONTARGET [Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial] comparing telmisartan with placebo). They classified patients into possible or confirmed ARB‐induced angioedema cases based on the likelihood of the angioedema diagnosis. The combined data revealed an overall rate of 2.5% risk of angioedema from ARBs (95% CI, 0%–6.6%) and a rate of 1.5% for confirmed cases (95% CI, 0%–5.1%). However, a sensitivity analysis of the randomized clinical trials alone revealed no significant differences in angioedema rates with ARBs or placebo (odds ratio, 1.1; 95% CI, 0.07–17).25 These results were validated in a more recent meta‐analysis in which 216 patients with a history of ACEI‐induced angioedema experienced similar rates of angioedema with ARBs as with placebo (4.1% vs 0.8%; risk ratio, 3.01 [95% CI, 0.41–22.4]).26
Finally, a systematic literature review by Knecht and colleagues27 in 2014 found the cross‐reactivity rate between ACEIs and ARBs to be <10% (ranging between 0 to 17%) and the native incidence of ARB‐induced angioedema to be less than one half that of ACEI‐induced angioedema (3%‐8%).
Meta‐analyses and retrospective chart reviews are limited by numerous factors including the heterogeneity of the patient population studied, specific medications used, dosing, and duration of therapy. Overall, while it is difficult to ascertain the true size of the population at risk for ACEI‐ARB angioedema cross‐reactivity, multiple studies have reported relatively low rates of native angioedema with ARBs (approximately half that of ACEIs, or 0.1%) and a low incidence of cross‐reactivity (<10%). The decision to initiate ARBs in patients who experienced angioedema with ACEIs must be met with careful consideration of the medication risk‐benefit ratio as well as a discussion of possible side effects.
6. TREATMENT
Classical treatment for ACEI‐induced angioedema involves the use of antihistamines, glucocorticoids, and epinephrine. Glucocorticoids have been shown to induce the expression of ACE28 and could theoretically accelerate bradykinin metabolism and thus alleviate angioedema that is mediated by bradykinin. Because ACEI‐induced angioedema is not a histamine‐mediated process, the role of antihistamines is unclear and they are usually used with minimal response.29 Treatments that have shown utility in the treatment of hereditary angioedema and target the factors that cause ACEI‐mediated angioedema are being explored.
6.1. Fresh frozen plasma
Fresh frozen plasma (FFP), which contains kininase II or ACE, has been used in the treatment of hereditary angioedema. Several groups have reported the rapid improvement of symptoms after two units of FFP in patients with ACEI‐induced angioedema refractory to standard treatment with steroids, antihistamines, and epinephrine and even more aggressive therapy with cyclosporine and intravenous immunoglobulin.30, 31 One such case series described seven patients who had improvement in angioedema symptoms 2 to 4 hours after the administration of one to three units of FFP.32 However, in addition to kininase II, FFP also contains HMW‐kininogen and kallikrein, the substrates that form bradykinin. There are rare reports of worsening symptoms of ACEI‐induced angioedema after FFP administration.33
6.2. Ecallantide
Ecallantide is a direct inhibitor of plasma kallikrein, and therefore reduces the production of bradykinin. Several randomized trials have sought to determine whether this agent might have a role in the resolution of ACEI‐induced angioedema. A multicenter, randomized, double‐blind trial of 79 patients from an emergency department with ACEI‐induced angioedema found that the addition of a single subcutaneous dose of ecallantide to standard therapy did not cause statistically significant improvement in angioedema compared with placebo.34 Ecallantide has not been proven to be effective in the treatment of refractory angioedema caused by an ACEI, although larger studies may be useful.
