Abstract
This 52‐week, randomized, open‐label study evaluated long‐term safety/tolerability of fixed‐dose combination azilsartan medoxomil/chlorthalidone (AZL‐M/CLD) vs fixed‐dose combination olmesartan medoxomil/hydrochlorothiazide (OLM/HCTZ) in patients with essential hypertension (stage 2; clinic systolic blood pressure 160–190 mm Hg). Initial AZL‐M/CLD 40/12.5 mg/d (n=418) or OLM/HCTZ 20/12.5 mg/d (n=419) could be uptitrated during weeks 4 to 52 (AZL‐M/CLD to 80/25 mg; OLM/HCTZ to 40/25 mg [United States] or 20/25 mg [Europe]) to meet blood pressure targets. Treatment‐emergent adverse events/serious adverse events occurred in 78.5%/5.7% of patients taking AZL‐M/CLD vs 76.4%/6.2% taking OLM/HCTZ. The most frequent adverse events were dizziness (16.3% vs 12.6%), blood creatinine increase (21.5% vs 8.6%), headache (7.4% vs 11.0%), and nasopharyngitis (12.2% vs 11.5%). Hypokalemia was uncommon (1.0% vs 0.7%). Greater blood pressure reductions with AZL‐M/CLD by week 2 were maintained throughout the study, despite less uptitration (32.3% vs 48.9% with OLM/HCTZ). Fixed‐dose combination AZL‐M/CLD showed an encouraging benefit‐risk profile when used per standard clinical practice in a titrate‐to‐target strategy.
Keywords: angiotensin receptor blocker, azilsartan, chlorthalidone, diuretic, fixed‐dose combination
1. INTRODUCTION
Most patients with hypertension require treatment with more than one antihypertensive drug to achieve blood pressure (BP) goals.1, 2, 3 Over 75% will require treatment with at least two agents, and over 25% will require triple therapy.1, 2, 3, 4 Evidence‐based hypertension treatment guidelines advise initiation of combination therapies in patients with systolic BP (SBP) >160 mm Hg (or >20 mm Hg above goal) and/or high cardiovascular risk. Such therapies can include the use of fixed‐dose combinations (FDCs) to simplify the treatment schedule and favor adherence.5, 6, 7 Combination therapy with a renin‐angiotensin system (RAS) inhibitor (either an angiotensin‐converting enzyme inhibitor or an angiotensin II receptor blocker [ARB]) plus a diuretic is a widely used and effective approach that has become an established component of evidence‐based hypertension treatment guidelines.3, 5, 7, 8, 9
Azilsartan medoxomil (AZL‐M) is a potent ARB approved for the management of hypertension at a dose of 20 to 80 mg daily (40–80 mg in the United States), alone or in combination with other antihypertensive agents.10, 11, 12 Thiazide‐like/type diuretics, such as chlorthalidone (CLD) and hydrochlorothiazide (HCTZ), respectively, are important options for use in combination BP‐lowering therapy, but they have significant pharmacokinetic and pharmacodynamic differences.7
For instance, CLD is 1.5 to 2.0 times more potent than HCTZ on an mg:mg basis, and has a considerably longer half‐life (45–60 h vs 8–15 h) and duration of action (48–72 h vs 16–24 h) after long‐term dosing.13, 14, 15 Meta‐analyses also suggest that CLD is superior to HCTZ in preventing cardiovascular events.16, 17 The combination of AZL‐M with CLD has been shown to be more effective at lowering SBP than AZL‐M plus HCTZ.18
The combination of AZL‐M and CLD as an FDC was approved for use in December 2011 in the United States, where it is currently available in two dose strengths (40/12.5 mg and 40/25 mg).19, 20 In an 8‐week, phase 3, randomized, double‐blind factorial study in patients with stage 2 hypertension, FDC AZL‐M/CLD provided near‐additive SBP reductions (29 mm Hg) compared with the respective AZL‐M and CLD monotherapy components (14 and 13 mm Hg, respectively).21 In a 12‐week, phase 3, randomized, 3‐arm, double‐blind study in patients with stage 2 hypertension, FDC AZL‐M/CLD (force titrated to a high dose of either 40/25 mg or 80/25 mg) provided superior antihypertensive efficacy compared with the maximum dose of FDC olmesartan (OLM)/HCTZ (40/25 mg) approved in the United States (−42.5 mm Hg vs −37.1 mm Hg change in clinic SBP for AZL‐M/CLD 40/25 mg vs OLM/HCTZ 40/25 mg; P<.001).22
The FDC of AZL‐M/CLD was safe and well tolerated in these short‐term studies; however, safety and tolerability in the longer term as part of a typical titrate‐to‐target BP‐lowering strategy remains an important consideration. The current study evaluated the long‐term (52‐week) safety, tolerability, and efficacy of FDC AZL‐M/CLD in comparison with FDC OLM/HCTZ using a titrate‐to‐target treatment approach in patients with stage 2 essential hypertension.
2. RESEARCH DESIGN AND METHODS
2.1. Patient eligibility
The present study included men and women older than 18 years with stage 2 essential hypertension. Participants were required to discontinue their current antihypertensive medications, if any, 2 to 3 weeks before enrollment and have a post‐washout clinic SBP ≥160 and ≤190 mm Hg. Clinical laboratory evaluations (including clinical chemistry, hematology, and complete urinalysis) had to be within the reference ranges for the testing laboratory or the investigator did not consider the results to be clinically significant for the patient to be randomized. The main exclusion criteria were diastolic BP (DBP) >119 mm Hg; current use of more than two antihypertensive agents; anticipated use of an ARB or thiazide‐type diuretic other than study medication; hypersensitivity to ARBs or thiazide‐type diuretics or other sulfonamide‐derived compounds; clinically relevant or unstable cardiovascular diseases within 6 months of enrollment; secondary hypertension of any etiology; history of cancer not in remission for at least the previous 5 years; known or suspected unilateral or bilateral renal artery stenosis; severe renal dysfunction or disease (based on estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2) at screening; history of drug or alcohol abuse within the past 2 years; poorly controlled diabetes mellitus at screening (glycated hemoglobin >8.5%); alanine aminotransferase or aspartate aminotransferase >2.5 times the upper limit of the normal (ULN) laboratory range, active liver disease, or jaundice; and serum potassium level outside of the normal reference range (ie, hypokalemia or hyperkalemia). Pregnant or lactating women were also excluded. Women of childbearing potential were given pregnancy avoidance counseling and a serum pregnancy test (hCG) obtained at screening, day 1, and at all time points during the treatment period (weeks 2, 4, 8, 12, 16, 24, 32, 42, and 52).
