Abstract
Pulse wave velocity (PWV) is a biomarker of arterial stiffness. Findings from prior studies are conflicting regarding the impact of obesity on PWV in children. The authors measured carotid‐femoral PWV in 159 children aged 4 to 18 years, of whom 95 were healthy, 25 were obese, 15 had hypertension (HTN), and 24 were both obese and hypertensive. Mean PWV increased with age but did not differ by race or sex. In adjusted analyses in children 10 years and older (n=102), PWV was significantly higher in children with hypertension (PWV±standard deviation, 4.9±0.7 m/s), obesity (5.0±0.9 m/s), and combined obesity‐hypertension (5.2±0.6 m/s) vs healthy children (4.3±0.7 m/s) (each group, P<.001 vs control). In our study, obesity and HTN both significantly and independently increased PWV, while African American children did not have a higher PWV than Caucasian children.
Cardiovascular disease (CVD) has become a clinical concern in younger children. The antecedents of CVD such as early atherosclerosis are known to begin in childhood, as shown in the prospective Bogalusa Heart Study.1 Obesity is also a major contributor to CVD, with a higher body mass index (BMI) contributing along with atherosclerosis to higher mortality in the Münster Heart Study.2 Obesity has increased in prevalence and currently affects 30% of children in the United States.3, 4 Concomitantly, hypertension (HTN) prevalence has also increased significantly.5, 6 Additional studies are needed to better understand the epidemiology and risks of CVD in children.
Arterial stiffness, or reduced arterial compliance, can be measured as pulse wave velocity (PWV), which is the speed at which the pulse wave is transmitted across the length of the arterial tree. A higher PWV indicates a stiffer blood vessel, contributing to increased afterload and subsequent cardiac remodeling.7 In adults, higher PWV predicts the risk of CVD events such as stroke, ischemic heart disease, and HTN.3, 4 Higher PWV has been found in African Americans8, 9 and may contribute to worse cardiovascular outcomes for African Americans with HTN.10
Existing data regarding PWV in children11 have two limitations. First, normative reference value data are largely derived from European Caucasian populations,12, 13 with unknown applicability to younger children of African American race. Second, some prior studies provided conflicting results on the effects of obesity on PWV. Several studies, including those by Lurbe and colleagues14 in Spain, Dangardt and colleagues15 in Sweden, and Charakida and colleagues16 in the United Kingdom, showed decreased PWV in obese children. In contrast, Sakuragi and colleagues17 showed that obesity increased PWV in Australian youth. Similarly, Urbina and colleagues18 and Shikha and colleagues19 found that obesity increased PWV in populations with diabetes mellitus in age ranges of 10–26 and 14–16 years of mixed races.
We undertook this study with the primary aim to determine whether obesity and/or HTN significantly increase PWV in healthy and affected (obese and hypertensive) North American children from different racial backgrounds, especially those of African American heritage.
Methods
Patients
We recruited patients aged between 4 and 18 years into four groups: (1) healthy controls, (2) obese only, (3) HTN only, and (4) both obese and hypertensive. We excluded patients who had any acute illness, were taking oral steroids, or had any of the following: seizure disorder, known heart disease, diabetes, multiorgan disease including genetic syndromes, and pregnancy, each of which might independently impact PWV or blood pressure (BP). We included patients with asthma, who were not taking oral steroids at the time of the study; patients taking attention deficit hyperactivity disorder (ADHD) medications; and adolescent females taking contraceptives.
Healthy control patients were defined as children who met our inclusion and exclusion criteria and did not have obesity or HTN, as defined below.
The HTN only group included children with primary HTN, either new‐onset (untreated) or well controlled on up to a maximum of two medications. HTN was defined by the treating pediatric nephrologist as per appropriate criteria and standard methodology,20 using the 95th percentile cutoff for age, sex, and height per standard BP reference tables.21 Use of multiple clinic BP measurements and/or ambulatory 24‐hour BP monitoring to define HTN was at the discretion of the treating physician.
The obese only group included children with a BMI >95th percentile for age, sex, and height, with normal BP for age, sex, and height.