6.3. Icatibant
For ACEI‐induced angioedema mediated by bradykinin, the role of icatibant, a bradykinin B2 receptor antagonist primarily used in the treatment of hereditary angioedema, has been evaluated. Case reports have shown that icatibant causes a statistically significant reduction in time until symptom relief when used in the treatment of ACEI‐induced angioedema.35, 36 Bas and colleagues37 conducted a randomized study of 27 patients that demonstrated a median time to complete resolution of angioedema of 8 hours with icatibant vs 27.1 hours with glucocorticoids and antihistamines (Figure 2). The French National Center for Angioedema has recommended the use of B2 receptor antagonists as first‐line therapy for ACEI‐induced angioedema.38 However, a randomized study by Straka and colleagues39 treated 31 patients, mostly blacks, with either icatibant or placebo. The time to resolution of symptoms was not statistically significant between the two groups (Figure 3). Further studies will be needed to demonstrate whether icatibant is effective in certain groups with ACEI‐induced angioedema and not in others.
Figure 2.
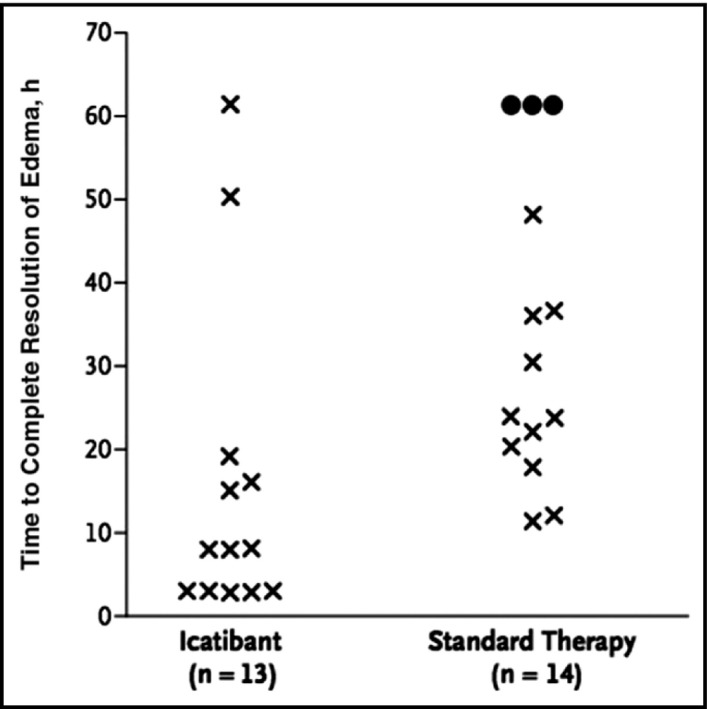
Time to complete resolution of edema according to study treatment for three patients in the standard‐therapy group who required rescue intervention (circles). The time to complete resolution was set at 61.2 hours
Figure 3.
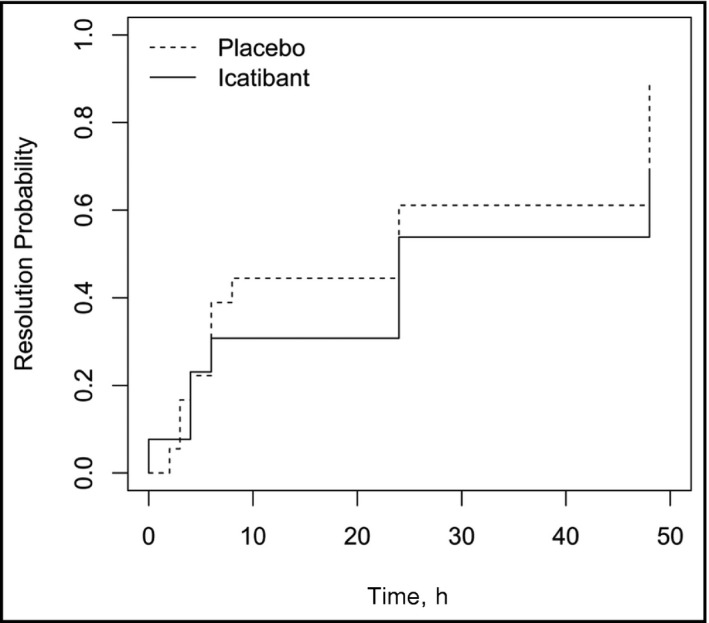
Time to resolution of primary symptoms as assessed by patients using visual analog scale in patients treated with either placebo or icatibant. P = .192 for effect of treatment
6.4. C1 inhibitor concentrate
Angioedema caused by both C1 esterase deficiency and ACEIs is a result of excess bradykinin, and therefore C1 inhibitor concentrate has been explored as a potential therapy in ACEI‐induced angioedema. Case reports have noted the successful utilization of C1 inhibitor concentrate to treat ACEI‐induced angioedema.40, 41, 42 A point‐of‐concept case series compared 10 patients with ACEI‐induced angioedema treated with C1 inhibitor concentrate to a historical control group of patients with ACEI‐induced angioedema who had been treated with corticosteroids and antihistamines. It demonstrated that the time to complete resolution of symptoms was 10.1 hours after C1 inhibitor administration and 33.1 hours in the control group. In addition, no patients treated with C1 inhibitors required intubation, while in the control group, three required tracheotomy and two required intubation.43 Although there are no prospective trials of the use of C1 inhibitor concentrate for the treatment of ACEI‐induced angioedema, case reports support its use.