2.2. Study design
This was a phase 3, randomized, parallel‐group, open‐label, multicenter, multinational study (NCT00996281) consisting of a 21‐day screening period (including a 14‐day drug washout), a 52‐week open‐label treatment period, and a 2‐week adverse event (AE) follow‐up period. A total of 1423 patients were screened at 79 sites—56 in the United States, nine in The Netherlands, six in Poland, five in the United Kingdom, and three in Germany. The study was approved by the applicable institutional review boards or ethics committees and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients gave written informed consent to participate in the study.
Participants were randomized to receive either FDC AZL‐M/CLD (initial dose 40/12.5 mg/d) or FDC OLM/HCTZ (initial dose 20/12.5 mg/d) and were stratified by race (black vs nonblack). Target BP was SBP <130 mm Hg and DBP <80 mm Hg for patients with diabetes mellitus or chronic kidney disease and SBP <140 mm Hg and DBP <90 mm Hg for those without diabetes or chronic kidney disease, where chronic kidney disease was defined as baseline eGFR <60 mL/min/1.73 m2 or urinary albumin:creatinine ratio [UACR] >200 mg/g at screening. If target BP was not achieved, doses could be uptitrated during weeks 4 to 52 according to the schedule in Figure 1. The maximum permitted dose of AZL‐M/CLD was 80/25 mg/d and the maximum permitted dose of OLM/HCTZ was 40/25 mg/d (for sites in the United States) or 20/25 mg/d (for sites in Europe). If BP remained uncontrolled after maximum titration, other antihypertensive agents could be added (excluding other ARBs or thiazide‐type diuretics). Investigators were also allowed to downtitrate based on tolerability.
Figure 1.
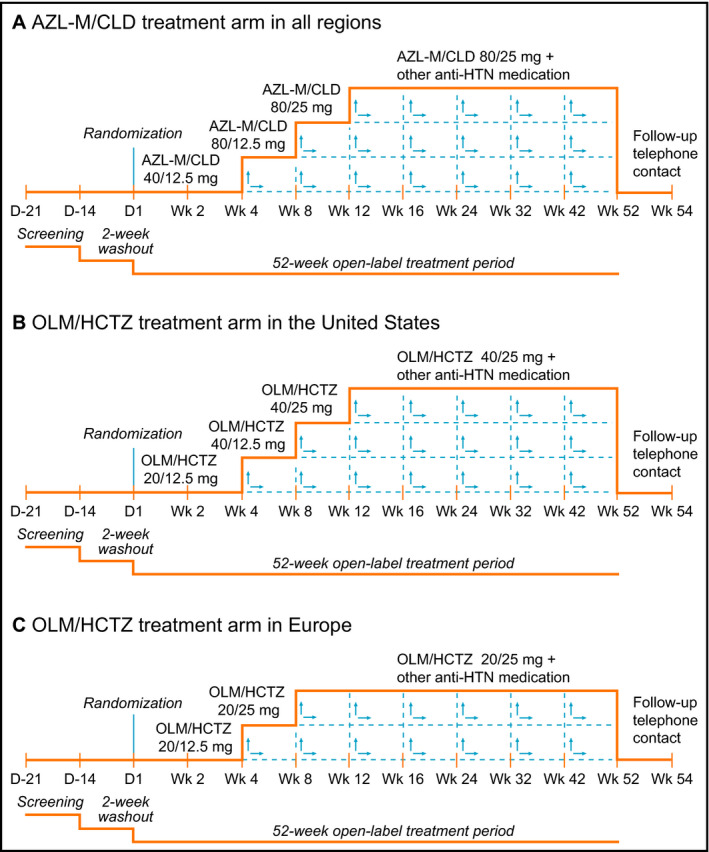
Study designs for the United States and European centers. (A) Azilsartan medoxomil/chlorthalidone (AZL‐M/CLD) treatment arm in all regions. (B) Olmesartan medoxomil/hydrochlorothiazide (OLM/HCTZ) treatment arm in the United States. (C) OLM/HCTZ treatment arm in Europe. HTN indicates hypertension
2.3. End points and assessments
The primary end point was the percentage of patients with one or more treatment‐emergent AE (TEAE) from week 0 (day 1) to week 52, where TEAEs were defined as AEs, regardless of intensity or relationship to study drug, which occurred after the first dose of study medication and no later than 14 days after the last dose of study medication (30 days for serious AEs). Subjective AEs were assessed by the investigator using a neutral nonleading question at each visit from the time that study drug was started until the follow‐up AE assessment. Patients could also report AEs occurring at any other time during the study. Data were presented as an overall summary (patients with ≥1 TEAE) and according to MedDRA system organ class and preferred term. Patients reporting more than one occurrence for the term being summarized were counted only once. If a patient reported more than one episode for one term with different intensity and/or different relationship, only the most severe and/or the most related event was counted.
Secondary safety variables included AEs, clinical safety laboratory tests, 12‐lead electrocardiographic findings, vital signs (including orthostatic vital signs), and the percentage of patients with creatinine elevations ≥50% from baseline and >ULN. Investigators were instructed to report single creatinine elevations ≥30% from baseline and >ULN as AEs, and were advised to withdraw patients with two consecutive creatinine elevations ≥50% from baseline and >ULN.
Clinic SBP and DBP were assessed at each study visit. Three serial seated BP measurements were taken for all participants approximately 24 hours after the previous dose and prior to dosing or blood collection on the day of clinic visits.
