Abstract
The US Preventive Services Task Force cholesterol guideline recommended statins for fewer adults than the 2013 American College of Cardiology/American Heart Association (ACC/AHA) guideline by setting a higher 10‐year atherosclerotic cardiovascular disease threshold (≥10.0% vs ≥7.5%) and requiring concomitant diabetes mellitus, hypertension, dyslipidemia, or cigarette smoking. The 2017 ACC/AHA hypertension guideline lowered the hypertension threshold, increasing 2016 guideline statin‐eligible adults. Cross‐sectional data on US adults aged 40 to 75 years enabled estimated numbers for the 2013 guideline and 2016 guideline with hypertension thresholds of ≥140/≥90 mm Hg and ≥130/80 mm Hg, respectively, on: (1) untreated, statin‐eligible adults for primary atherosclerotic cardiovascular disease prevention (25.40, 14.72, 15.35 million); (2) atherosclerotic cardiovascular disease events prevented annually (124 000, 70 852, 73 199); (3) number needed to treat (21, 21, 21); and (4) number needed to harm (38, 143, 143) per 1000 patient‐years for incident diabetes mellitus (42 800, 6700, 7100 cases per year). Despite the lower hypertension threshold, the 2013 cholesterol guideline qualifies approximately 10 million more adults for statins and prevents approximately 50 600 more primary atherosclerotic cardiovascular disease events but induces approximately 35 700 more diabetes mellitus cases annually than the 2016 guideline.
Keywords: atherosclerotic cardiovascular disease, cholesterol guideline, incident diabetes mellitus, prevention, statins
1. INTRODUCTION
In the United States, approximately 1.5 million strokes and myocardial infarctions occur annually.1, 2 In 2010, coronary heart disease was the leading cause of years of life lost in the United States at 7.2 million, with stroke being third at 1.9 million years.3 Hypercholesterolemia is a major risk factor for atherosclerotic cardiovascular disease (ASCVD).4 Statins reduce fatal and nonfatal ASCVD.5, 6 Applying the 2013 American College of Cardiology/American Heart Association (ACC/AHA) cholesterol guideline, which calls for moderate‐ to high‐dose statins, to 32 million statin‐eligible but untreated adults in the United States with 10‐year ASCVD risk ≥7.5% would prevent approximately 218 000 cardiovascular disease (CVD) events annually.7, 8
The 2016 US Preventive Services Task Force (USPSTF) cholesterol guideline qualifies approximately 9% fewer adults for statin therapy as the 10‐year estimated ASCVD event threshold was raised from ≥7.5% to ≥10%.9, 10 Moreover, one or more of concomitant diabetes mellitus (DM), dyslipidemia, hypertension, or cigarette smoking was required for statin eligibility by the 2016 but not the 2013 cholesterol guideline. The ACC/AHA 2017 hypertension guideline lowered the diagnostic threshold for hypertension from ≥140/≥90 mm Hg to ≥130/≥80 mm Hg, which would increase the number of adults with hypertension eligible for statins by the 2016 cholesterol guideline.
A meta‐analysis of cholesterol‐lowering trials for the 2016 guideline noted that low‐, moderate‐, and high‐intensity statins collectively reduced composite ASCVD events 30%, myocardial infarction 36%, and stroke 21%.10 The 2016 cholesterol guideline authors found no significant evidence for a statin‐dose ASCVD prevention benefit relationship and recommended low‐moderate doses of statins rather than moderate‐high doses in the 2013 guideline.9 Yet, the meta‐analysis11 included two trials using high‐dose statins.12, 13 JUPITER (Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin)13 was a large trial using high‐intensity statins that had more favorable outcomes than the overall meta‐analysis. Moreover, the 2013 cholesterol guideline cited evidence that low‐density lipoprotein cholesterol (LDL‐C) reduction rises as statin intensity is increased from low to moderate to high.7 Data from the Cholesterol Treatment Trialists' Collaboration show that larger reductions of LDL‐C prevent more ASCVDs.14
The main goal was to estimate and compare numbers of adults who are statin eligible for primary ASCVD prevention, ASCVD events prevented, and number needed to treat (NNT) to prevent an ASCVD event by the 2013 and 2016 cholesterol guidelines. Both diagnostic thresholds for hypertension of ≥140/≥90 mm Hg and ≥130/≥80 mm Hg were used in the calculations for the 2016 guideline. Given concerns about incident DM, especially with high‐dose statins, the number needed to harm (NNH) for incident DM was also estimated for both cholesterol guidelines.9, 14, 15, 16
2. METHODS
National Health and Nutrition Examination Survey (NHANES) reports assess a representative sample of the US noninstitutionalized civilian population. All adults provided written consent approved by the National Center for Health Statistics.17
Participants included adults aged 40 to 75 years in NHANES 2009–2014 with recorded blood pressure (BP) and a complete lipid profile (fasting sample) available on roughly half of the NHANES participants.
