Abstract
The aim of this study was to investigate whether arterial stiffness plays a role in retaining normal diastolic function in a middle‐aged and elderly Korean population. A total of 267 patients without documented cardiovascular disease, 50 years and older (mean age, 57.3±6.3 years; 69.8% men) were retrospectively analyzed. All patients underwent both transthoracic echocardiography and brachial‐ankle pulse wave velocity measurement on the same day. Patients with septal annular peak velocity (e’) ≥8 cm/s and left atrial volume index <34 mL/m2 were considered as having normal diastolic function. Ninety‐eight patients (36.7%) had normal diastolic function. Low brachial‐ankle pulse wave velocity (<1314 cm/s) was an independent factor for determining normal diastolic function even after controlling for potential confounders in multiple logistic regression analysis (odds ratio, 2.58; 95% confidence interval, 1.46–4.57; P=.001). Our results suggest that compliant arteries may play an important role in maintaining normal left ventricular diastolic function in middle‐aged and elderly patients without documented cardiovascular disease.
Keywords: arterial stiffness, diastolic function, elderly, pulse wave velocity
1. INTRODUCTION
Left ventricular (LV) diastolic dysfunction (LVDDF) is highly prevalent and is an independent risk factor for mortality even in general populations.1, 2, 3 In addition, LVDDF can lead to overt heart failure syndrome in spite of normal or mildly impaired LV systolic function at the time of symptoms.4 Recently, the clinical significance of heart failure with preserved ejection fraction has been emphasized because of its high prevalence and poor prognosis, which are similar to those of heart failure with reduced ejection fraction.5, 6 Therefore, early recognition of subclinical LV diastolic dysfunction is important. Transthoracic echocardiography (TTE) is the most useful single method in the diagnosis of LV diastolic function. TTE gives objective information on mechanical and functional abnormalities associated with decreased ventricular relaxation and increased ventricular stiffness.7 For this reason, evaluation of diastolic function became a routine and essential component of TTE.7
It has been shown that aging is the most important determinant of LV diastolic function, and the prevalence of LVDDF proportionally increases with age.4, 5, 8, 9 From a histopathologic aspect, aging‐related LVDDF is associated with a decreased number of cardiac myocytes by increased apoptosis and necrosis with age. It has been proposed that the LV wall becomes more thickened due to remaining myocyte hypertrophy, increased collagen deposits, and decreased elastin to compensate for myocardial loss.10 These aging‐related myocardial changes reduce ventricular compliance, leading to decreased diastolic filling and increased cardiac filling pressure.11 In addition, the increased prevalence of traditional risk factors, such as hypertension, diabetes mellitus, and coronary artery disease, also contribute to the development of LVDDF in patients at advanced age.5, 12
The association between increased arterial stiffness and LVDDF has been well characterized, suggesting a close interaction between the arterial system and the left ventricle.13, 14, 15, 16 Although the mechanism of this association has not yet been fully determined, it has generally been suggested that increased arterial stiffness promotes LVDDF. Greater arterial stiffness can lead to increased pulse pressure and LV afterload, which contributes to LV hypertrophy and subendocardial ischemia.14, 15 A stiffened artery also reduces aortic diastolic pressure and coronary flow.16
It is to be noted that normal diastolic function could be detected in 44% to 72% of an advanced age group of healthy patients.1, 12, 17 Our study investigated whether there is a relationship between retaining normal diastolic function and preservation of normal arterial compliance in a group of middle‐aged and elderly Korean patients.