2.4. Statistical analyses
All data were summarized using descriptive statistics performed with SAS version 9.1.3 (SAS Institute Inc, Cary NC), and no between‐group statistical comparisons were performed. The safety analysis set used for the primary end point and secondary safety variables included all participants who received at least one dose of study medication, with patients analyzed according to actual study medication received. Efficacy data were derived from the full analysis set, which included all randomized participants who received at least one dose of study medication; patients were analyzed according to the treatment group to which they were randomized. All patients in the safety population were included in the full analysis set. There was no formal statistical sample size justification for this study, although a target of approximately 800 to 880 participants was set, with at least 250 patients per treatment group expected to complete 1 year of treatment. All data are presented as mean±standard deviation, unless otherwise stated.
3. RESULTS
3.1. Patient disposition and demographics
A total of 1423 patients were screened and 837 were randomized (Figure S1). Demographic and baseline characteristics of the AZL‐M/CLD and OLM/HCTZ groups were generally comparable (Table 1). Mean SBP was approximately 168 mm Hg in both groups. The majority of participants (61%) were aged 45 to 64 years, 28% were 65 years and older, and 15% had diabetes. The majority of participants (88%) had normal or only mildly impaired kidney function (eGFR ≥60 mL/min/1.73 m2), with the remainder having moderate impairment (eGFR ≥30 to <60 mL/min/1.73 m2), and 13% met the study criteria for chronic kidney disease (baseline eGFR <60 mL/min/1.73 m2 or UACR >200 mg/g at screening). A total of 292 patients (70%) in the AZL‐M/CLD group and 339 patients (81%) in the OLM/HCTZ group completed at least 294 days of treatment. The mean duration of treatment was 283 days for the AZL‐M/CLD group and 313 days for the OLM/HCTZ group. More patients in the OLM/HCTZ group required one or more (49%), two or more (29%), or three or more (11%) treatment uptitrations during the study compared with patients in the AZL‐M/CLD group (32%, 17%, and 8%, respectively).
Table 1.
Demographic and Baseline Characteristics
| Demographic Variable | AZL‐M/CLD (n=418) | OLM/HCTZ (n=419) |
|---|---|---|
| Men/women, % | 54/46 | 59/41 |
| Age, mean±SD, y | 58.5±10.8 | 57.6±10.8 |
| SBP, mean±SD, mm Hg | 168.2±7.1 | 167.6±7.0 |
| DBP, mean±SD, mm Hg | 95.7±9.2 | 95.7±9.6 |
| SBP categories, % | ||
| ≥140 to <160 mm Hg | <1 | <1 |
| ≥160 to <180 mm Hg | 91 | 92 |
| ≥180 mm Hg | 8 | 8 |
| DBP categories, % | ||
| <90 mm Hg | 26 | 25 |
| ≥90 mm Hg | 74 | 75 |
| Region, United States/Europe, % | 60/40 | 59/41 |
| Race, white/black/other, % | 82/17/3 | 80/18/4 |
| BMI, mean±SD, kg/m2 | 31.4±6.2 | 31.9±6.6 |
| eGFR categories, %a | ||
| ≥30 to <60 mL/min/1.73 m2 | 13 | 10 |
| ≥60 to <90 mL/min/1.73 m2 | 66 | 63 |
| ≥90 mL/min/1.73 m2 | 21 | 26 |
| Chronic kidney disease, %b | 14 | 12 |
| Diabetes, % | 15 | 14 |
Abbreviations: AZL‐M/CLD, azilsartan medoxomil/chlorthalidone; BMI, body mass index; DBP, diastolic blood pressure; OLM/HCTZ, olmesartan medoxomil/hydrochlorothiazide; SBP, systolic blood pressure; SD, standard deviation.
Estimated glomerular filtration rate (eGFR) data were not available for three patients.
Chronic kidney disease=baseline eGFR <60 mL/min/1.73 m2 or urinary albumin:creatinine ratio (UACR) >200 mg/g at screening.
A total of 220 patients (26%) permanently and prematurely discontinued treatment during the study (131 patients [31%] in the AZL‐M/CLD group and 89 patients [21%] in the OLM/HCTZ group), most commonly due to AEs (see Figure S1 for details). The higher percentage of discontinuations in the AZL‐M/CLD group was accounted for primarily by more withdrawals due to protocol‐specified criteria to discontinue patients with creatinine elevations (which were reversible upon study drug discontinuation) and slightly more events associated with greater BP reduction (dizziness, hypotension).
3.2. Efficacy
Numerically greater reductions in SBP and DBP were observed by weeks 2 to 4 in the AZL‐M/CLD group compared with the OLM/HCTZ group and this separation was maintained through to week 52 (Figure 2). Mean±standard deviation changes in SBP from baseline to week 4 (observed cases) were −38.7±14.8 mm Hg in the AZL‐M/CLD group vs −32.2±14.9 mm Hg in the OLM/HCTZ group, and changes in DBP were −15.9±8.8 mm Hg vs −12.0±9.5 mm Hg, respectively. The week 4 visit was just prior to the option to uptitrate treatment to achieve BP goals. Consequently, all patients were still taking starting doses of randomized study treatment at this point. Mean changes in SBP from baseline to week 52 (observed cases) were −44.2±12.1 mm Hg (AZL‐M/CLD) vs −39.4±12.1 mm Hg (OLM/HCTZ), and mean changes in DBP were −19.0±8.5 mm Hg vs −16.0±9.3 mm Hg, respectively. Mean changes in SBP from baseline to the final visit (last observation carried forward) were −42.3±14.2 mm Hg (AZL‐M/CLD) vs −38.0±14.1 mm Hg (OLM/HCTZ), and mean changes in DBP were −18.4±9.0 mm Hg (AZL‐M/CLD) vs −15.6±9.8 mm Hg (OLM/HCTZ), respectively.
Figure 2.