Statin use was determined from medications reportedly taken in the prior 30 days and a match to statins marketed in the United States.8
Race/ethnicity was determined by self‐report and separated into non‐Hispanic white (white), non‐Hispanic black (black), Hispanic ethnicity, and other.8
BP was measured and analyzed according to NHANES guidelines. Hypertension was defined as described17 and included a diagnostic threshold of ≥140/≥90 mm Hg and ≥130/≥80 mm Hg.18
Prevalent DM included: (1) diagnosed DM defined by positive response(s) to one or more of the following questions: “Have you ever been told by a doctor that you have diabetes?” or “Are you now taking insulin?” or “Are you now taking diabetic pills to lower your blood sugar? and (2) undiagnosed DM defined as negative responses to the above questions and a fasting glucose of ≥126 mg/dL or glycated hemoglobin ≥6.5%.19
Dyslipidemia is defined as LDL‐C >130 mg/dL or high‐density lipoprotein cholesterol < 40 mg/dL.9
ASCVD risk factors include dyslipidemia, DM, hypertension, and current smoking.9
2.1. Inclusions and exclusion criteria and ASCVD risk assessment
Adults aged 40 to 75 years were included with a valid BP and complete lipid profile (fasting sample only). Exclusion criteria included: (1) prior coronary heart disease or stroke (secondary prevention); (2) LDL‐C ≥ 190 mg/dL, as these groups were not included in the 2016 cholesterol guideline9; and (3) self‐reported congestive heart failure or estimated glomerular filtration rate <15 mL/1.73 m2 per minute (stage 5 chronic kidney disease), as the 2013 cholesterol guideline did not address primary prevention for these groups.7
Chronic kidney disease (stage 3 or 4) was defined by an estimated glomerular filtration rate 15 to 59 mL/1.73 m2 per minute or urine albumin ≥300 mg/d or albumin:creatinine ≥300 mg/g creatinine.20, 21
Statin eligibility by the 2013 ACC/AHA cholesterol guideline included adults aged 40 to 75 years with LDL‐C 70 to 189 mg/dL. Ten‐year ASCVD (ASCVD10) risk was calculated using the Pooled Cohort Risk Assessment Equations.7
DM irrespective of ASCVD10 risk, moderate‐intensity statins, level IA, benefit ≫> risk (treatment is effective and recommended).
No DM, ASCVD10 risk ≥7.5%, moderate‐ to high‐intensity statin, level IA.
Statin eligibility by the 2016 cholesterol guideline included adults aged 40 to 75 years with at least one ASCVD risk factor.9 Statin eligibility by the 2016 guideline was calculated separately using BP thresholds of ≥140/≥90 mm Hg and ≥130/≥80 mm Hg.18
ASCVD10 risk ≥10%, low‐moderate–intensity statin, grade B, ie, “the USPSTF recommends the service. There is high certainty that net benefit is moderate, or moderate certainty that net benefit is moderate to substantial.”9
ASCVD10 risk 7.5% to 10%, low‐moderate–intensity statin, grade C––“selectively offer this service to individual patients based on professional judgment and patient preferences. There is at least moderate certainty net benefit is small.”9
2.2. Estimated decline in ASCVDs with statins
In a group of patients, estimated LDL‐C declines were <30% with low doses, 30% to <50% with moderate doses, and ≥50% with high doses.7 The reduction in LDL‐C was conservatively estimated at 30% with low‐ to moderate‐intensity statins (mean of 25% reduction with low and 35% with moderate intensity). LDL‐C was estimated to decline 42.5% with moderate‐ to high‐intensity statins (mean of 35% with moderate and 50% with high intensity). For each 39‐mg/dL decline in LDL‐C, ASCVD events were assumed to decline 20% when ASCVD10 was >20% and 33% when ASCVD10 was ≤20%.7, 22
Estimates of incident DM with statins23, 24, 25 were obtained using a meta‐analysis with odds ratios (ORs) for incident DM with reductions of LDL‐C of 20% to 30% (OR, 0.98; 95% confidence interval [CI], 0.83–1.16), 30% to 40% (OR, 1.13; 95% CI, 1.01–1.26), and 40% to 50% (OR, 1.29; 95% CI, 1.13–1.47).24 These LDL‐C changes equate roughly to low‐, moderate‐, and high‐intensity statins, respectively.7 Absolute incident DM risk was estimated using ORs provided and a basal incidence DM rate of 12/1000 patient‐years in recent meta‐analysis25, 26 and similar to two trials with patients having a mean age and body mass index similar to statin‐eligible adults in this report.13, 27, 28
2.3. Data reporting and analysis
SAS Enterprise Guide 7.1 was used and accounts for complex sampling characteristics of NHANES. Only adults with fasting samples were studied, therefore the fasting sample weight (WTSAF2YR) was used. Descriptive statistics including mean and standard errors were calculated. Wald's F test was applied for continuous variables and the Rao‐Scott modified chi‐square test was used for categorical variables. ASCVD events prevented were calculated with PROC SURVEYMEANS using assumptions described. Absolute risk reduction and NNTs and NNHs were calculated using PROC SURVEYMEANS. Two‐sided P values < .05 were accepted as significant.
3. RESULTS
Figure 1 depicts the selection of adults aged 40 to 75 years in the NHANES fasting sample who were eligible for primary prevention of ASCVD by the 2013 and 2016 cholesterol guidelines. After exclusions, 963 adults had a level 1A statin recommendation by the 2013 guideline. By the 2016 guideline, 622 had a grade B (ASCVD10 ≥10% and one or more risk factors) and 206 had a grade C statin (ASCVD 10 ≥ 7.5% to <10% and one or more risk factors) recommendation using ≥140/≥90 mm Hg as the diagnostic threshold for hypertension. The number with a grade B recommendation rose by 15 patients and with a grade C recommendation by eight when ≥130/≥80 mm Hg was used as the diagnostic threshold for hypertension.
Figure 1.