The fourth group had both obesity and HTN (obese + HTN), using the same criteria for obesity and HTN listed above.
Healthy control patients and obese patients were recruited from the Washington University Pediatric and Adolescent Ambulatory Research Consortium (WU PAARC). This is a practice‐based research network of 33 community practices in the greater St. Louis area that participate in pediatric research. Control patients were also recruited from the Washington University Research Volunteer database. We recruited additional obese participants from the preventive cardiology and gastroenterology clinics at Washington University School of Medicine and St. Louis Children's Hospital. Hypertensive patients were all recruited from pediatric nephrology clinics at St. Louis Children's Hospital.
This cross‐sectional study was approved by the institutional review board of Washington University School of Medicine.
Study Methods
After a brief eligibility survey, patients were invited to participate. One parent provided written informed consent, and patients older than 7 years provided assent. The parent completed a detailed 11‐question health questionnaire that provided information on age, sex, race, medical history, and current medications. One member of the study team (NKM) measured each patient's height, weight, brachial BP at rest, and carotid‐to‐femoral length. We calculated the BMI percentile and z score and categorized the patients to one of our study groups.
PWV Measurements
Carotid‐to‐femoral PWV was measured noninvasively for each patient by the same individual (NKM) using the Sphygmocor device (AtCor Medical, Sydney, New South Wales, Australia), which is a Food and Drug Administration–approved applanation tonometer device. This machine records both pressure wave tracings over the carotid and femoral artery with simultaneous three‐lead electrocardiography. We obtained three readings for all patients at rest. One reading and PWV result was selected for final analysis by one investigator (NKM) based on contour analysis of waves and reproducibility, assessed as a low coefficient of variation (≤10%) and good wave contour. If more than one waveform of sufficient good quality was obtained on an individual patient, the mean of the acceptable PWV measurements was used for analysis.
Statistical Analysis
All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC). To test for univariable differences among groups (Tables 1 and 2), we used analysis of variance (ANOVA) for continuous variables and either a chi‐square statistic or Fisher exact test, as appropriate, for categorical variables. We used Pearson's correlation coefficient to assess the relationship between BMI and PWV.
Table 1.
Summary Statistics of Traits for All 159 Patients
| Variable | Control (n=95) | Hypertensive Only (n=15) | Obese Only (n=25) | Hypertensive + Obese (n=24) | P Value |
|---|---|---|---|---|---|
| PWV, m/s | 4 (0.7) | 4.7 (0.8) | 4.7 (0.9) | 5.1 (0.7) | <.001 |
| Age, y | 10.4 (4) | 14.1 (2.3) | 11.8 (2.9) | 14.3 (2.8) | <.001 |
| BMI, kg/m2 | 18.4 (3.6) | 22.1 (3.8) | 30.1 (5.9) | 35 (7.2) | <.001 |
| SBP, mm Hg | 98.8 (10.7) | 121.7 (12) | 113 (10.9) | 126.8 (9.5) | <.001 |
| DBP, mm Hg | 60.7 (9.6) | 71.7 (11.9) | 67.8 (10.1) | 72.1 (12) | <.001 |
| Pulse rate, beats per min | 75.2 (12.1) | 71.2 (15.1) | 77.1 (13.4) | 75 (12.2) | .575 |
| Male/female | 51/44 | 9/6 | 16/9 | 16/8 | .602 |
| Race | |||||
| Other | 7 (7) | 2 (13) | 2 (8) | 1 (4) | |
| African American | 32 (34) | 6 (40) | 7 (28) | 7 (29) | .874 |
| Caucasian | 56 (59) | 7 (47) | 16 (64) | 16 (67) | |
| ADHD medication | 8 (8) | 3 (20) | 4 (16) | 2 (8) | .372 |
| HTN medication | 0 | 9 (60) | 0 | 17 (71) | <.001 |
Abbreviations: ADHD, attention deficit hyperactivity disorder; BMI, body mass index; DBP, diastolic blood pressure; HTN, hypertension; PWV, pulse wave velocity; SBP, systolic blood pressure. Estimates of continuous variables are presented as mean (standard deviation) and categorical factors are presented as number (percentage).