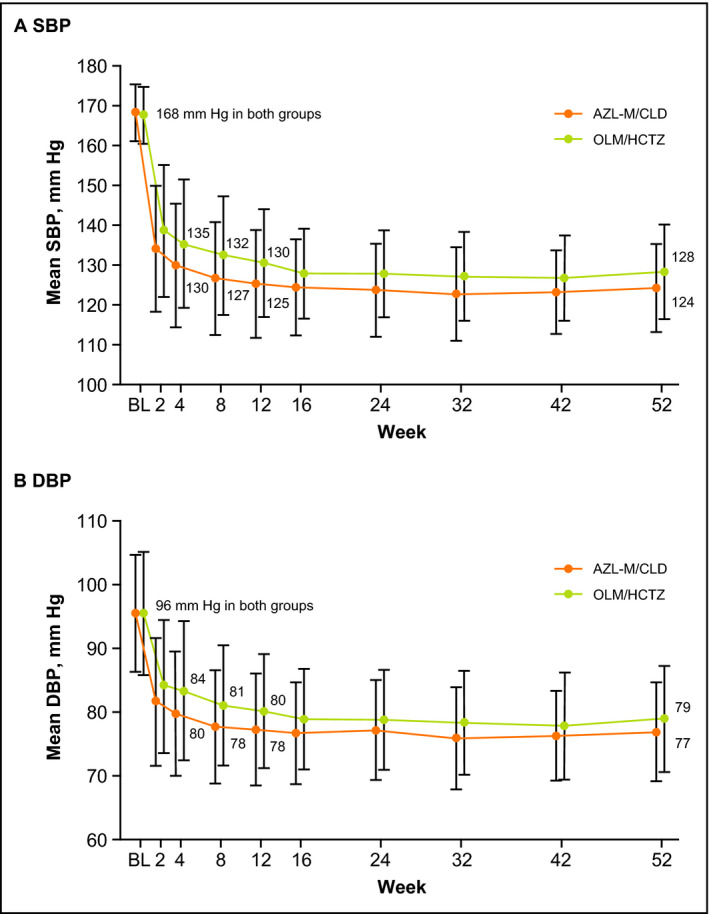
Mean trough clinic sitting systolic blood pressure (SBP) (A) and diastolic blood pressure (DBP) (B) by study visit (observed cases). For azilsartan medoxomil/chlorthalidone (AZL‐M/CLD), n=410 at baseline (BL) and n=289 at week 52. For olmesartan medoxomil/hydrochlorothiazide (OLM/HCTZ), n=416 at BL and n=331 at week 52. Data are expressed as mean±standard deviation
This difference in BP change was observed despite a smaller percentage of patients taking AZL‐M/CLD (32%) needing to titrate beyond the initial dose in order to achieve target BP compared with those taking OLM/HCTZ (49%). Greater reductions in DBP were seen in patients with baseline DBP ≥90 mm Hg in both treatment groups compared with those with baseline DBP <90 mm Hg.
3.3. Safety and tolerability
The percentage of patients who reported one or more TEAE (the primary end point) was 78.5% in the AZL‐M/CLD group and 76.4% in the OLM/HCTZ group (Table 2). The most commonly reported AEs (AZL‐M/CLD vs OLM/HCTZ) were dizziness (16.3% vs 12.6%), blood creatinine increase (21.5% vs 8.6%), headache (7.4% vs 11.0%), and nasopharyngitis (12.2% vs 11.5%) (Table 2). Events of hypokalemia were uncommon in both treatment groups (1.0% vs 0.7%).
Table 2.
Overview of Treatment‐Emergent AEs (Safety Population)
| Adverse Event | AZL‐M/CLD (n=418) | OLM/HCTZ (n=419) | ||
|---|---|---|---|---|
| No. of Events | No. of Patients (%) | No. of Events | No. of Patients (%) | |
| Death | – | 2 (0.5)c | – | 2 (0.5)d |
| Serious AE | 47 | 24 (5.7) | 40 | 26 (6.2) |
| Any AE | 1279 | 328 (78.5) | 1213 | 320 (76.4) |
| Mild | 786 | 126 (30.1) | 721 | 117 (27.9) |
| Moderate | 421 | 157 (37.6) | 439 | 172 (41.1) |
| Severe | 72 | 45 (10.8) | 53 | 31 (7.4) |
| Leading to discontinuationa | – | 79 (18.9) | – | 43 (10.3) |
| AE (preferred term) in ≥5% of patients in either group | No. of patients (%) | No. of patients (%) | ||
| Blood creatinine increasedb | 90 (21.5) | 36 (8.6) | ||
| Dizziness | 68 (16.3) | 53 (12.6) | ||
| Nasopharyngitis | 51 (12.2) | 48 (11.5) | ||
| Headache | 31 (7.4) | 46 (11.0) | ||
| Upper respiratory tract infection | 20 (4.8) | 27 (6.4) | ||
| Diarrhea | 20 (4.8) | 21 (5.0) | ||
| Nausea | 20 (4.8) | 21 (5.0) | ||
| Fatigue | 21 (5.0) | 17 (4.1) | ||
Abbreviations: AZL‐M/CLD, azilsartan medoxomil/chlorthalidone; OLM/HCTZ, olmesartan medoxomil/hydrochlorothiazide.
Temporary drug interruption or permanent discontinuation. Investigators were advised to withdraw patients with two consecutive creatinine elevations ≥50% from baseline and upper limit of normal (ULN).
Investigators were instructed to report single creatinine elevations ≥30% from baseline and >ULN as adverse events (AEs).
Accidental drowning (1) and septic shock (1).
Gunshot wound (1) and atherosclerotic cardiovascular disease (1).
Serious AEs were reported by 5.7% of patients in the AZL‐M/CLD group and 6.2% in the OLM/HCTZ group (Tables 2 and 3). Few patients (2 [0.4%] in the AZL‐M/CLD group and 5 [1.2%] in the OLM/HCTZ group) experienced serious AEs that were considered by the investigator to be possibly or probably related to the study drug (Table 3). One patient taking AZL‐M/CLD 40/12.5 mg developed postural hypotension on day 43, followed by several brief episodes of syncope on day 44. The study drug was withdrawn permanently the following day and the event was considered resolved. A patient taking AZL‐M/CLD 80/12.5 mg had an event of lung embolism (diagnosed after experiencing symptoms of dyspnea and thoracic pain) on day 95. The study drug was withdrawn permanently and the patient received anticoagulation therapy. There were no clinical sequelae and the event was confirmed as resolved 1 month later. One patient taking OLM/HCTZ 40/25 mg had an episode of presyncope that was deemed resolved after 3 days; the patient continued the study without change to treatment. The other four serious AEs occurred in patients taking OLM/HCTZ 20/12.5 mg. Two patients had serious AEs of “blood creatinine increased”; one patient continued without change to treatment and the event was deemed resolved 8 days later, while the second patient was withdrawn permanently from the study and the event was considered resolved at follow‐up 11 days later. One patient experienced a “transient ischemic attack” on study day 3 that resolved on the same day; the patient was withdrawn permanently from the study the following day. Finally, one patient had a serious AE of “acute renal failure” based on elevated serum creatinine and abnormal serum urea nitrogen. The study drug was withdrawn permanently 2 days later and the event was deemed resolved after a further 15 days. This was the only event among all serious AEs in either treatment group considered probably related to the study drug by the investigator. There were four deaths reported during the study (2 in each treatment group), but none were considered by the investigator to be related to study treatment.