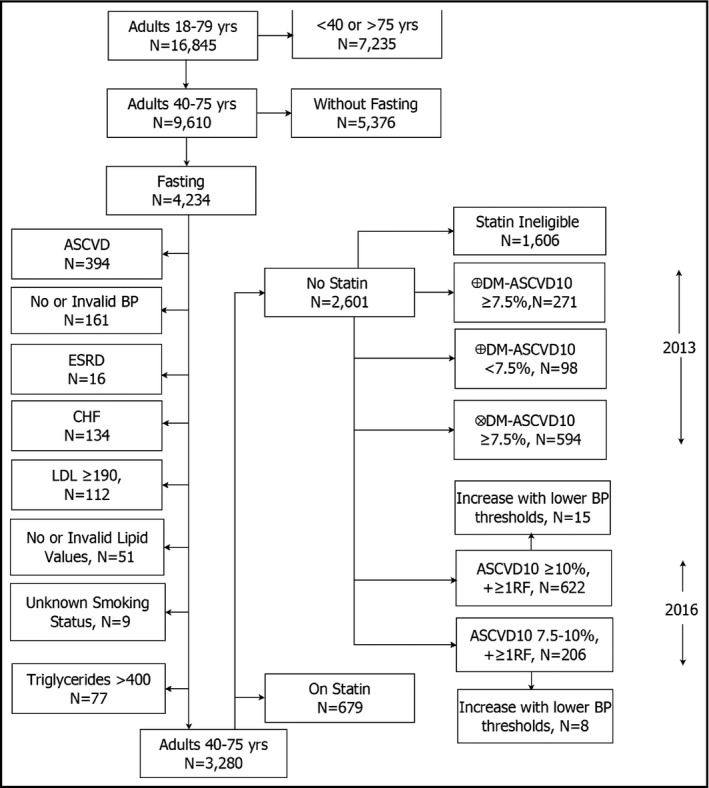
The diagram reflects the process for identifying statin‐untreated adults aged 40 to 75 years who are statin‐eligible by the 2013 and the 2016 cholesterol guidelines. Numbers of statin‐eligible adults for the 2013 or 2016 cholesterol guideline do not sum to the number of statin‐eligible patients as some individuals are eligible for both, one, or the other guideline. For statin‐eligible adults in the 2016 cholesterol guideline, hypertension (untreated) was defined either by blood pressure (BP): * ≥140/≥90 mm Hg or † ≥130/≥80 mm Hg. CHF, congestive heart failure; DM, diabetes mellitus; ESRD, end‐stage renal disease; LDL, low‐density lipoprotein; RF, atherosclerotic cardiovascular disease (ASCVD) risk factors
3.1. 2013 Guideline statin‐eligible adults
Approximately 25.4 million untreated adults had a 1A recommendation for statins by the 2013 guideline (Table 1). The total included 6.34 million with DM and ASCVD10 ≥7.5%, 2.78 million with DM and ASCVD10 <7.5%, and 16.28 million without DM and ASCVD10 ≥7.5%. The nondiabetic subset was the oldest and those with DM and ASCVD10 < 7.5% the youngest. The lower‐risk diabetic group had a higher proportion of women, Hispanics, and other race‐ethnicity than the other two groups. Both DM groups had lower incomes, higher BMI, and more obesity than the nondiabetic group. The lower‐risk diabetic group had the least hypertension, cigarette smoking, and chronic kidney disease, and they had lower total and LDL‐C than the other two groups. The higher‐risk diabetic group had the highest prevalence of hypertension and chronic kidney disease and highest ASCVD10 risk. The nondiabetic subgroup had the highest high‐density lipoprotein cholesterol and lowest triglyceride values but the most cigarette smokers.
Table 1.
US adults aged 40 to 75 years untreated but 2013 or 2016 cholesterol guideline statin eligible for primary ASCVD prevention
| Group | Adults not taking statins | |||||||
|---|---|---|---|---|---|---|---|---|
| Cholesterol guideline | 2013 Eligible | 2016 Eligible ≥140/≥90 mm Hg | 2016 Eligible 130–9/80–9 mm Hg | |||||
| Variable/subgroup | DM‐ASCVD10 ≥7.5% | DM‐ASCVD10 <7.5% | No DM‐ASCVD10 ≥7.5% | ASCVD10 ≥10% + ≥1RF | ASCVD10 ≥10% + ≥1RF excludes DMa | ASCVD10 7.5%–<10% + ≥1RF | ASCVD10 ≥10% + ≥1RF | ASCVD10 7.5%–<10% + ≥1RF |
| NHANES sample, No. | 271 | 98 | 594 | 622 | 387 | 206 | 15 | 8 |
| US population 40–75 y, No. | 6 341 983 | 2 783 193 | 16 278 179 | 14 715 706 | 9 523 078 | 6 155 754 | 635 366 | 330 400 |
| US population 40–75 y, % | 6.7 | 2.9 | 17.2 | 15.5 | 10.0 | 6.5 | 0.7 | 0.4 |
| Age, y | 59.8 ± 0.5 | 49.5 ± 0.7 | 62.9 ± 0.5 | 63.7 ± 0.3 | 65.0 ± 0.4 | 56.2 ± 0.8 | 66.7 ± 0.8 | 64.3 ± 0.8 |
| Men, % | 161 (55.9) | 31 (33.8) | 403 (66.8) | 401 (63.0) | 257 (65.4) | 131 (59.6) | 13 (91.9) | 7 (84.5) |
| Women, % | 110 (44.1) | 67 (66.2) | 191 (33.2) | 221 (37.0) | 130 (34.6) | 75 (40.4) | 2 (8.1) | 1 (15.5) |
| Race/ethnicity, white, No. (%) | 77 (56.8) | 22 (45.5) | 233 (69.7) | 208 (62.5) | 145 (66.6) | 71 (65.3) | 9 (87.8) | 4 (81.8) |
| Black, No. (%) | 81 (20.0) | 12 (8.3) | 165 (16.0) | 187 (19.0) | 112 (17.6) | 63 (18.8) | 1 (2.9) | 2 (8.8) |
| Hispanic, No. (%) | 91 (17.4) | 42 (28.4) | 140 (9.9) | 172 (12.8) | 96 (10.6) | 53 (12.2) | 3 (5) | 2 (9.4) |
| Other, No. (%) | 22 (5.8) | 22 (17.8) | 56 (4.4) | 55 (5.7) | 34 (5.2) | 19 (3.7) | 2 (4.2) | 0 (0) |
| BMI, kg/m2 | 33.5 ± 0.9 | 35.6 ± 1.3 | 28.5 ± 0.3 | 30.3 ± 0.5 | 28.9 ± 0.4 | 30.1 ± 0.9 | 26.6 ± 1.7 | 27 ± 1.3 |
| Obese (BMI ≥30), % | 135 (58.5) | 67 (70.6) | 196 (32.4) | 248 (44.3) | 137 (39.0) | 90 (38.9) | 3 (20.7) | 2 (22.4) |
| Hypertension, % | 188 (72.8) | 35 (38.7) | 354 (56.8) | 458 (74.4) | 287 (73.3) | 108 (55.5) | 0 (0)b | 0 (0)b |
| SBP, mm Hg | 133.6 ± 1.6 | 119.1 ± 1.6 | 132.0 ± 1.1 | 134.9 ± 1.2 | 134.7 ± 1.4 | 129.9 ± 1.8 | 133.1 ± 0.8 | 127.2 ± 2.8 |
| DBP, mm Hg | 74.2 ± 1.0 | 72.9 ± 1.7 | 72.3 ± 0.7 | 72.2 ± 0.8 | 71.6 ± 1.1 | 75.0 ± 0.9 | 74.9 ± 1.6 | 77.1 ± 2.6 |