7. CONCLUSIONS
Despite the array of newer therapies that have been examined, there are no currently approved algorithms for the treatment of ACEI‐induced angioedema in the United States. It is important for primary care providers to be aware of the varying presentations of ACEI‐induced angioedema, to recognize the association of angioedema with ARB therapy, and to have knowledge of the newer agents and therapies being investigated for the treatment of this adverse reaction to one of the most commonly prescribed antihypertensive agents in the United States.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Brown T, Gonzalez J, Monteleone C. Angiotensin‐converting enzyme inhibitor–induced angioedema: A review of the literature. J Clin Hypertens. 2017;19:1377–1382. 10.1111/jch.13097
REFERENCES
- 1. Miller DR, Oliveria SA, Berlowitz DR, et al. Angioedema incidence in US veterans initiating angiotensin‐converting enzyme inhibitors. Hypertension. 2008;51:1624‐1630. [DOI] [PubMed] [Google Scholar]
- 2. Faisant C, Armengol G, Bouillet L, et al. Angioedema triggered by medication blocking the renin/angiotensin system: retrospective study using the French national pharmacovigilance database. J Clin Immunol. 2016;36:95‐102. [DOI] [PubMed] [Google Scholar]
- 3. Bavishi C, Ahmed M, Trivedi V, et al. Meta‐analysis of randomized trials on the efficacy and safety of angiotensin‐converting enzyme inhibitors in patients > 65 years of age. Am J Cardiol. 2016;118:1427‐1436. [DOI] [PubMed] [Google Scholar]
- 4. Gu Q, Burt V, Dillon C, Yoon S. Trends in antihypertensive medication and blood pressure control among United States adults with hypertension. Circulation. 2012;126:2105‐2114. 10.1161/circulationaha.112.096156. [DOI] [PubMed] [Google Scholar]
- 5. Hoover T, Lippmann M, Grouzmann E, et al. Angiotensin converting enzyme inhibitor induced angio‐oedema: a review of the pathophysiology and risk factors. Clin Exp Allergy. 2010;40:50‐61. [DOI] [PubMed] [Google Scholar]
- 6. Kitamura S, Carbini LA, Simmons WH, Scicli AG. Effects of aminopeptidase P inhibition on kinin‐mediated vasodepressor responses. Am J Physiol. 1999;276:H1664‐H1671. [DOI] [PubMed] [Google Scholar]
- 7. Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin‐converting enzyme inhibitors. J Eval Clin Pract. 2004;10:499‐509. [DOI] [PubMed] [Google Scholar]
- 8. Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165:1637‐1642. [DOI] [PubMed] [Google Scholar]
- 9. Brown NJ, Byiers S, Carr D, et al. Dipeptidyl peptidase‐IV inhibitor use associated with increased risk of ACE inhibitor‐associated angioedema. Hypertension. 2009;54:516‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duerr M, Glander P, Diekmann F, et al. Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients. Clin J Am Soc Nephrol. 2010;5:703‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Slater EE, Merrill DD, Gues HA, et al. Clinical profile of angioedema associated with angiotensin converting‐enzyme inhibition. JAMA. 1988;260:967‐970. [PubMed] [Google Scholar]