Table 3.
Treatment‐Emergent Serious AEs (Safety Population)
| No. of Patients (%) | ||
|---|---|---|
| System Organ Classa Preferred Term | AZL‐M/CLD (n=418) | OLM/HCTZ (n=419) |
| Any serious AE | 24 (5.7) | 26 (6.2) |
| Related | 2 (0.4) | 5 (1.2) |
| Cardiac disorders | 3 (0.7) | 5 (1.2) |
| Angina pectoris | 0 | 2 (0.5) |
| Atrial fibrillation | 1 (0.2) | 1 (0.2) |
| Cardiac arrest | 1 (0.2) | 0 |
| Cardiogenic shock | 1 (0.2) | 0 |
| Coronary artery disease | 0 | 1 (0.2) |
| Mitral valve incompetence | 1 (0.2) | 0 |
| Myocardial infarction | 0 | 1 (0.2) |
| Renal and urinary disorders | 0 | 1 (0.2) |
| Renal failure acute | 0 | 1 (0.2) |
| Vascular disorders | 1 (0.2) | 1 (0.2) |
| Arteriosclerosis | 0 | 1 (0.2) |
| Orthostatic hypotension | 1 (0.2) | 0 |
| Nervous system disorders | 3 (0.7) | 2 (0.5) |
| Syncope | 2 (0.5) | 0 |
| Loss of consciousness | 1 (0.2) | 0 |
| Presyncope | 0 | 1 (0.2) |
| Radicular syndrome | 1 (0.2) | 0 |
| Transient ischemic attack | 0 | 1 (0.2) |
Abbreviations: AE, adverse event; AZL‐M/CLD, azilsartan medoxomil/chlorthalidone; OLM/HCTZ, olmesartan medoxomil/hydrochlorothiazide.
Selected system organ classes only.
The percentage of patients who discontinued due to AEs was higher in the AZL‐M/CLD group (79 patients, 18.9%) than in the OLM/HCTZ group (43 patients, 10.3%) (Table 2). Discontinuation was permanent in 75 patients (17.9%) taking AZL‐M/CLD and 37 patients (8.8%) taking OLM/HCTZ. The AEs most often leading to discontinuation (AZL‐M/CLD vs OLM/HCTZ) were blood creatinine increase (6.0% [n=21 taking AZL‐M/CLD 40/12.5 mg; n=2 taking AZL‐M/CLD 80/12.5 mg; n=2 taking AZL‐M 80/25 mg] vs 0.7% [n=2 taking OLM/HCTZ 20/12.5 mg; n=1 taking OLM/HCTZ 20/25 mg]), dizziness (4.1% vs 1.7%), and hypotension (2.2% vs 1.0%). Of the 70 patients who underwent uptitration to the highest AZL‐M/CLD dose (80/25 mg), five (7.1%) discontinued due to AEs.
3.4. Laboratory data, electrocardiographic parameters, and vital signs
Key laboratory parameters are described in Table 4. Shifts from normal (at baseline) to high (at final visit) uric acid (consistent with combined ARB and diuretic use) were reported more often in the AZL‐M/CLD group (Table 4), although AEs of gout were slightly less frequent in the AZL‐M/CLD group (1.4% vs 2.1% in the OLM/HCTZ group). Shifts from normal to high serum urea nitrogen were also reported more frequently in the AZL‐M/CLD group (18.7% vs 8.8% in the OLM/HCTZ group).
Table 4.
Key Serum Laboratory Parameters (Safety Population)
| Parameter | AZL‐M/CLD (n=418) | OLM/HCTZ (n=419) |
|---|---|---|
| Creatinine | ||
| Two or more consecutive elevations (≥1.5 × BL and >ULN), n/N (%) | 21/408 (5.1) | 5/414 (1.2) |
| Potassium | ||
| Baseline, mean±SD, mmol/La | 4.33±0.43 | 4.32±0.37 |
| Change at final visit, mean±SD, mmol/La | −0.04±0.44 | −0.06±0.41 |
| Shift from normal to low, n/N (%)b | 3/400 (0.8) | 2/410 (0.5) |
| Shift from normal to high, n/N (%)b | 7/400 (1.8) | 2/410 (0.5) |
| Sodium | ||
| Shift from normal to low, n/N (%)b | 13/365 (3.6) | 5/382 (1.3) |
| Uric acid | ||
| Shift from normal to high, n/N (%)c , d | 95/379 (25.1) | 60/373 (16.1) |
| Fasting glucose | ||
| Baseline, mean±SD, mmol/Le | 5.98±1.42 | 5.70±0.94 |
| Change at final visit, mean±SD, mmol/Le | 0.16±1.19 | 0.24±1.22 |
| Shift from <7.0 to ≥7.0 mmol/L, n/N (%)e | 29/348 (8.1) | 26/375 (6.8) |
| Shift from ≥7.0 to <7.0 mmol/L, n/N (%)e | 23/57 (39.7) | 11/33 (31.4) |
| Glycosylated hemoglobin (diabetic patients only) | ||
| Shift from <7.0% to ≥7.0%, n/N (%)f | 6/36 (16.7) | 9/40 (22.5) |
| Shift from ≥7.0% to <7.0%, n/N (%)f | 8/24 (33.3) | 3/15 (20.0) |
Abbreviations: AZL‐M/CLD, azilsartan medoxomil/chlorthalidone; BL, baseline; OLM/HCTZ, olmesartan medoxomil/hydrochlorothiazide; SD, standard deviation; ULN, upper limit of the normal.