| Total cholesterol, mg/dL | 202.3 ± 2.2 | 191.2 ± 4.1 | 210.8 ± 1.9 | 205.1 ± 2.0 | 208.4 ± 2.7 | 215.4 ± 3.1 | 187.1 ± 5.5 | 201.9 ± 4.0 |
| HDL‐C, mg/dL | 47.9 ± 1 | 49.0 ± 1.9 | 54.8 ± 1.0 | 51.6 ± 0.7 | 53.9 ± 1.1 | 51.9 ± 1.8 | 61.4 ± 3.6 | 65.6 ± 4.8 |
| LDL‐C, mg/dL | 123.5 ± 2.2 | 112.9 ± 4.1 | 130.2 ± 1.6 | 125.7 ± 1.9 | 127.7 ± 2.3 | 134.2 ± 2.9 | 111.6 ± 5.3 | 114.9 ± 2.2 |
| Triglycerides, mg/dL | 154.1 ± 7.6 | 147.1 ± 14.2 | 128.7 ± 5 | 138.5 ± 3.6 | 133.8 ± 4.4 | 146.3 ± 8.6 | 70.6 ± 3.8 | 107.1 ± 24.6 |
| DM, No. (%) | 271 (100) | 98 (100) | 0 (0) | 235 (35.3) | 0 (0) | 48 (21.7) | 0 (0) | 0 (0) |
| Glycated hemoglobin <8%, No. (%) | 210 (74.6) | 73 (70.1) | NA | 187 (77.0) | NA | 33 (67.2) | NA | NA |
| Cigarette smokers, No. (%) | 67 (18.6) | 4 (6.4) | 177 (28.9) | 183 (26.7) | 122 (30.6) | 71 (34.4) | 0 (0) | 0 (0) |
| CKD, No. (%) | 33 (13.3) | 3 (2.4) | 30 (5.7) | 59 (10.7) | 30 (8.5) | 7 (4.1) | 0 (0) | 0 (0) |
| 10‐y ASCVD risk, No. (%) | 19.7 ± 0.8 | 4.3 ± 0.2 | 13.4 ± 0.2 | 18.3 ± 0.4 | 16.2 ± 0.3 | 8.8 ± 0.1 | 13.3 ± 0.6 | 9.2 ± 0.1 |
BMI, body mass index; CKD, chronic kidney disease with estimated glomerular filtration rate 15–59 mL/1.73 m2 per minute; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol; NA, not applicable; NHANES, National Health and Nutrition Examination Survey; RF, atherosclerotic cardiovascular disease (ASCVD) risk factors; SBP, systolic blood pressure.
Second column under “Eligible 2016” includes individuals in the first column but excludes patients with diabetes mellitus (DM), ie, the group at risk for DM if given a statin.
Not hypertensive at ≥140/≥90, but Stage 1 hypertension at 130–139 systolic or 80–89 diastolic.18
3.2. 2016 Guideline statin‐eligible adults with hypertension defined by treatment or BP ≥140/≥90 mm Hg
Table 1 shows that the total of 622 NHANES patients representing approximately 14.72 million US adults had a level B statin recommendation. The second column under 2016 includes only adults in the first column without DM, ie, the group (at risk for statin‐induced DM). The third column describes adults with a level C statin recommendation.11 Of 622 adults with a level B statin recommendation, the mean age was 63.7 years, 63% were men, 62.5% were white, 19% were black, and 12.8% were Hispanic. The subset without DM had a mean age of 65 years, 65.4% were male, and two thirds were white. Since all of them are included in the first column, between‐group comparisons are less informative. The lower‐risk (level C) statin‐eligible group was younger than the higher‐risk group.
3.3. 2016 Guideline statin‐eligible adults with hypertension defined by BP 130 to 139/80 to 89 mm Hg
There were 15 statin‐eligible patients with ASCVD10 ≥10%, representing approximately 635 000 US adults when the lower threshold for incident hypertension was applied. None of this group had DM. The second column in Table 1 includes eight individuals with ASCVD10 risk 7.5 to <10%, representing approximately 330 000 US adults who were statin eligible with the lower hypertension threshold of 130 to 139/80 to 89 mm Hg. Given the small numbers, data for patients in the two columns were not compared.
The estimated numbers of statin‐eligible adults and ASCVD events prevented were greater with the 2013 than the 2016 cholesterol guideline using either BP threshold for hypertension (Table 2). Absolute risk reduction and NNT were similar with both guidelines and both BP thresholds, while relative risk reduction was greater with the 2013 than 2016 guideline given use of higher‐intensity statins under the 2013 guideline. Lowering the threshold for untreated hypertension to ≥130/≥80 mm Hg qualified roughly 635 000 more adults with hypertension for statin therapy and led to an estimated prevention of approximately 2350 more primary ASCVD events annually than when untreated hypertension was defined as ≥140/≥90 mm Hg.
Table 2.