Table 2.
Summary Statistics of Traits for All Patients 10 Years and Older
| Variable | Control (n=49) | Hypertensive Only (n=13) | Obese Only (n=18) | Hypertensive + Obese (n=22) | P Value |
|---|---|---|---|---|---|
| PWV, m/s | 4.3 (0.7) | 4.9 (0.7) | 5 (0.9) | 5.2 (0.6) | <.001 |
| Age, y | 13.3 (2.4) | 14.6 (1.7) | 13.2 (1.7) | 14.7 (2.1) | .024 |
| BMI, kg/m2 | 20.4 (3.3) | 22.8 (3.4) | 32.9 (4.2) | 35.8 (6.9) | <.001 |
| SBP, mm Hg | 102.1 (10.6) | 120.6 (12.5) | 117.3 (7.8) | 127.9 (9.1) | <.001 |
| DBP, mm Hg | 63.4 (9.2) | 70.2 (12) | 70.7 (9.5) | 72.5 (12.2) | .002 |
| Pulse rate, beats per min | 71.5 (12) | 69.3 (11.6) | 76.4 (13.9) | 74.7 (12.4) | .318 |
| Male/female | 24/25 | 8/5 | 11/7 | 16/6 | .602 |
| Caucasian/African American | 29/20 | 7/6 | 12/6 | 15/7 | .624 |
| ADHD medication | 4 (8) | 2 (15) | 2 (11) | 2 (9) | .372 |
| HTN medication | 0 (0) | 8 (62) | 0 (0) | 15 (68) | <.001 |
Abbreviations: ADHD, attention deficit hyperactivity disorder; BMI, body mass index; DBP, diastolic blood pressure; HTN, hypertension; PWV, pulse wave velocity; SBP, systolic blood pressure. Estimates of continuous variables are presented as the mean (standard deviation) and categorical factors are presented as number (percentage).
We analyzed differences in PWV between groups (healthy controls, HTN alone, obesity alone and combined HTN‐obesity) for patients 10 years and older only. Younger children were not included in these analyses because of the lack of data to support that measures of cardiovascular disease show a change in children this young.
To investigate the influence of obesity and HTN on PWV we considered two separate statistical approaches. While a case can be made that the two disease processes are different and independent of each other, the majority of recent childhood HTN cases may be biologically related to obesity, perhaps through sympathetic nervous system activity.6
We considered a two‐way ANOVA model that included both of the main effects (HTN + obesity) and their interaction (eg, if HTN has a different response whether a patient is also obese). Hence, we conducted an explicit test for independence of the two variables via the interaction. The interaction test was not significant.
As we were primarily interested in comparisons of all the disease groups (HTN only, obese only, and HTN + obese) with healthy controls, we performed one‐way ANOVA, where the different disease groups were all considered mutually exclusive, and tested whether the group assignment significantly influenced PWV. We then added categorical and continuous demographic variables to this model including race (Caucasian, African American), sex, and age (10 to 18 years), and retained significant demographic variables in this analysis of covariance (ANCOVA) model. Interactions were assessed between age and all remaining factors to test for slope heterogeneity among groups. To evaluate the magnitude of the effects, means adjusted for covariates (ie, least‐squares means) were estimated. All results are presented from the one‐way ANOVA model. A P value <.05 was considered significant.
Results
Patient Traits
A total of 162 patients met the inclusion criteria for our study and provided consent. Three patients were excluded because of an inability to measure PWV accurately, leaving a total of 159 patients aged 4 to 18 years in the final study group (Table 1). The male to female ratio was 92:67 (males 57.8%). The self‐reported racial breakdown was Caucasian 95 (59.7%), African American 52 (32.7%), and other 12 (7.5%). Of the total 159 patients, 95 (59.7%) were healthy controls, 25 (15.7%) were obese only, 15 (9.4%) had HTN only, and 24 (15.0%) had HTN and obesity. Estimates of the unadjusted PWV means showed that each of the study groups had, on average, PWV values higher by at least 0.7 m/s compared with the control group, as shown in Table 1.