For potassium, 1 mmol/L=1 mEq/L.
Definitions of “low” sodium: (mmol/L) <132 (18–59 y) and <135 (>59 y); potassium (mmol/L) <3.4.
Definitions of “high” uric acid: (μmol/L) >125 (18–50 y) and >149 (>50 y).
To convert μmol/L to mg/dL, divide by 59.5.
To convert mmol/L to mg/dL, multiply by 18.
For glycated hemoglobin, 7%=53 mmol/mol.
Shifts from normal to low potassium were uncommon in both groups and mean changes were negligible (Table 4); ≤1% developed hypokalemia in either group. Shifts from normal to low sodium were uncommon but slightly more frequent with AZL‐M/CLD (Table 4). Shifts in fasting glucose from the diabetic (≥7.0 mmol/L) to nondiabetic (<7.0 mmol/L) range were more common with AZL‐M/CLD. Similarly, in patients with known diabetes, shifts from above to below the typical glycated hemoglobin goal of 7% were more common with AZL‐M/CLD, whereas shifts in the opposite direction were more common with OLM/HCTZ (Table 4). There were no notable changes in lipid parameters in either group (data not shown). The incidence of creatinine elevations ≥50% and >ULN at any visit was higher for AZL‐M/CLD vs OLM/HCTZ (Table 5). However, the majority of creatinine elevations were transient and reversible, as reflected by the lower incidence at two consecutive visits or the final visit compared with any postbaseline visit and the large percentage that resolved after the final visit for both treatment groups (Table 5). Patients with creatinine elevations ≥50% tended to have greater SBP reductions at week 52 compared with those without elevations (mean change −48.4 mm Hg vs −41.6 mm Hg, respectively).
Table 5.
Transience and Reversibility of Creatinine Elevations (Safety Population)
| Parameter | AZL‐M/CLD (n=418) | OLM/HCTZ (n=419) |
|---|---|---|
| Patients with creatinine elevations ≥50% from baseline and >ULN, n/N (%) | ||
| Any postbaseline visitc | 58/408 (14.2) | 24/414 (5.8) |
| Final visit | 24/408 (5.9) | 4/414 (1.0) |
| Two or more consecutive elevationsc | 21/408 (5.1) | 5/414 (1.2) |
| Reversibility of creatinine elevations ≥30% from baseline and >ULN at final visit, n/N (%) | ||
| Resolved after final visitc | 30/35 (85.7) | 5/9 (55.6) |
| Unresolved | 2/35 (5.7) | 1/9 (11.1) |
| Voluntary withdrawala | 1/35 (2.6) | 1/9 (11.1) |
| Lost to follow‐upb | 0/35 | 2/9 (22.2) |
| Early termination due to other reasonc | 2/35 (5.7) | 0/9 |
Abbreviations: AZL‐M/CLD, azilsartan medoxomil/chlorthalidone; OLM/HCTZ, olmesartan medoxomil/hydrochlorothiazide.
Experienced one or more creatinine elevation with a prespecified percentage ≥baseline and >upper limit of normal (ULN).
Two or more creatinine values that met the criteria at consecutive visits (may include final visit and unscheduled visits through 7 days after the last dose of study medication).
Primarily patients who resolved during follow‐up, but also patients who were considered resolved at final visit relative to screening values; all patients resolved to ≤0.2 mg/dL above the baseline or screening value.
Patient withdrew for personal reasons. Creatinine elevation at final visit with no follow‐up available.
Patients completed the study and had creatinine elevation at final visit but lost to follow‐up.
Patients had creatinine elevation at final visit but was subsequently discharged due to adverse event of renal cancer (1) and noncompliance (1).
No clinically relevant differences in urinalysis parameters, electrocardiographic parameters, or vital signs (including heart rate, body weight, or orthostatic BP) were observed between AZL‐M/CLD and OLM/HCTZ. The percentage of patients with markedly altered hematology parameter values was low in both groups (hematocrit <0.8 of baseline, 1.7% AZL‐M/CLD, 0.5% OLM/HCTZ; hemoglobin 3 g/dL decrease from baseline, 1.7% AZL‐M/CLD, 0% OLM/HCTZ; red blood cell count <0.8 of baseline, 1.7% AZL‐M/CLD, 0.2% OLM/HCTZ). Shifts from normal to high liver enzymes were slightly more common in the OLM/HCTZ group (alanine aminotransferase, 6.9% AZL‐M/CLD, 9.0% OLM/HCTZ; aspartate aminotransferase, 4.3% AZL‐M/CLD, 7.9% OLM/HCTZ).
4. DISCUSSION
In this 52‐week, open‐label, randomized, titrate‐to‐target BP study comparing the long‐term safety and tolerability of FDC AZL‐M/CLD vs FDC OLM/HCTZ in patients with stage 2 essential hypertension, both AZL‐M/CLD and OLM/HCTZ were well tolerated, with a similar percentage of patients in each group experiencing AEs (the primary end point) or serious AEs. Slightly fewer patients in the AZL‐M/CLD group (0.4% vs 1.2%) experienced serious AEs that were considered by the investigator to be related to study drug. Although creatinine elevations were more frequent in the AZL‐M⁄CLD group, these were generally nonprogressive or reversible after treatment discontinuation, and there were no differences in syncope or hypotension. There was a higher frequency of discontinuations due to AEs in the AZL‐M⁄CLD group; however, the difference was mostly accounted for by discontinuations for creatinine elevations, which were likely influenced by the protocol guidance advising investigators to withdraw patients with two consecutive creatinine elevations ≥50% from baseline and >ULN. Of note, all three patients who experienced a renal‐related serious AE (two involved a blood creatinine increase) were OLM/HCTZ‐treated patients; two were withdrawn from the study and one had acute renal failure deemed probably related to study drug.