Predicted impact of 2013 and 2016 cholesterol guideline–recommended statins on primary ASCVD prevention in adults aged 40 to 75 years
| NHANES, No. | Population, No. | ASCVD events per 10 y, % | ASCVD events per 10 y, No. | ASCVD events prevented per 10 y with statin, No. | RRR, % | ARR, % | NNT | |
|---|---|---|---|---|---|---|---|---|
| 2013 Cholesterol guideline group | ||||||||
| ⊕DM ASCVD10 7.5%a | 271 | 6 341 983 | 19.7 | 1 249 326 | 342 149 | 30.5 | 5.4 | 19 |
| ⊕DM ASCVD10 <7.5%a | 98 | 2 783 193 | 4.3 | 118 754 | 39 101 | 33.1 | 1.4 | 71 |
| ∅DM ASCVD10 ≥7.5%b | 594 | 16 278 179 | 13.4 | 2 173 740 | 857 195 | 41.1 | 5.3 | 19 |
| 2013 Primary prevention | 963 | 25 403 355 | 13.9 | 3 541 820 | 1 238 445 | 37.6 | 4.9 | 21 |
| 2016 Cholesterol guideline group | ||||||||
| ≥ 1 risk and ASCVD10 ≥10%c | 622 | 14 715 706 | 18.3 | 2 699 313 | 708 523 | 28.3 | 4.8 | 21 |
| ≥ 1 risk and ASCVD10 ≥10%c,d | 637 | 15 351 072 | 18.1 | 2 783 771 | 731 991 | 28.3 | 4.8 | 21 |
| ≥1 RF and ASCVD10 7.5% to <10%c | 206 | 6 155 754 | 8.8 | 543 464 | 181 880 | 33.5 | 3.0 | 34 |
| ≥1 RF and ASCVD10 7.5% to <10%c,d | 214 | 6 486 154 | 8.8 | 573 730 | 190 879 | 33.3 | 2.9 | 34 |
⊕, with; ∅, without; ARR, absolute risk reduction; ASCVD, atherosclerotic cardiovascular disease; DM, diabetes mellitus; NNT, number needed to treat; RF, risk factor 2016 guideline; RRR, relative risk reduction.
Assumes moderate‐dose statins reduce low‐density lipoprotein cholesterol (LDL‐C) 35%.
Assumes moderate‐ to high‐dose statins reduce LDL‐C 42.5%.
Assumes low‐ to moderate‐dose statins reduce LDL‐C 30%.
Hypertension diagnosed at ≥130/≥80 mm Hg. Lines in italics represent “what‐if” scenarios. For 2013, the effects of statins were assessed in the subset with ASCVD10 7.5% to <10% and adults with and without one risk factor in the 2016 guideline and ASCVD10 7.5% to <10%, and ≥10%.
3.4. New‐onset DM
Absolute excess risk of incident DM was calculated using the mean and 95% confidence limits for ORs of incident DM with basal rates for incident DM (6, 12, 18/1000 patient‐years), and three levels of LDL‐C reduction, corresponding roughly to low‐, moderate‐, and high‐intensity statin therapy (Table 3). Greater basal rates for incident DM and higher statin doses led to larger estimates for incident DM.
Table 3.
Estimate of incident DM with low‐, moderate‐, and high‐intensity statin therapy btop panel) and estimates of new‐onset DM (bottom panel)24
| Statin dose decline LDL‐C OR incident DM | Statin intensity (fall LDL‐C, %), [OR, 95% CI] Incident DM | ||
|---|---|---|---|
| Low (20%–30%), 0.98 [0.83–1.16] | Moderate (30%–40%), 1.13 [1.01–1.26] | High (40%–50%), 1.29 [1.13–1.47] | |
| Baseline DM risk/1000 person‐y | Absolute excess risk/1000 person‐y | ||
| 6 | −0.1 (−1.0 to +1.0) | 0.8 (0.1–1.6) | 1.7 (0.8–1.8) |
| 12 | −0.2 (−2.0 to +1.9) | 1.6 (0.1–3.1) | 3.5 (1.6–5.1) |
| 18 | −0.3 (−3.1 to +2.9) | 2.3 (0.2–4.7) | 5.2 (2.3–8.5) |
| Risk group | Statin dose | NOD/1000 patient‐ya | NNH/1000 patient‐y | Statin‐related DMs, No./10 y |
|---|---|---|---|---|
| 2013∅DM ASCVD10 ≥7.5% | Moderate‐high | 2.6 (0.8–4.1)b | 38 (24–100) | 428 373c162 782–678 257 |
| 2016 ASCVD10 ≥10% + 1RF∅DM | Low‐moderate | 0.7 (−0.9 to +2.5)b | 143 (negative 40) | 66 595c(negative 238 077) |
| 2016 ASCVD10 ≥10% + 1RF∅DMd | Low‐moderate | 0.7 (−0.9 to +2.5)b | 143 (negative 40) | 71 038(negative 253 959) |
⊕, with; ∅, without; ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; LDL‐C, low‐density lipoprotein cholesterol; NA, not applicable (individuals have diabetes mellitus [DM]); negative, ie, lower confidence limit suggests DM prevention with low‐moderate–intensity statins; NNH, number needed to treat; NOD, new‐onset (incident) DM; OR, odds ratio; RF, (cardiovascular) risk factor.
1000 person‐years roughly equivalent to 100 persons × 10 years recognizing that intervention trials included in the analysis ranged from a mean of approximately 2 to 6 years in duration.
Estimate based on incident DM risk of 12/1000 person‐years in an untreated (placebo) group (using data from bold line in upper panel).
Estimate based on the numbers of untreated, statin‐eligible adults without DM by the 2013 guideline (16 278 179) and 2016 (9 523 078) by the 2016 cholesterol guideline.
Hypertension defined by blood pressure ≥130/≥80 mm Hg rather than ≥140/≥90 mm Hg.
Bold line represents data assumptions used to calculate NOD and NNH in lower half
3.5. New‐onset DM––bottom panel
Assuming a basal incident rate of 12/1000 patient‐years for incident DM, absolute excess risk per 1000 patient‐years for incident DM is provided for nondiabetic adults in the 2013 and 2016 cholesterol guidelines. The mean point estimate for NNH is lower (greater risk) with moderate‐high– (38) than low‐moderate (143)–intensity statin therapy. The 10‐year mean estimate for excess incident DM cases with the 2013 guideline was 428 373 vs 66 595 with the 2016 guideline at a hypertension threshold of ≥140/≥90 mm Hg and 71 038 at a threshold of ≥130/≥80 mm Hg. Thus, the 2013 guideline could lead to roughly 35 700 more DM cases annually than the 2016 guideline.