2. PATIENTS AND METHODS
2.1. Study patients and protocol
This retrospective and single‐center study was performed at Boramae Medical Center (Seoul, Korea). Between October 2009 and July 2013, medical records of patients older than 50 years with stable medical conditions and no documented cardiovascular disease (myocardial infarction, coronary revascularization, positive results on cardiac tests including myocardial perfusion imaging, exercise treadmill test, coronary angiography, stroke, and peripheral artery disease [ankle‐brachial index <0.9]) were reviewed. All study patients underwent TTE, brachial‐ankle pulse wave velocity (baPWV) measurement, and various blood tests for their routine checkup on the same day. Patients were excluded when they had the following cardiac conditions: (1) LV ejection fraction <50%; (2) LV regional wall motion abnormalities; (3) valvular dysfunction more than mild degree; (4) nonsinus rhythm; (5) pericardial effusion; and (6) unavailable information on parameters for determining LV diastolic function. After exclusion, a total of 267 patients were analyzed in this study. Among those analyzed, 72 apparently healthy patients without cardiovascular risk factors (26.9%) had been enrolled in a prior study13 investigating the association between baPWV and LV filling pressure (E/e'), performed by our group. Each checkup consisted of a medical history taking, physical examinations, blood pressure (BP) measurement, and laboratory tests. We assessed, through a medical interview, whether study patients had hypertension, diabetes mellitus, or dyslipidemia and whether they were smokers. Hypertension was defined as the use of antihypertensive medications or systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg. Diabetes mellitus was defined as the use of oral hypoglycemic agents or insulin or serum fasting glucose level ≥126 mg/dL. Dyslipidemia was defined as known but untreated dyslipidemia or current treatment with lipid‐lowering agents. Patients were classified as smokers if they had smoked regularly during the past 12 months. A trained nurse checked brachial BP, and Korotkoff phases I and V were used to determine systolic and diastolic pressures. Body mass index (BMI) was calculated as body weight in kilograms divided by square of height in meters (kg/m2). All blood samples were acquired in the morning after more than eight hours of fasting. White blood cell count and blood levels of hemoglobin, fasting glucose, uric acid, serum creatinine, glycated hemoglobin, and C‐reactive protein were measured. Estimated glomerular filtration rate was calculated by the Modification of Diet in Renal Disease equation.18 Approval of the study protocol was obtained from the institutional review board of Boramae Medical Center (Seoul, Korea). Written informed consent was waived because of the retrospective design of the study.
2.2. baPWV measurement
Pulse wave velocity (PWV), which is defined as velocity at which a pulse wave propagates to the periphery, was designed to estimate systemic arterial stiffness index. The baPWV is defined by the distance between the two sites divided by the time difference between the two pulses.19 The baPWV was measured with a volume‐plethysmographic apparatus (VP‐1000; Colin Co. Ltd., Komaki, Japan).13 Patients rested in the supine position for more than 5 minutes before the examination. Daily medication, caffeine consumption, and cigarette smoking were not allowed before the examination. To measure BP and semiconductor pulse wave, the measurement cuffs were placed around the bilateral antecubital areas and ankles. Pulse volume waveform, phonogram, BP, and heart rate were recorded simultaneously. The mean of the left and right baPWV values was used in the analysis. All measures were conducted by the same experienced operator who was blinded to patients’ information. The coefficient of variance for interobserver reliability of baPWV was 5.1% in our laboratory.
2.3. TTE
TTE was performed using commercially available devices (Sequoia, Siemens Medical Solutions, Mountain View, CA, USA, or Vivid 7; GE Medical Systems, Milwaukee, WI, USA) according to the recommendations of current guidelines.7, 20 M‐mode echocardiography was used to measure LV dimensions from the parasternal short‐axis view, and biplane Simpson's method was used to measure LV ejection fraction from the apical four‐ and two‐chamber views. All patients underwent pulsed wave Doppler examination at the tip of the mitral leaflets to measure the peak early mitral inflow velocity during early diastole (E), late diastole (A), and deceleration time. Color‐coded tissue Doppler imaging was performed from the apical four‐chamber view to calculate early velocity (e') at the septal mitral annulus. The left atrial volume index (LAVI) was measured with the biplane method from the apical four‐ and two‐chamber views at ventricular end systolic phases. Patients with septal annular peak velocity (e') ≥8 cm/s and LAVI <34 mL/m2 were considered as having normal LV diastolic function.7 The coefficient of variation for interobserver reliability of septal e' velocity was 6.8% in our laboratory.