Analysis of PWV Between Groups
After excluding patients younger than 10 years (n=48, of whom 40 were in the control group) for the reason stated above and the “other” race category (n=12) because of very few patients in that group, 102 patients remained in the dataset for the between‐group analysis of PWV. Overall, the final model accounted for more than 30% of the variation in the data (R 2=0.32) and described a significant amount of variation (P<.001).
In the initial model, there was a significant group main effect for the ANOVA (P<.001), with all study groups having significantly higher PWV means than the control group in post hoc analysis (adjusted mean=4.25 m/s [control], 4.85 [hypertensive only], 5.00 [obese only], 5.16 [HTN + obese]). After adjusting for age, all study groups remained significantly different from the control group (Table 2), but none of the three study groups were significantly different from each other, based on post hoc pairwise comparisons (t tests) of age‐adjusted means.
Neither race nor sex was significant and was not retained in the final model. This is consistent with estimates of the unadjusted mean and 95% confidence intervals [CIs] that indicate similar PWV means and substantial overlap among races in the children 10 years and older (African American race [n=39], unadjusted mean PWV, 4.55 [95% CI, 4.31–4.79] and Caucasian race [n=63], unadjusted mean PWV, 4.72 [95% CI, 4.52–4.93]) and sex (male sex unadjusted mean PWV, 4.71 [95% CI, 4.49–4.94] and female unadjusted mean PWV, 4.58 [95% CI, 4.36–4.79]). Repeating these analyses for the entire 4–18 age study group yielded the same overlap between races (African American race [n=52], unadjusted mean PWV, 4.31 [95% CI, 4.08–4.54]; Caucasian race [n=95], unadjusted mean PWV, 4.38 [95% CI, 4.20–4.56]). Age was significant with a slope of 0.10, but there was no significant interaction between disease group and age (Table 3).
Table 3.
Parameter Estimates From the Final Model Where Disease Was Treated as a Single Factor With Four Groups
| Parameter | Regression Coefficient | P Value |
|---|---|---|
| Hypertensive + obese | 0.77 (0.42–1.12) | <.001 |
| Obese | 0.75 (0.39–1.11) | <.001 |
| Hypertensive | 0.47 (0.05–0.89) | .03 |
| Age, y | 0.10 (0.04–0.16) | .002 |
Regression coefficients (95% confidence intervals) correspond to the covariate‐adjusted mean difference relative to the control group, and the coefficient for age corresponds to the slope. Each condition adds to the baseline pulse wave velocity (control) by the amount of the regression coefficient. Race, sex, and interaction (disease × age) were not significant and were not retained in the final model.
In these 102 patients, PWV showed a significant association with untransformed BMI (Figure a, r=0.543 [P<.001]). PWV retained a significant association with BMI z scores (Figure b, r=0.458 [P<.001]).
Figure 1.