Figure 2 depicts estimates of statin‐eligible adults, ASCVD events prevented, NNT to prevent an ASCVD event, and NNH for incident DM with both cholesterol guidelines.
Figure 2.
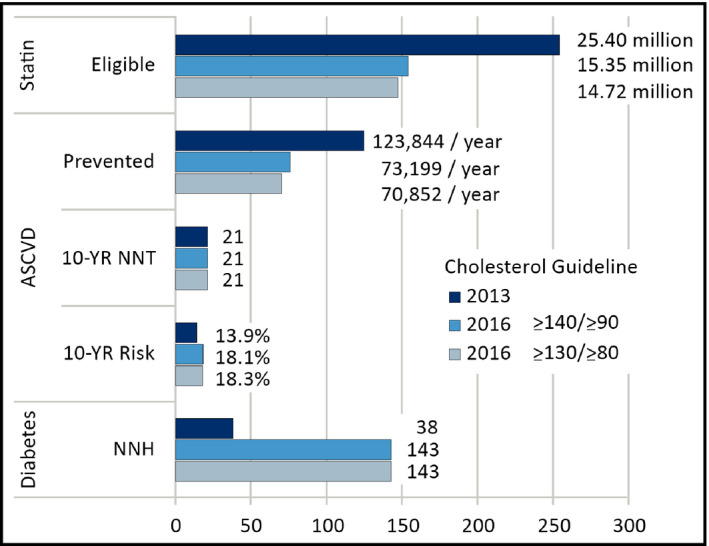
Lowering the diagnostic threshold for hypertension from ≥140/≥90 mm Hg* to ≥130/≥80 mm Hg† increases the number of adults eligible for statins and the number of atherosclerotic cardiovascular disease (ASCVD) events prevented by the 2016 cholesterol guideline, yet the number of statin‐eligible adults is greater with the 2013 cholesterol guideline. By treating more adults and with higher‐dose statins, the 2013 guideline prevents more ASCVD events and induces more diabetes mellitus (lower number needed to harm [NNH]) than the 2016 guideline regardless of the blood pressure threshold for hypertension
4. DISCUSSION
The principal objective was to compare numbers of statin‐eligible adults, primary ASCVD events, and NNT prevented in the US population with the 2013 ACC/AHA and 2016 USPSTF cholesterol guidelines.7, 9 The analysis was also prompted by the 2017 ACC/AHA hypertension guideline, which defined hypertension by BP ≥130/≥80 mm Hg rather than ≥140/≥90 mm Hg.18 The 2017 hypertension guideline recommended treatment for adults with BP ≥130/≥80 mm Hg if they had clinical CVD, DM, or 10‐year ASCVD risk ≥10%. This ASCVD risk level matches that in the 2016 cholesterol guideline for a strong (grade B) recommendation for low‐moderate–dose statins. Thus, all individuals aged 40 to 75 years with BP 130 to 139/80 to 89 mm Hg and 10‐year ASCVD risk ≥10% qualify for antihypertensive therapy and statins for primary CVD prevention.
4.1. Statin‐eligible adults for primary prevention of ASCVD
Approximate 25.4 million statin‐untreated US adults aged 40 to 75 years had a strong (level IA) statin recommendation for primary prevention in the 2013 guideline.7 The estimated number of adults with a strong (grade B) statin recommendation for primary prevention by the 2016 guideline was 14.72 million and 15.35 million with hypertension defined by BPs ≥140/≥90 mm Hg and ≥130/≥80 mm Hg,9, 18 respectively. Thus, the 2017 hypertension guideline, which defined hypertension at the lower threshold of ≥130/≥80 mm Hg qualifies approximately 635 000 more adults with hypertension for statins.
Yet, the 2013 guideline provides a strong statin recommendation for approximately 10 million more adults than the 2016 guideline even with the lower BP threshold for hypertension.
The difference in numbers of statin‐eligible adults between the two guidelines is reduced to approximately 2.7 million adults if the group with 10‐year ASCVD risk 7.5 to <10% and one or more risk factors including BP ≥130/≥80 mm Hg received statins under the less compelling “grade C” recommendation in the 2016 cholesterol guideline. The residual difference in numbers of statin‐eligible adults between the two cholesterol guidelines is largely explained by those with DM and 10‐year ASCVD risk <7.5%. This lower‐risk diabetic group has a strong (level IA) statin recommendation under the 2013 guideline but no statin recommendation under the 2016 guideline.
4.2. ASCVD events prevented
Full implementation of the 2013 cholesterol guideline in untreated, statin‐eligible adults would prevent approximately 124 000 primary ASCVD events annually (Figure 2). Of roughly 14.7 million statin‐untreated adults aged 40 to 75 years with a grade B statin recommendation for primary prevention in the 2016 cholesterol guideline, approximately 71 000 primary ASCVD events would be prevented annually. Lowering the hypertension threshold to ≥130/≥80 mm Hg would prevent approximately 2350 additional ASCVD events annually. Even with the lower BP threshold, the 2013 cholesterol guideline would prevent approximately 50 600 more primary ASCVD events annually than the 2016 guideline. The difference in primary ASCVD events prevented would be reduced to approximately 31 600 annually if the roughly 6.75 million adults with 10‐year ASCVD risk ≥7.5 to 10% and one risk factor including BP ≥130/≥80 mm Hg received low‐moderate–dose statins (grade C in the 2016 guideline). The greater reduction in ASCVD events with more vs less intense statin therapy coincides with previous reports.16, 22, 23 The 2016 guideline noted that individual responses to statin therapy vary widely.9 Yet, at a population level, higher statin doses produce larger decreases in LDL‐C,7, 16 which are associated with greater declines in ASCVD events.7, 14, 16, 22, 23
4.3. Statin‐related adverse events: focus on DM
More intense statin therapy increases adverse events,16, 23, 24, 25, 26 although rhabdomyolysis risk is debated when high‐intensity simvastatin7 is excluded.15 Statin‐related DM risk has raised concerns.23, 24, 25, 26 Our estimates, found in the Methods section, indicate that incident DM risk is greater for moderate‐high– (NNH 38, 2013 cholesterol guideline) than low‐moderate–intensity (NNH 143, 2016 cholesterol guideline) statin therapy). Implementing the 2013 guideline for primary CVD prevention in all statin‐eligible untreated adults would lead to an excess of approximately 43 000 incident DM cases annually vs 6700 or 7100 with the 2016 guideline and hypertension thresholds of ≥140/≥90 mm Hg and ≥130/≥80 mm Hg, respectively.