- 12. Hedner T, Samuelsson O, Lindholm L, et al. Angioedema in relation to treatment with angiotensin converting enzyme inhibitors. Br Med J. 1992;304:941‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Orr KK, Myers J. Intermittent visceral edema induced by long‐term enalapril administration. Ann Pharmacother. 2004;38:825‐827. [DOI] [PubMed] [Google Scholar]
- 14. Norman J, Holmes W, Bell W, Finks S. Life‐threatening ACE inhibitor‐induced angioedema after eleven years on lisinopril. J Pharm Pract. 2013;26:382‐388. [DOI] [PubMed] [Google Scholar]
- 15. Howarth D. ACE inhibitor angioedema: a very late presentation. Aust Fam Physician. 2013;42:860‐862. [PubMed] [Google Scholar]
- 16. Dyer PD. Late‐onset angioedema after interruption of angiotensin converting enzyme inhibitor therapy. J Allergy Clin Immunol. 1994;93:947‐948. [DOI] [PubMed] [Google Scholar]
- 17. Beltrami L, Zanichelli A, Zingale L, et al. Long‐term follow‐up of 111 patients with angiotensin‐converting enzyme inhibitor‐related angioedema. J Hypertens. 2011;29:2273‐2277. [DOI] [PubMed] [Google Scholar]
- 18. Fuchs SA, Meyboom RH, van Puijenbroek EP, Guchelaar HJ. Use of angiotensin receptor antagonists in patients with ACE inhibitor induced angioedema. Pharm World Sci. 2004;26:191‐192. [DOI] [PubMed] [Google Scholar]
- 19. Abdi R, Dong VM, Lee CJ, Ntsoso KA. Angiotensin II receptor blocker‐associated angioedema: on the heels of ACE inhibitor angioedema. Pharmacotherapy. 2002;22:1173‐1175. [DOI] [PubMed] [Google Scholar]
- 20. Makani H, Messerli FH, Romero J, et al. Meta‐analysis of randomized trials of angioedema as an adverse event of renin‐angiotensin system inhibitors. Am J Cardiol. 2012;110:383‐391. [DOI] [PubMed] [Google Scholar]
- 21. Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howes LG, Tran D. Can angiotensin receptor antagonists be used safely in patients with previous ACE inhibitor‐induced angioedema? Drug Saf. 2002;25:73‐76. [DOI] [PubMed] [Google Scholar]
- 23. Van Rijnsoever EW, Kwee‐Zuiderwijk WJM, Feenstra J. Angioneurotic edema attributed to the use of losartan. Arch Intern Med. 1998;158:2063‐2065. [DOI] [PubMed] [Google Scholar]
- 24. Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in patients with ACE inhibitor‐induced angioedema. Ann Pharmacother. 2000;34:526‐528. [DOI] [PubMed] [Google Scholar]
- 25. Haymore BR, DeZee KJ. Use of angiotensin receptor blockers after angioedema with an angiotensin‐converting enzyme inhibitor. Ann Allergy Asthma Immunol. 2009;103:83‐84. [DOI] [PubMed] [Google Scholar]
- 26. Caldeira D, David C, Sampaio C. Tolerability of angiotensin‐receptor blockers in patients with intolerance to angiotensin‐converting enzyme inhibitors: a systematic review and meta‐analysis. Am J Cardiovasc Drugs. 2012;12:263‐277. [DOI] [PubMed] [Google Scholar]
- 27. Knecht SE, Dunn SP, Macaulay TE. Angioedema related to angiotensin inhibitors. J Pharm Pract. 2014;27:461‐465. [DOI] [PubMed] [Google Scholar]
- 28. Dasarathy Y, Lanzillo JJ, Fanburg BL. Stimulation of bovine pulmonary artery endothelial cell ACE by dexamethasone: involvement of steroid receptors. Am J Physiol. 1992;263:L645‐L649. [DOI] [PubMed] [Google Scholar]
- 29. Bas M, Greve J, Stelter K, et al. Therapeutic efficacy of icatibant in angioedema induced by angiotensin‐converting enzyme inhibitors: a case series. Ann Emerg Med. 2010;56:278‐282. [DOI] [PubMed] [Google Scholar]