2.4. Statistical analysis
Continuous variables are presented as mean±standard deviation, and categorical variables as percentages. Continuous variables were compared using Student t test, and categorical variables using the chi‐square test between patients with and without LVDDF. To assess the cutoff value of baPWV as a predictor of normal diastolic function, receiver operating characteristic (ROC) curve analysis was performed. Multiple logistic regression analysis was performed to investigate whether baPWV is an independent factor for determining normal diastolic function by adjusting for age, sex, BMI, hypertension, diabetes mellitus, dyslipidemia, and estimated glomerular filtration rate, which were known to be associated with arterial stiffness.21 Integrated discrimination improvement index was used to evaluate the additional value of baPWV to traditional risk factors including hypertension, diabetes mellitus, dyslipidemia, and smoking in the identification of normal diastolic function.22 A P value <.05 was considered statistically significant. All statistical analyses were conducted using SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
3. RESULTS
Among the total study patients, septal annular (e') peak velocity was negatively correlated with age (r=−.331, P<.001), and LAVI and baPWV were positively correlated with age (r=.183 and P=.003 for LAVI; r=.369 and P<.001 for baPWV [Figure 1]). Ninety‐eight patients (36.7%) had normal LV diastolic function. The baseline characteristics of the study patients according to the presence of LVDDF are summarized in Table 1. Patients with normal LV diastolic function were younger (57.0±4.9 years vs 59.6±6.5 years, P=.001) and had lower BMI (23.7±3.0 kg/m2 vs 25.4±2.9 kg/m2, P=.001) than those with LVDDF. There were no significant differences in the incidences of traditional risk factors, including hypertension, diabetes mellitus, dyslipidemia, or smoking between the two groups (P>.05 for each). Laboratory results were also similar between the two groups. Echocardiographic findings showed significant differences in diastolic parameters including E and A wave velocities, E/A, E wave deceleration time, e' velocity, E/e', and LAVI between the two groups (P<.05 for each diastolic parameter). The mean baPWV value was significantly lower in patients with normal diastolic function than in those with LVDDF (1440±440 cm/s vs 1346±346 cm/s, P<.001 [Figure 2]). In ROC curve analysis, a baPWV of 1314 cm/s was the best cutoff value for the detection of LVDDF (sensitivity, 69.8%; specificity, 57.1%; area under the ROC curve, 0.634; 95% confidence interval [CI], 0.56–0.70; P<.001 [Figure 3]). Multiple logistic regression analysis demonstrated that younger age (<65 years; odds ratio [OR], 3.74; 95% CI, 1.30–10.75; P=.014), lower BMI (<25 kg/m2; OR, 2.95; 95% CI, 1.62–5.37; P<.001), and lower baPWV (<1314 cm/s; OR, 2.58; 95% CI, 1.46‐4.57; P=.001) were independent predictors of normal diastolic function after controlling for potential confounders (Table 2). Even after further stratification of the study patients into four groups by baPWV quartiles, the independent association between low baPWV and normal diastolic function in multivariable analysis was still significant (fourth quartile [the highest] vs first quartile [the lowest]: OR, 2.29; 95% CI, 1.46–5.56; P=.040 [Table S1]). The integrated discrimination improvement index showed better performance of the model that included baPWV compared with the reference model without baPWV in identifying normal diastolic function (P=.001).
Figure 1.
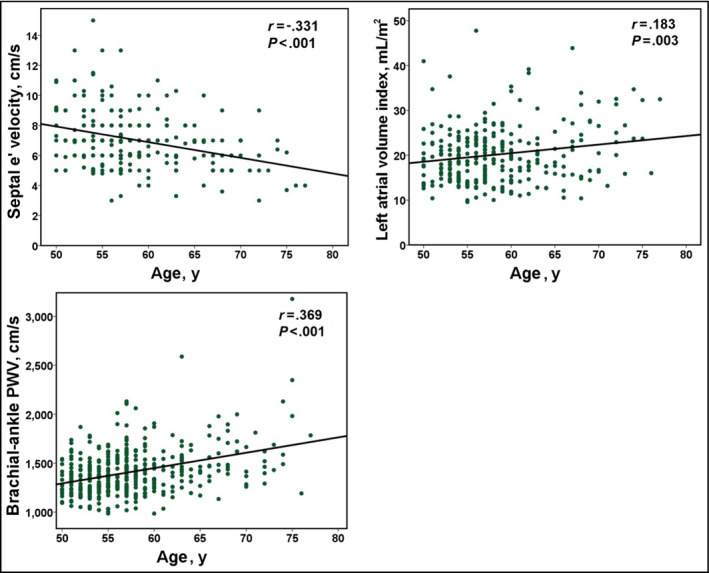
Changes in septal e' velocity, left atrial volume index, and brachial‐ankle pulse wave velocity (PWV) according to age in study patients
Table 1.