Perceptions of incremental DM risk with statins are also affected by whether risk is simply accelerated by a few weeks, as suggested from JUPITER, or whether statin‐induced DM would not have occurred absent statins during the remaining lifetime.28, 29 Longer‐term follow‐up studies are required to determine whether statin‐associated DM adversely impacts clinical outcomes. For example, adults with hypertension who develop DM while taking chlorthalidone did not show adverse effects on cardiovascular outcomes or all‐cause mortality with long‐term follow‐up.30
4.4. Limitations
Our report reflects US guidelines applied to white, black and Hispanic residents of the United States. The results may be less applicable to other populations. For several reasons, it is unlikely that 100% of statin‐eligible untreated adults will take statins. As previously noted, treating half of this group would result in statin therapy for 70% to 75% of the statin‐eligible group, which is comparable to the percentage of patients with hypertension receiving pharmacotherapy. This projection is even more credible when considering evidence that the proportion of adults taking pharmacotherapy is growing faster for hypercholesterolemia than hypertension.31 Thus, lowering the annual estimate of ASCVD events prevented and incident DM by 50% represent more realistic goals over the next 5 to 10 years. This report did not assess primary prevention of ASCVD in adults with LDL‐C ≥ 190 mg/dL or secondary prevention, which were not addressed in the 2016 guideline. Estimates of ASCVD prevention in these two groups were previously reported.8
As noted in the Methods section, approximately half of adults in NHANES were studied in the afternoon and did not have fasting laboratory values required for calculating LDL‐C. The fasting sample weight was used to estimate the relationship of our findings in the US population. Our previous analysis of NHANES 2005–2010 indicated that adults studied in the morning (fasting blood sample) had lower systolic and diastolic BPs and were more likely to receive antihypertensive medications and have hypertension controlled than patients studied in the afternoon. Patients studied in the morning also had lower total cholesterol and higher high‐density lipoprotein cholesterol rates than adults studied in the afternoon but similar rates of statin therapy and demographic characteristics.32 The extent to which differences in risk factor values reflect effects and interactions of feeding and diurnal variation is, to our knowledge, unknown.
Estimates of incident DM are dependent on basal (placebo) rates in nondiabetic populations and the relative increase in risk with statins, which vary widely between studies.23, 24, 25, 26, 33 To address variability, 95% CIs were calculated for NNH. Our analysis did not include cost‐effectiveness. Prior estimates, assuming an average statin cost of $68 per year indicated that an ASCVD threshold of ≥7.5% had an incremental cost‐effectiveness ratio of $37 000 per quality‐adjusted life‐year when compared with an ASCVD risk threshold of ≥10%.34 Thus, statins for individuals with ASCVD risk of 7.5% to <10% falls within the commonly accepted range of <$50 000 per quality‐adjusted life‐year.
5. CONCLUSIONS
Approximately 1.5 million atherosclerotic cardiovascular events occur annually in the United States and national goals aim for a 20% reduction.35, 36 Statins are indicated for the primary prevention of CVD. In comparing the benefits and risks of the 2013 and 2016 cholesterol guidelines, both guidelines have similar NNTs for ASCVD prevention but the 2013 guideline prevents approximately 50 800 more primary ASCVD events annually than the 2016 guideline by treating more adults and using higher‐dose statins. Lowering the threshold for hypertension to ≥130/≥80 mm Hg (2017 hypertension guideline) increases the number of statin‐eligible adults by approximately 635 000 under the 2016 guideline and raises the number of primary ASCVD events prevented by approximately 2350 annually. The estimated benefits of the 2013 guideline for primary ASCVD prevention are counterbalanced by an estimated excess of approximately 35 700 incident DM cases annually relative to the 2016 guideline. Practical tools that facilitate shared informed decisions by patients and their clinicians on the benefits and risks of statin therapy could enhance implementation of guidelines for ASCVD prevention, while respecting individual preferences in balancing benefits and risks.
DISCLOSURES
Dr Egan has received royalties from UpToDate; research support from Medtronic and Quintiles; income as a consultant from AstraZeneca, Medtronic, and Valencia; and honoraria for lectures from Merck‐Serono and Emcure. The rest of the authors have nothing to disclose.
ACKNOWLEDGMENTS
None.
Egan BM, Li J, Davis RA, et al. Differences in primary cardiovascular disease prevention between the 2013 and 2016 cholesterol guidelines and impact of the 2017 hypertension guideline in the United States. J Clin Hypertens. 2018;20:991–1000. 10.1111/jch.13314
Funding information
Centers for Disease Control and Prevention (CDC), “State public health actions to prevent and control diabetes, heart disease, obesity and associated risk factors and promote school health.” CDC 1305 to the South Carolina Department of Health and Environmental Control with subcontract to CCI.
CDC, “State and local public health actions to prevent obesity, diabetes, and heart disease and stroke.” CDC, 1422 to the South Carolina Department of Health and Environmental Control with subcontract to CCI.