Acute serum creatinine elevations have been previously reported in patients receiving RAS‐inhibiting drugs, including both angiotensin‐converting enzyme inhibitors and ARBs.23, 24, 25, 26, 27 This might be expected based on their mechanism of action—as RAS blockade inhibits angiotensin II‐mediated vasoconstriction of efferent glomerular arterioles, it causes a decrease in intraglomerular pressure, thus leading to a reversible acute decrease of glomerular filtration rate.23 Furthermore, creatinine elevations may be exaggerated further in states of volume depletion, as provided by concomitant use of a potent diuretic, such as CLD.23 Thus, acute creatinine elevations observed in the setting of goal‐directed combination therapy with a RAS inhibitor and a potent diuretic are most likely to reflect antihypertensive drug efficacy, as in the present study, rather than any undesirable adverse effect on the kidney.27, 28 Indeed, in rodent studies, the more effective decrease in intraglomerular pressure provided by RAS inhibitors provides protection from progressive renal injury.28 Clinical studies also suggest that the acute increases in serum creatinine after starting RAS inhibitors, or more aggressive RAS inhibitor/diuretic combination therapy, are strongly associated with long‐term preservation of renal function in patients with chronic renal disease.23, 26, 27, 28
In the current study, patients treated with AZL‐M/CLD had greater sustained BP reductions, despite more patients requiring uptitration in the OLM/HCTZ group. As expected, the greater BP reductions with AZL‐M/CLD were associated with more AEs of dizziness and increased creatinine. These results are consistent with previous findings from a 12‐week, randomized controlled trial showing significantly greater reductions in clinic and ambulatory SBP with the FDC AZL‐M/CLD force‐titrated to doses of either 40/25 mg or 80/25 mg compared with the FDC OLM/HCTZ force‐titrated to the maximum dose approved in the United States of 40/25 mg.22 In that short‐term study, the FDC OLM/HCTZ was associated with a lower frequency of total AEs compared with the FDC AZL‐M/CLD. However, the current study suggests that when used as part of a more clinically relevant titrate‐to‐target approach over the longer term, overall tolerability is similar between the two FDC treatments. It should be noted that the recommended starting dose of the FDC AZL‐M/CLD in the United States is 40/12.5 mg and the maximum approved dose is 40/25 mg, although AZL‐M 80 mg is approved for use in free combinations.11, 20 In the current study, a 40/25‐mg dose was not part of the titration scheme (doses went from 40/12.5 mg to 80/12.5 mg to 80/25 mg), although it was not necessary to titrate beyond the 40/12.5 mg starting dose in the majority of patients (68%), and only 17% of patients required the 80/25‐mg dose (with or without additional antihypertensive drugs) to achieve BP goals. The design of the current study does not allow any meaningful comparisons of tolerability between the different AZL‐M/CLD doses required by patients within the titrate‐to‐target algorithm, although few patients who required the highest dose (80/25 mg) discontinued due to AEs. In a previous 8‐week, factorial study, the percentage of patients with any AE was similar between the FDC AZL‐M/CLD doses of 40/25 mg and 80/25 mg (67.9% and 62.1%, respectively) and between doses of 40/12.5 mg and 80/12.5 mg (56.8% and 54.9%, respectively), with few serious AEs reported (n=1 for 40/12.5 mg; n=2 each for the other doses).21 Two open‐label, treat‐to‐target studies of AZL‐M permitted patients to receive additional antihypertensives from week 8, if required, to reach BP targets (a 56‐week study allowed CLD 25 mg or HCTZ 12.5–25 mg29 and a 26‐week study allowed CLD 25 mg30). AZL‐M was well tolerated over the longer term and provided stable BP improvements when combined with either of the diuretics.29, 30
Previous data suggest that AZL‐M is a more efficacious BP‐lowering agent than the ARBs valsartan and OLM when used at maximal approved doses.31, 32 The recently published EARLY registry (a prospective, observational, German, multicenter registry of 3849 patients with essential arterial hypertension; 1‐year follow‐up) found that AZL‐M monotherapy had a comparable safety profile to that of angiotensin‐converting enzyme inhibitors, but the BP target of <140/90 mm Hg was achieved by significantly more patients in the AZL‐M group (61.1%) than in the angiotensin‐converting enzyme inhibitor group (56.4%).33 In addition, CLD has been shown to be a more potent and longer‐lasting diuretic than HCTZ at the doses used in this study,13, 14, 15 which may partly explain the superiority of CLD over HCTZ in preventing cardiovascular events, as demonstrated in a meta‐analysis.16, 17 Consequently, there is a strong rationale for using AZL‐M and CLD together in an FDC formulation.
Despite the known potential benefits of CLD, few FDC therapies have incorporated this diuretic. The hypokalemic effects of diuretics, especially at high doses, are well‐known.34 Perceptions about differences in propensity to cause hypokalemia may be one reason why HCTZ has traditionally been the thiazide‐type diuretic of choice over CLD.14 However, hypokalemia was uncommon (≤1.0%) with both AZL‐M/CLD and OLM/HCTZ in the current study and mean changes in potassium levels were negligible and comparable between groups at the doses used. This is consistent with the results of a previous 6‐week study comparing the FDC AZL‐M/CLD with the free combination of AZL‐M and HCTZ.18 Combination therapy with a RAS inhibitor would be expected to decrease the incidence of hypokalemia, as these agents decrease potassium excretion.34 Indeed, combination with a RAS inhibitor is one of several recognized strategies for treating and preventing thiazide diuretic–induced hypokalemia.34 Accordingly, hypokalemia was reported to be less frequent with the FDC AZL‐M/CLD than with CLD monotherapy in a factorial trial.21 Historically, there has been considerable controversy over the benefits of lowering BP to <140 mm Hg. However, the well‐conducted Systolic Blood Pressure Intervention Trial (SPRINT) has recently shown that a lower BP target does translate into further cardiovascular risk reduction.35 In patients at high risk for cardiovascular events, but without diabetes, there were clear cardiovascular benefits with lower (target SBP <120 mm Hg) vs higher (target SBP <140 mm Hg) BP targets.35 If, based on SPRINT, guidelines now recommend lower SBP goals than previously suggested, AZL‐M/CLD could be an important component of an effective antihypertensive armamentarium.
CONCLUSIONS
These results demonstrate the encouraging benefit‐risk profile of the FDC AZL‐M/CLD used in accordance with standard clinical practice in a titrate‐to‐target BP treatment strategy in patients with stage 2 essential hypertension. Reversible creatinine elevations, as a marker of efficacy, might be expected based on the mechanisms of action of both AZL‐M and the potent thiazide‐type diuretic CLD, and, contrary to previous preconceptions, hypokalemia was uncommon with CLD at the recommended doses for the commercially available FDC formulation used in this study.