- 30. Karim MY, Masood A. Fresh‐frozen plasma as a treatment for life‐threatening ACE‐inhibitor angioedema. J Allergy Clin Immunol. 2002;109:370. [DOI] [PubMed] [Google Scholar]
- 31. Warrier M, Copilevitz C, Dykewicz M, Slavin R. Fresh frozen plasma in the treatment of resistant angiotensin‐converting enzyme inhibitor angioedema. Ann Allergy Asthma Immunol. 2004;92:573‐575. [DOI] [PubMed] [Google Scholar]
- 32. Hassen G, Kalantari H, Parraga M, et al. Fresh frozen plasma for progressive and refractory angiotensin‐converting enzyme inhibitor‐induced angioedema. J Emerg Med. 2013;44:764‐772. [DOI] [PubMed] [Google Scholar]
- 33. Adebayo O, Wilkerson RG. Angiotensin‐converting enzyme inhibitor‐induced angioedema worsened with fresh frozen plasma. Am J Emerg Med. 2017;35:192.e1‐192.e2. [DOI] [PubMed] [Google Scholar]
- 34. Lewis LM, Graffeo C, Crosley P, et al. Ecallantide for the acute treatment of angiotensin‐converting enzyme inhibitor‐induced angioedema: a multicenter, randomized controlled trial. Ann Emerg Med. 2015;65:204‐213. [DOI] [PubMed] [Google Scholar]
- 35. Javaud N, Fain O, Bernot B, et al. Bradykinin‐mediated angioedema secondary to angiotensin converting enzyme: initiate treatment from the prehospital phase. Ann Fr Anesth Reanim. 2011;30:848‐850. [DOI] [PubMed] [Google Scholar]
- 36. Schmidt PW, Hirschl MM, Trautinger F. Treatment of angiotensin‐converting enzyme inhibitor‐related angioedema with the bradykinin B2 receptor antagonist icatibant. J Am Acad Dermatol. 2010;63:913‐914. [DOI] [PubMed] [Google Scholar]
- 37. Bas M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE‐inhibitor‐induced angioedema. N Engl J Med. 2015;372:418‐425. 10.1056/nejmoa1312524. [DOI] [PubMed] [Google Scholar]
- 38. Nosbaum A, Bouillet L, Floccard B, et al. Management of angiotensin‐converting enzyme inhibitor‐related angioedema: recommendations from the French national center for angioedema. Rev Med Interne. 2013;34:209‐213. [DOI] [PubMed] [Google Scholar]
- 39. Straka BT, Ramirez CE, Byrd JB, et al. Effect of bradykinin receptor antagonism on ACE inhibitor‐associated angioedema. J Allergy Clin Immunol. 2017;140:242‐248.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rasmussen ER, Bygum A. ACE‐inhibitor induced angio‐oedema treated with complement C1‐inhibitor concentrate. BMJ Case Rep. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lipski S, Casimir G, Vanlommel M, et al. Angiotensin‐converting enzyme inhibitors‐induced angioedema treated by C1 esterase inhibitor concentrate (Berinert®): about one case and review of the therapeutic arsenal. Clin Case Rep. 2015;3:126‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nielsen EW, Gramstad S. Angioedema from angiotensin‐converting enzyme inhibitor treated with complement 1 (C1) inhibitor concentrate. Acta Anaesthesiol Scand. 2006;50:120‐122. [DOI] [PubMed] [Google Scholar]
- 43. Greve J, Bas M, Hoffmann T, et al. Effect of C1‐esterase‐inhibitor in angiotensin‐converting enzyme inhibitor‐induced angioedema. Laryngoscope. 2015;125:E198‐E202. [DOI] [PubMed] [Google Scholar]