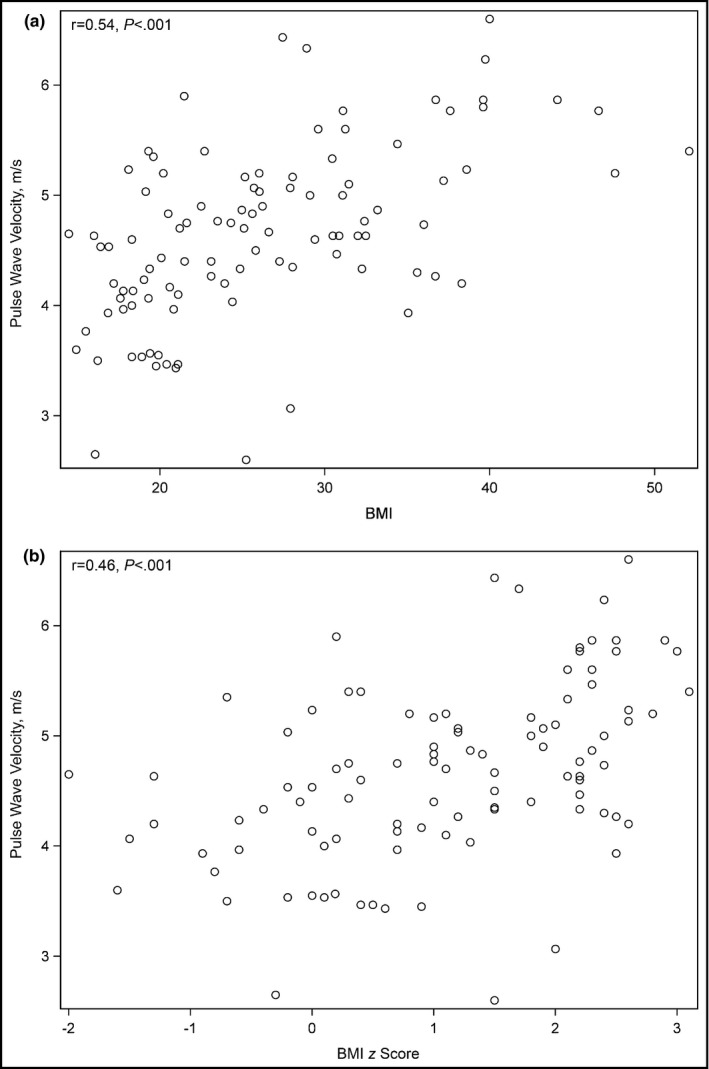
Scatter plot and Pearson's correlation coefficient (r) for body mass index (BMI) and pulse wave velocity (PWV) (patients 10 years and older) (a). Scatter plot and Pearson's correlation coefficient (r) for BMI z score and PWV (patients 10 years and older) (b).
Discussion
Our study reported the following novel findings: (1) in our study population, both obesity and HTN independently increased PWV; (2) African American children did not have a higher PWV than Caucasian children.
Study Strengths
Strengths of our study include the homogeneity of technique and measurement. We utilized what others described as a “gold standard” technique of carotid‐femoral PWV.8, 11 In contrast, carotid‐radial PWV, used in many of the studies that showed lower PWV with obesity,15, 22, 23, 24, 25 may not capture those segments of the arterial tree that are impacted earliest and most by obesity or HTN. All measurements of carotid‐femoral length and PWV were performed by a single author (NKM), thereby removing some of the heterogeneity of measurements generated when multiple operators perform the measurements, as postulated to occur in other studies.11, 14 These variations in methodology between studies may also account for the conflict in literature regarding the relationship of PWV and obesity.
The change in PWV relative to obesity alone in children is associated with multiple conflicting reports in the literature. Obesity introduces several structural and hemodynamic changes that may impact the blood vessel wall. Fat may deposit around the vessel wall and change compliance. Obesity‐associated inflammation, mediated by adipocytokines such as endothelin and nitric oxide, may lead to vasodilatation in resistance vessels,26 although PWV is more a measure of stiffness in conducting vessels. Studies by some groups have shown an increase in PWV (ie, a decrease in arterial compliance) with obesity alone,17, 18, 27 in accordance with our results. Other studies have shown a “paradoxical” decrease in PWV with obesity alone, an unexpected result since adult studies consistently show higher PWV with obesity.28 One hypothesis for the paradoxical decrease was earlier puberty leading to earlier maturation to peak arterial compliance and increased body size in obese children.26 Another hypothesis is that the decrease in PWV is a short‐term adaptation, not sustainable in the long‐term, and eventually reverses to a higher PWV with time in longitudinal studies.15 Variation in PWV changes are also associated with body region, with studies of carotid artery more likely to show increased PWV with obesity, vs studies of proximal to distal upper or lower limb.29
Some studies have demonstrated that African American young adults have higher PWV values than Caucasian adults.8, 9 A study by Yan and colleagues8 performed carotid‐femoral PWV measurements in 52 African American and 48 Caucasian patients aged 18 to 37 years. The study by Li and colleagues9 performed brachial‐ankle PWV in a study group aged 24 to 44 years that included 231 African American patients. Yet, our study in children showed that African American children did not have elevated PWV relative to Caucasian children. Our results are also in contrast with those of Urbina and colleagues,18 who showed a significantly higher PWV in African American children aged 10 to 26 years, but in a population that was highly enriched for diabetes, a disease that may change vessel wall characteristics. To our knowledge, our study is the first to look at African American children without diabetes. We speculate that the increased PWV compared with Caucasians may not be present in African Americans until early adulthood either as a developmental feature or as a difference in obesity or hypertensive disease progression.