Baseline Characteristics of Study Patients According to the Presence of Diastolic Dysfunction
| Characteristic | Patients With Normal Diastolic Function (n=98) | Patients With Diastolic Dysfunction (n = 169) | P Value |
|---|---|---|---|
| Age, y | 57.0±4.9 | 59.6±6.5 | .001 |
| Men, No. (%) | 66 (67.3) | 111 (65.7) | .781 |
| Body mass index, kg/m2 | 23.7±3.0 | 25.4±2.9 | .001 |
| Traditional risk factors, No. (%) | |||
| Diabetes mellitus | 8 (8.9) | 26 (15.6) | .132 |
| Hypertension | 31 (34.4) | 75 (45.2) | .096 |
| Dyslipidemia | 20 (22.2) | 54 (32.5) | .082 |
| Current smoking | 39 (47.0) | 56 (40.9) | .375 |
| Systolic blood pressure, mm Hg | 121±12 | 124±14 | .081 |
| Diastolic blood pressure, mm Hg | 78±9 | 80±10 | .097 |
| Major laboratory findings | |||
| White blood cell count, per μL | 5832±2294 | 5863±1722 | .907 |
| Hemoglobin, g/dL | 14.5±1.4 | 14.7±1.4 | .205 |
| Fasting glucose, mg/dL | 97.3±19.9 | 100.7±21.6 | .188 |
| Uric acid, mg/dL | 5.2±1.5 | 5.4±1.4 | .198 |
| Glycated hemoglobin, % | 5.8±0.9 | 5.8±0.7 | .728 |
| C‐reactive protein, mg/dL | 0.15±0.27 | 0.16±0.27 | .845 |
| eGFR, mL/min/1.73 m2 | 81.7±13.4 | 78.6±14.1 | .072 |
| Echocardiographic findings | |||
| LV end‐diastolic dimension, mm | 47.9±3.9 | 48.0±4.2 | .811 |
| LV end‐systolic dimension, mm | 29.9±2.8 | 29.6±3.1 | .040 |
| LV ejection fraction, % | 66.8±5.2 | 67.1±5.3 | .616 |
| LV mass index, g/m2 | 89.2±25.2 | 93.0±19.9 | .263 |
| E, cm/s | 73.1±16.3 | 62.4±14.3 | <.001 |
| A, cm/s | 61.5±12.1 | 71.0±16.4 | <.001 |
| E/A | 1.22±0.31 | 0.90±2.37 | <.001 |
| Deceleration time, ms | 219±47 | 233±40 | .021 |
| Septal e’ velocity, cm/s | 9.14±1.57 | 6.06±1.07 | <.001 |
| E/e’ | 8.01±2.03 | 10.51±2.76 | <.001 |
| LA volume index, mL/m2 | 18.0±4.3 | 21.4±6.9 | <.001 |
| PA systolic pressure, mm Hg | 26.2±4.7 | 25.1±4.6 | .112 |
Abbreviations: eGFR, estimated glomerular filtration rate; LA, left atrial; LV, left ventricular; PA, pulmonary artery.
Figure 2.