Agency for Healthcare Research and Quality, “N2: Building a Network of Safety Net PBRNs” (grant No. 1 P30‐HS‐021667) to the Clinical Directors Network.
National Heart, Lung and Blood Institute, “Blood Pressure‐Visit Intensification for Successful Improvement of Treatment (BP‐Visit)” (grant No. 1 R18‐HL‐117801) to the Clinical Directors Network.
REFERENCES
- 1. Stroke Facts. http://www.cdc.gov/stroke/facts.htm. Accessed September 22, 2017.
- 2. Heart Disease Facts. http://www.cdc.gov/heartdisease/facts.htm. Accessed September 22, 2017.
- 3. US Burden of Disease Collaborators . The State of US Health, 1990–2010. Burden of disease, injuries and risk factors. JAMA. 2013;310:591‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wadhere RK, Steen DL, Khan I, Giugliano RP, Foody JM. A review of low‐density cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J Clin Lipidol. 2016;10:472‐489. [DOI] [PubMed] [Google Scholar]
- 5. Taylor F, Huffman MD, Macedo TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Data Syst Rev. 2013;1:1‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lardizabal JA, Deedwania P. Lipid‐lowering therapy with statins for the primary and secondary prevention of cardiovascular disease. Cardiol Clin. 2011;29:87‐103. [DOI] [PubMed] [Google Scholar]
- 7. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1‐S45. [DOI] [PubMed] [Google Scholar]
- 8. Egan BM, Li J, White K, et al. 2013 ACC/AHA cholesterol guideline and implications for healthy people 2020 cardiovascular disease prevention goals. J Am Heart Assoc. 2016;5:e003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. US Preventive Services Task Force , Bibbins‐Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults. JAMA. 2016;316:1997‐2007. [DOI] [PubMed] [Google Scholar]
- 10. Pagidipati NJ, Navar AM, Mulder H, Sniderman AD, Peterson ED, Pencina MJ. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA guideline. JAMA. 2017;317:1563‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chou R, Dana T, Blazina I, et al. Statin use for the prevention of cardiovascular disease in adults: A systematic review for the U.S. Preventive Service Task Force. Evidence Synthesis No. 139. AHRQ Publication No. 14‐05206‐EF‐2, Nov. 2016. [PubMed]
- 12. Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306‐314. [DOI] [PubMed] [Google Scholar]
- 13. Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359:2195‐2207. [DOI] [PubMed] [Google Scholar]
- 14. Cholesterol Treatment Trialists Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Downs JR, O'Malley PG. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2014 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann Intern Med. 2015;163:291‐297. [DOI] [PubMed] [Google Scholar]
- 16. Mills EJ, O'Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta‐analysis of >40,000 patients. Eur Heart J. 2011;32:1409‐1415. [DOI] [PubMed] [Google Scholar]
- 17. National Center for Health Statistics, Centers for Disease Control and Prevention . NCHS research ethics review board (ERB) approval. https://www.cdc.gov/nchs/nhanes/irba98.htm. Accessed March 20, 2018.
- 18. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. Hypertension. 2018;71:1269‐1324. [DOI] [PubMed] [Google Scholar]
- 19. American Diabetes Association . Standards of medical care in diabetes––2017. Diabetes Care. 2017;40(suppl 1):S1‐S135.27979885 [Google Scholar]
- 20. Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713‐735. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA. Estimating GFR using the CKD epidemiology collaboration (CKD‐EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cholesterol Treatment Trialists (CTT) Collaborators . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Preiss D, Seshasia SRK, Welsh P, et al. Risk of incident diabetes with intensive‐dose compared with moderate‐dose statin therapy. JAMA. 2011;305:2556‐2564. [DOI] [PubMed] [Google Scholar]
- 24. Dormuth CR, Fillon KB, Paterson JM, et al. Higher potency statins and the risk of new diabetes: multicentre, observational study of administrative databases. BMJ. 2014;348:g3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang S, Cai R, Yuan Y, Varghese Z, Moorhead J, Ruan XZ. Association between reductions in low‐density lipoprotein cholesterol with statin therapy and the risk of new‐onset diabetes: a meta‐analysis. Sci Rep. 2017;7:39982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomized statin trials. Lancet. 2010;374:735‐742. [DOI] [PubMed] [Google Scholar]
- 27. Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Lipid Lowering ARM (ASCOT–LLA): a multicenter randomized controlled trial. Lancet. 2003;36:1149‐1158. [DOI] [PubMed] [Google Scholar]
- 28. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rochlani Y, Kattoor AJ, Pothineni NV, Palagiri RDR, Romeo F, Mehta JL. Balancing primary prevention and statin‐induced diabetes mellitus prevention. Am J Cardiol. 2017;120:1122‐1128. [DOI] [PubMed] [Google Scholar]
- 30. Barzilay JI, Davis BR, Pressel SL, et al. Long‐term effects of incident diabetes mellitus on cardiovascular outcomes in people treated for hypertension: the ALLHAT diabetes extension study. Circ Cardiovasc Qual Outcomes. 2012;5:153‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kantor ED, Rehm CD, Haas JS, Chan AT, Glovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818‐1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Egan BM, Li J, Qanungo S, Wolfman TE. Blood pressure and cholesterol control in hypertensive hypercholesterolemic patients. National Health and Nutrition Examination Surveys 1988–2010. Circulation. 2013;128:29‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldstein MR, Mascitelli L. Do statins cause diabetes? Curr Diab Rep. 2013;13:381‐390. [DOI] [PubMed] [Google Scholar]
- 34. Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost‐effectiveness of 10‐year risk threshold for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Heart Association: 2020 impact goal. https://www.heart.org/idc/groups/heart-public/@wcm/@swa/documents/downloadable/ucm_425189.pdf. Accessed September 23, 2017.
- 36. Million Hearts® 2022: Preventing 1 Million Heart Attacks and Strokes by 2022. https://millionhearts.hhs.gov/files/MH-2022-Fact-Sheet.pdf. Accessed September 23, 2017.