Study Limitations
The limitations of our study include the cross‐sectional design, the use of tape rather than calipers to measure the carotid‐to‐femoral distance, the lack of other vessel compliance analyses such as aortic index/pulse waveform analyses, and lack of other biological measures such as carotid‐intimal thickness. Other studies have utilized other measures of PWV, such as cardiac impedance, augmentation index, and brachial distensibility.30 We had few African American in the age group younger than 10 years. We also did not have enough children of other races (eg, Hispanic and Asian) to make meaningful conclusions in those groups. We have not attempted to evaluate the newly diagnosed hypertensive patients separately as a result of the small sample size. Lastly, we did not have an adequate number of children in each group to assess the effects of asthma or use of ADHD medication or oral contraceptives.
Understanding the changes in PWV may impact practice, such as future choice of antihypertensive agent. In one study, β‐blocker agents did not reverse abnormalities in PWV in the same manner as angiotensin‐converting enzyme inhibitors and calcium channel blockers.31 However, these single study results need validation in other studies. Future directions suggested by our results include longitudinal studies that assess the change in carotid‐femoral PWV in healthy and children with disease over time, especially the change with increasing obesity in patients, or with control of HTN in newly diagnosed hypertensive patients.
Conclusions
In our study population, higher PWV was seen in children younger than 18 years with both obesity and hypertension acting independently and together.
Financial Disclosure
None of the authors have any relevant financial disclosures.
Funding Source
The Sphygmocor device used in this study was purchased through a grant from the Children's Discovery Institute, St. Louis, to Dr Kozel.
Conflict of Interest
The authors have no conflicts of interest relevant to this article to disclose.
Acknowledgments
We would like to acknowledge the support and effort of Ms Kathy Mandrell (WUPAARC Network Coordinator) in collaborating our study with local pediatric practices. We also thank Drs Isabell Rosenbloom and John Davis at Tots Thru Teens clinic in Bridgeton, Missouri, for allowing us to identify and enroll patients from their pediatric office. We presented the results of this study in a poster presentation at the Pediatric Academic Society/American Society of Pediatric Nephrology in April 2015.
J Clin Hypertens (Greenwich). 2017;19:221–226. DOI: 10.1111/jch.12892 © 2016 Wiley Periodicals, Inc.
References
- 1. Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338(23):1650–1656. [DOI] [PubMed] [Google Scholar]
- 2. Schulte H, Cullen P, Assmann G. Obesity, mortality and cardiovascular disease in the Munster Heart Study (PROCAM). Atherosclerosis. 1999;144:199–209. [DOI] [PubMed] [Google Scholar]
- 3. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011‐2012. JAMA 2014;311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Preventive Services Task Force , Barton M. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics 2010;125:361–367. [DOI] [PubMed] [Google Scholar]
- 5. Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999‐2000. JAMA 2002;288:1728–1732. [DOI] [PubMed] [Google Scholar]
- 6. Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. 2002;40:441–447. [DOI] [PubMed] [Google Scholar]
- 7. Townsend RR, Black HR, Chirinos JA, et al. Clinical use of pulse wave analysis: proceedings from a symposium sponsored by North American Artery. J Clin Hypertens (Greenwich). 2015;17:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yan H, Ranadive SM, Heffernan KS, et al. Hemodynamic and arterial stiffness differences between African‐Americans and Caucasians after maximal exercise. Am J Physiol Heart Circ Physiol. 2014;306:H60–H68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li S, Chen W, Srinivasan SR, Berenson GS. Childhood blood pressure as a predictor of arterial stiffness in young adults: the Bogalusa Heart Study. Hypertension. 2004;43:541–546. [DOI] [PubMed] [Google Scholar]