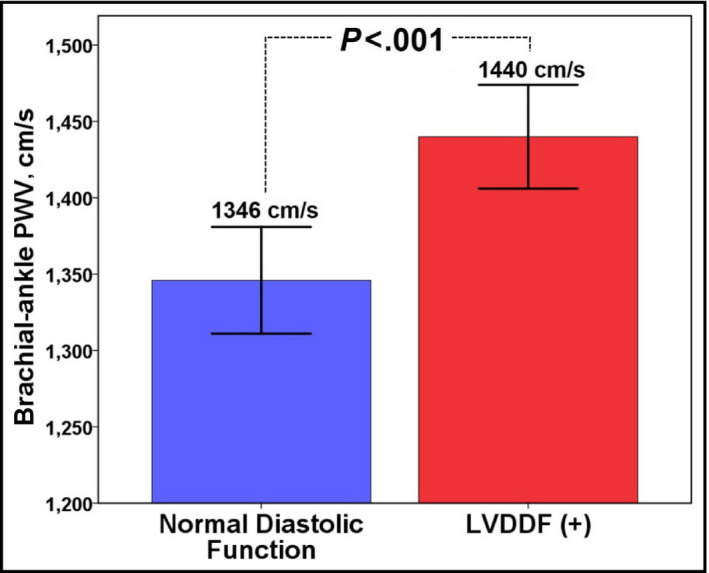
Brachial‐ankle pulse wave velocity (PWV) values according to the presence of diastolic dysfunction. LVDDF indicates left ventricular diastolic dysfunction
Figure 3.
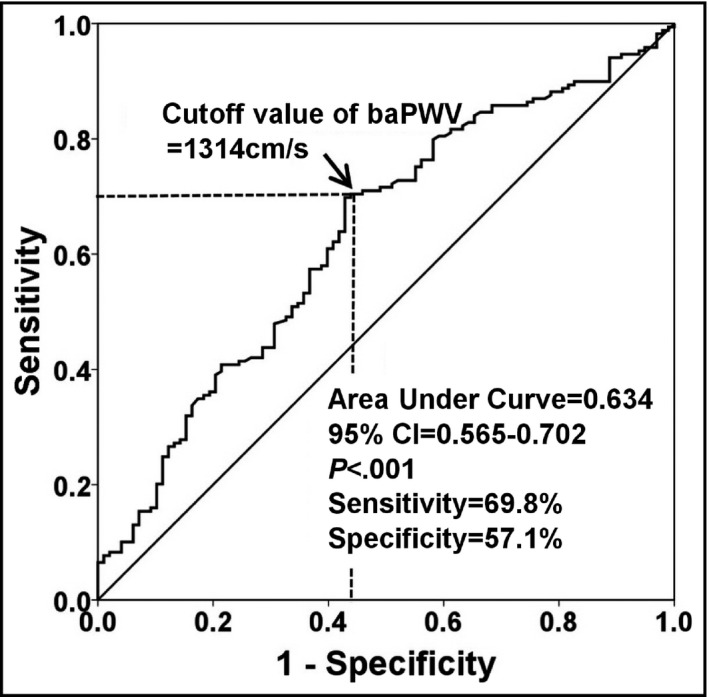
Cutoff value of brachial‐ankle pulse wave velocity (baPWV) in the prediction of normal left ventricular diastolic function. CI indicates confidence interval
Table 2.
Independent Factors Determining Normal Diastolic Function
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Age <65 y | 3.74 (1.30–10.75) | .014 |
| Men | 1.25 (0.68–2.30) | .472 |
| Body mass index <25 kg/m2 | 2.95 (1.62–5.37) | <.001 |
| Hypertension | 0.85 (0.45–1.63) | .632 |
| Diabetes mellitus | 1.64 (0.65–4.14) | .293 |
| Dyslipidemia | 1.12 (0.55–2.26) | .759 |
| eGFR ≥60 mL/min/1.73 m2 | 4.84 (0.54–43.02) | .157 |
| baPWV <1314 cm/s | 2.58 (1.46–4.57) | .001 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; CI, confidence interval; eGFR, estimated glomerular filtration rate; OR, odds ratio.
4. DISCUSSION
The results of this study showed that low baPWV was independently associated with normal diastolic function in Korean adults without documented cardiovascular disease older than 50 years. Adding information of baPWV to traditional cardiovascular risk factors improved diagnostic accuracy for normal diastolic function in these patients. This study suggests the role of baPWV in retaining normal diastolic function in this population, implicating the usefulness of baPWV as an indicator of LV diastolic function.