- 10. Flack JM. The epidemiology of hypertension and related conditions in the African‐American population. J Natl Med Assoc. 1995;87(8 suppl):606–609. [PMC free article] [PubMed] [Google Scholar]
- 11. Savant JD, Furth SL, Meyers KEC. Arterial stiffness in children: pediatric measurement and considerations. Pulse. 2015;2:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reusz GS, Cseprekal O, Temmar M, et al. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension. 2010;56:217–224. [DOI] [PubMed] [Google Scholar]
- 13. Thurn D, Doyon A, Sozeri B, et al. Aortic pulse wave velocity in healthy children and adolescents: reference values for the vicorder device and modifying factors. Am J Hypertens. 2015;28:1480–1488. [DOI] [PubMed] [Google Scholar]
- 14. Lurbe E, Torro I, Garcia‐Vicent C, et al. Blood pressure and obesity exert independent influences on pulse wave velocity in youth. Hypertension. 2012;60:550–555. [DOI] [PubMed] [Google Scholar]
- 15. Dangardt F, Chen Y, Berggren K, et al. Increased rate of arterial stiffening with obesity in adolescents: a five‐year follow‐up study. PLoS One. 2013;8:e57454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charakida M, Jones A, Falaschetti E, et al. Childhood obesity and vascular phenotypes: a population study. J Am Coll Cardiol. 2012;60:2643–2650. [DOI] [PubMed] [Google Scholar]
- 17. Sakuragi S, Abhayaratna K, Gravenmaker KJ, et al. Influence of adiposity and physical activity on arterial stiffness in healthy children: the lifestyle of our kids study. Hypertension. 2009;53:611–616. [DOI] [PubMed] [Google Scholar]
- 18. Urbina EM, Khoury PR, McCoy CE, et al. Triglyceride to HDL‐C ratio and increased arterial stiffness in children, adolescents, and young adults. Pediatrics. 2013;131:e1082–e1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shikha D, Singla M, Walia R, et al. Vascular compliance in lean, obese, and diabetic children and adolescents: a cross‐sectional study in a minority population. Cardiorenal Med. 2014;4:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 22. Tryggestad JB, Thompson DM, Copeland KC, Short KR. Obese children have higher arterial elasticity without a difference in endothelial function: the role of body composition. Obesity. 2012;20:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tryggestad JB, Thompson DM, Copeland KC, Short KR. Arterial compliance is increased in children with type 2 diabetes compared with normal weight peers but not obese peers. Pediatr Diabetes. 2013;14:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chalmers LJ, Copeland KC, Hester CN, et al. Paradoxical increase in arterial compliance in obese pubertal children. Angiology. 2011;62:565–570. [DOI] [PubMed] [Google Scholar]
- 25. Dangardt F, Osika W, Volkmann R, et al. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imaging. 2008;28:287–293. [DOI] [PubMed] [Google Scholar]
- 26. Tryggestad JB, Short KR. Arterial compliance in obese children: implications for cardiovascular health. Exerc Sport Sci Rev. 2014;42:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. [DOI] [PubMed] [Google Scholar]
- 28. Acree LS, Montgomery PS, Gardner AW. The influence of obesity on arterial compliance in adult men and women. Vasc Med. 2007;12:183–188. [DOI] [PubMed] [Google Scholar]
- 29. Hudson LD, Rapala A, Khan T, et al. Evidence for contemporary arterial stiffening in obese children and adolescents using pulse wave velocity: a systematic review and meta‐analysis. Atherosclerosis. 2015;241:376–386. [DOI] [PubMed] [Google Scholar]
- 30. Totaro S, Khoury PR, Kimball TR, et al. Arterial stiffness is increased in young normotensive subjects with high central blood pressure. J Am Soc Hypertens. 2015;9:285–292. [DOI] [PubMed] [Google Scholar]
- 31. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. [DOI] [PubMed] [Google Scholar]