The prevalence of LV diastolic dysfunction in the general population has been reported in prior studies; however, the results are variable. Redfield and coworkers1 investigated 2042 randomly selected community patients aged 45 years or older and showed that the prevalence of LVDDF was 28.1%. Abhayaratna and colleagues12 performed a cross‐sectional survey of 1275 randomly selected residents aged 60 to 86 years and showed that the prevalence of diastolic dysfunction was 34.7%. Kuznetsova and associates17 randomly recruited a population sample (n=539) and demonstrated that the prevalence of normal diastolic function in patients older than 50 years was 55.9%. Among adults without documented cardiovascular disease older than 50 years in our study, the prevalence of LVDDF was 63.3%, which was somewhat high compared with those of aforementioned studies. These discrepancies among studies may be attributed to the different study population and diagnostic criteria for LVDDF.
The association between arterial stiffness and LV diastolic function has been investigated in many studies. Although arterial stiffness has been assessed using different methods, such as aortic dimension and strain on echocardiography,23 the pulse pressure method,15 and baPWV,13, 24, 25, 26, 27 the close relationship between LV diastolic function and arterial stiffness is consistent among studies. A recent study performed by our group demonstrated an independent association between baPWV and global longitudinal strain of LV, supporting the evidence that increased arterial stiffness may result in impaired LV longitudinal function.28 A following mechanism by which an increased arterial stiffness leads to LV diastolic dysfunction has been suggested.15 Increased arterial stiffness results in the early return of the reflected pulse wave in late systole. This early arrival of returned wave during LV ejection leads to augmented aortic systolic BP and decreased aortic diastolic pressure. This increased systolic pressure (=increased afterload) promotes LV hypertrophy, and decreased diastolic BP impairs coronary blood flow, which accelerates LV remodeling and delays LV relaxation.29 In agreement with this theory, our study demonstrated that middle‐aged and elderly patients with more compliant or less stiff arteries tend to have normal diastolic function. We recognized that LV diastolic function is affected by many factors, such as age, sex, and other risk factors for cardiovascular disease.1 However, most of these potential confounders were controlled in multivariable analysis, and our study showed that a compliant artery can be an independent predictor of normal LV diastolic function in middle‐aged and elderly people. Although identification of close interaction between arterial stiffness and LV diastolic function is not a novel finding in our study, our results deserve attention because we provide additional evidence on “arterial‐LV coupling” from a different point of view.
Previous studies of Japanese patients demonstrated that a baPWV value >1700 cm/s is the risk factor for cardiovascular events such as acute coronary syndrome16 and heart failure.14 Another study of relatively healthy women documented that the cutoff value of baPWV for LVDDF is 1600 cm/s.30 However, in our study, the cutoff value of baPWV for LVDDF was 1314 cm/s, which is much lower than those of previous studies. Different study populations and end points may explain these differences among studies: patients without documented cardiovascular disease were enrolled and a soft end point, LVDDF, was used in our study. Our previous study showed that a baPWV of 1282 cm/s is the cutoff value for the detection of high LV filling pressure (E/e' ≥10) in an apparently healthy Korean population,13 which is in line with the results of this study.
We found that a low BMI was associated with normal LV diastolic function in our study population. In this regard, cardiac structural changes, including LV/left atrial enlargement, LV hypertrophy, alteration in the metabolism of glucose and fatty acids, and elevated proinflammatory cytokines, have been suggested to be risk factors for LVDDF and heart failure in obese patients.31
LVDDF has been reported as an independent risk factor for cardiovascular morbidity and mortality.1, 2, 3 Importantly, although cardiac structural changes are considered physiologic responses to the aging process, age‐related LVDDF is increasingly recognized as a risk factor for cardiovascular disease.4 Therefore, identification of risk factors for LVDDF would be valuable. Indeed, many factors that exert a significant impact on the development of LVDDF have been identified.1, 5, 9, 11, 12 On the other hand, there have been few studies aimed at analyzing which factors play a role in retaining normal diastolic function even at advanced age. In this context, our study results provide additional evidence that arterial stiffness is an important risk factor of LVDDF. Since the standard treatment for LVDDF that can lead to survival benefit has not yet been established,32 there have been many trials to improve LVDDF. Considering the presence of several effective ways to reduce arterial stiffness,33 our study results will allow us to develop an effective treatment strategy for preventing or delaying age‐related LVDDF.
Arterial stiffness can be estimated using PWV, the most widely used method in clinical and research fields.33 Given that carotid‐femoral PWV (cfPWV) is a more selective measurement of central arterial wall stiffness than muscular arterial stiffness,34 it has been considered the gold standard noninvasive parameter for arterial stiffness. However, cfPWV measurement can be uncomfortable to patients and requires time and skill. Otherwise, baPWV measurement is simpler and more reproducible than cfPWV. Therefore, baPWV is an effective tool for mass screening.19 In addition, baPWV showed a strong correlation with cfPWV and aortic stiffness measured using invasive methods.35 Furthermore, the clinical value and usefulness of baPWV have been demonstrated in many clinical studies13, 24, 36 and a meta‐analysis.37 Taking into account the close relationship between baPWV and parameters of LV diastolic function in our study, it is suggested that baPWV may be an efficient and convenient marker of LVDDF.
5. STUDY LIMITATIONS
In addition to the retrospective design, the results of this study are subjected to several limitations. First, the causal relationship between baPWV and LV diastolic function could not be confirmed in this cross‐sectional study. Second, the definition of LVDDF is somewhat arbitrary, even though the current guideline7 was applied to this study. In addition, the guideline firstly recommends the use of the average value of both septal and lateral e'7; however, septal eʹ was used in our study. Third, information on concomitant medications and exercise training, which might have an impact on arterial stiffness and LV diastolic function, was not obtained. Lastly, study patients were restricted to a middle‐aged and elderly population without overt cardiovascular disease and cardiac structural changes. Therefore, further studies are needed to generalize our results in the Korean general population as well as in other ethnic groups.
6. CONCLUSIONS
A low baPWV is associated with normal LV diastolic function in a Korean middle‐aged and elderly population without documented cardiovascular disease. Compliant artery may play an important role in maintaining normal LV diastolic function in this population.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
ACKNOWLEDGMENTS
The authors appreciate the help of Seon Kyung Lee, Min‐Seon Choi, Yu Nui Kim, and Hye Jin Park for data collection.
Park K‐T, Kim H‐L, Oh S, et al. Association between reduced arterial stiffness and preserved diastolic function of the left ventricle in Korean middle‐aged and elderly patients. J Clin Hypertens. 2017;19:620‐626.
REFERENCES
- 1. Redfield MM, Jacobsen SJ, Burnett JC Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 2. Bella JN, Palmieri V, Roman MJ, et al. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle‐aged and elderly adults: the Strong Heart Study. Circulation. 2002;105:1928–1933. [DOI] [PubMed] [Google Scholar]
- 3. Halley CM, Houghtaling PL, Khalil MK, Thomas JD, Jaber WA. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082–1087. [DOI] [PubMed] [Google Scholar]
- 4. Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–1048. [DOI] [PubMed] [Google Scholar]
- 5. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–1393. [DOI] [PubMed] [Google Scholar]
- 6. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 7. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 8. Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–2289. [DOI] [PubMed] [Google Scholar]
- 9. Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population‐based cohort. J Am Coll Cardiol. 1999;33:1948–1955. [DOI] [PubMed] [Google Scholar]
- 10. Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. [DOI] [PubMed] [Google Scholar]
- 11. Gardin JM, Arnold AM, Bild DE, et al. Left ventricular diastolic filling in the elderly: the cardiovascular health study. Am J Cardiol. 1998;82:345–351. [DOI] [PubMed] [Google Scholar]
- 12. Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim HL, Im MS, Seo JB, et al. The association between arterial stiffness and left ventricular filling pressure in an apparently healthy Korean population. Cardiovasc Ultrasound. 2013;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meguro T, Nagatomo Y, Nagae A, et al. Elevated arterial stiffness evaluated by brachial‐ankle pulse wave velocity is deleterious for the prognosis of patients with heart failure. Circ J. 2009;73:673–680. [DOI] [PubMed] [Google Scholar]
- 15. Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomiyama H, Koji Y, Yambe M, et al. Brachial‐ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J. 2005;69:815–822. [DOI] [PubMed] [Google Scholar]
- 17. Kuznetsova T, Herbots L, Lopez B, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. [DOI] [PubMed] [Google Scholar]
- 18. Lee CS, Cha RH, Lim YH, et al. Ethnic coefficients for glomerular filtration rate estimation by the Modification of Diet in Renal Disease study equations in the Korean population. J Korean Med Sci. 2010;25:1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Munakata M. Brachial‐ankle pulse wave velocity in the measurement of arterial stiffness: recent evidence and clinical applications. Curr Hypertens Rev. 2014;10:49–57. [DOI] [PubMed] [Google Scholar]
- 20. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 21. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 22. Kerr KF, McClelland RL, Brown ER, Lumley T. Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol. 2011;174:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eren M, Gorgulu S, Uslu N, Celik S, Dagdeviren B, Tezel T. Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart. 2004;90:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu L, Jiang CQ, Lam TH, et al. Thomas GN. Arterial stiffness and left‐ventricular diastolic dysfunction: Guangzhou Biobank Cohort Study‐CVD. J Hum Hypertens. 2011;25:152–158. [DOI] [PubMed] [Google Scholar]
- 25. Kang S, Fan HM, Li J, et al. Relationship of arterial stiffness and early mild diastolic heart failure in general middle and aged population. Eur Heart J. 2010;31:2799–2807. [DOI] [PubMed] [Google Scholar]
- 26. Lee JKS, Lee DJ, Kwon OW, et al. Association with left ventricular diastolic function parameters and right brachial‐ankle pulse wave velocity in hemodialysis patients. Korean J Nephrol. 2007;26:204–211. [Google Scholar]
- 27. Tsai WC, Lee KT, Kuo HF, et al. Association of increased arterial stiffness and p wave dispersion with left ventricular diastolic dysfunction. Int J Med Sci. 2013;10:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim HL, Seo JB, Chung WY, et al. Independent association between brachial‐ankle pulse wave velocity and global longitudinal strain of left ventricle. Int J Cardiovasc Imaging. 2015;31:1563–1570. [DOI] [PubMed] [Google Scholar]
- 29. Safar ME, Levy BI, Struijker‐Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. [DOI] [PubMed] [Google Scholar]
- 30. Wu J, Yu SY, Wo D, et al. Risks and predictors of mild diastolic dysfunction among middle‐aged and aged women: a population‐based cohort study. J Hum Hypertens. 2016;30:335–340. [DOI] [PubMed] [Google Scholar]
- 31. De Pergola G, Nardecchia A, Giagulli VA, et al. Obesity and heart failure. Endocr Metab Immune Disord Drug Targets. 2013;13:51–57. [DOI] [PubMed] [Google Scholar]
- 32. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 33. Cavalcante JL, Lima JA, Redheuil A, Al‐Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. [DOI] [PubMed] [Google Scholar]
- 34. Davies JI, Struthers AD. Pulse wave analysis and pulse wave velocity: a critical review of their strengths and weaknesses. J Hypertens. 2003;21:463–472. [DOI] [PubMed] [Google Scholar]
- 35. Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial‐ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. [DOI] [PubMed] [Google Scholar]
- 36. Cainzos‐Achirica M, Rampal S, Chang Y, et al. Brachial‐ankle pulse wave velocity is associated with coronary calcium in young and middle‐aged asymptomatic adults: the Kangbuk Samsung Health Study. Atherosclerosis. 2015;241:350–356. [DOI] [PubMed] [Google Scholar]
- 37. Vlachopoulos C, Aznaouridis K, Terentes‐Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with brachial‐ankle elasticity index: a systematic review and meta‐analysis. Hypertension. 2012;60:556–562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