Supporting information
ACKNOWLEDGMENTS
J.M.N. is a member of the speakers' bureau and consultant for Boehringer‐Ingelheim, Forest, Novartis, and Takeda. W.C.C. has received grant and research support from Lilly and has undertaken uncompensated work (member of steering committee and consultancy) for Takeda. E.L. is a full‐time employee of Takeda Development Center Americas, Inc. (Deerfield, IL). A.H. is a full‐time employee of Takeda Pharmaceuticals International, Inc. (Deerfield, IL). B.B. was an employee of Takeda at the time of the study and is currently employed at Abbvie, Inc. (North Chicago, IL). This study was sponsored by Takeda Development Center Americas, Inc. The authors would like to thank Stuart Kupfer for his critical review of the manuscript. Medical writing assistance was provided by Absolute Healthcare Communications, Ltd, Twickenham, UK. Preliminary results from this study were presented at the 24th Scientific Meeting of the International Society of Hypertension, September 30–October 4, 2012, in Sydney, Australia.
Neutel JM, Cushman WC, Lloyd E, Barger B, Handley A. Comparison of long‐term safety of fixed‐dose combinations azilsartan medoxomil/chlorthalidone vs olmesartan medoxomil/hydrochlorothiazide. J Clin Hypertens. 2017;19:874–883. 10.1111/jch.13009
Bruce Barger is currently an employee of AbbVie, Inc., North Chicago, IL.
Clinicaltrials.gov Identifier: NCT00996281
REFERENCES
- 1. Mancia G, De Backer G, Dominiczak A, et al. 2007 ESH‐ESC practice guidelines for the management of arterial hypertension: ESH‐ESC task force on the management of arterial hypertension. J Hypertens. 2007;25:1751‐1762. [DOI] [PubMed] [Google Scholar]
- 2. Gradman AH. Rationale for triple‐combination therapy for management of high blood pressure. J Clin Hypertens (Greenwich). 2010;12:869‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gradman AH, Basile JN, Carter BL, et al. Combination therapy in hypertension. J Am Soc Hypertens. 2010;4:90‐98. [DOI] [PubMed] [Google Scholar]
- 4. Cushman WC, Ford CE, Einhorn PT, et al. Blood pressure control by drug group in the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich). 2008;10:751‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206‐1252. [DOI] [PubMed] [Google Scholar]
- 6. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159‐2219. [DOI] [PubMed] [Google Scholar]
- 7. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507‐520. [DOI] [PubMed] [Google Scholar]
- 8. Weir MR, Bakris GL. Combination therapy with renin‐angiotensin‐aldosterone receptor blockers for hypertension: how far have we come? J Clin Hypertens (Greenwich). 2008;10:146‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sood N, Reinhart KM, Baker WL. Combination therapy for the management of hypertension: a review of the evidence. Am J Health Syst Pharm. 2010;67:885‐894. [DOI] [PubMed] [Google Scholar]
- 10. Zaiken K, Cheng JW. Azilsartan medoxomil: a new angiotensin receptor blocker. Clin Ther. 2011;33:1577‐1589. [DOI] [PubMed] [Google Scholar]
- 11. Edarbi (azilsartan medoxomil) tablets . U.S. prescribing information. Arbor Pharmaceuticals, LLC, Atlanta, GA, USA. July 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200796s006lbl.pdf. Accessed July 12, 2016.
- 12. Edarbi (azilsartan medoxomil) tablets. Summary of product characteristics. Takeda Pharma A/S, Taastrup, Denmark. July 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002293/WC500119204.pdf. Accessed July 12, 2016.
- 13. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43:4‐9. [DOI] [PubMed] [Google Scholar]
- 14. Ernst ME, Carter BL, Zheng S, Grimm RH Jr. Meta‐analysis of dose‐response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens. 2010;23:440‐446. [DOI] [PubMed] [Google Scholar]
- 15. Peterzan MA, Hardy R, Chaturvedi N, Hughes AD. Meta‐analysis of dose‐response relationships for hydrochlorothiazide, chlorthalidone, and bendroflumethiazide on blood pressure, serum potassium, and urate. Hypertension. 2012;59:1104‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roush GC, Buddharaju V, Ernst ME. Is chlorthalidone better than hydrochlorothiazide in reducing cardiovascular events in hypertensives? Curr Opin Cardiol. 2013;28:426‐432. [DOI] [PubMed] [Google Scholar]
- 17. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta‐analyses. Hypertension. 2012;59:1110‐1117. [DOI] [PubMed] [Google Scholar]
- 18. Bakris GL, Sica D, White WB, et al. Antihypertensive efficacy of hydrochlorothiazide vs chlorthalidone combined with azilsartan medoxomil. Am J Med. 2012;125:1229.e1‐1229.e10. [DOI] [PubMed] [Google Scholar]
- 19. Shuster JE, Bleske BE, Dorsch MP. Clinical utility of azilsartan‐chlorthalidone fixed combination in the management of hypertension. Vasc Health Risk Manag. 2012;8:381‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edarbyclor (azilsartan medoxomil and chlorthalidone) tablets . U.S. prescribing information. Arbor Pharmaceuticals, LLC, Atlanta, GA, USA. July 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202331s006lbl.pdf. Accessed July 12, 2016.
- 21. Sica D, Bakris GL, White WB, et al. Blood pressure‐lowering efficacy of the fixed‐dose combination of azilsartan medoxomil and chlorthalidone: a factorial study. J Clin Hypertens (Greenwich). 2012;14:284‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cushman WC, Bakris GL, White WB, et al. Azilsartan medoxomil plus chlorthalidone reduces blood pressure more effectively than olmesartan plus hydrochlorothiazide in stage 2 systolic hypertension. Hypertension. 2012;60:310‐318. [DOI] [PubMed] [Google Scholar]
- 23. Bakris GL, Weir MR. Angiotensin‐converting enzyme inhibitor‐associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685‐693. [DOI] [PubMed] [Google Scholar]
- 24. Kidney Disease Outcomes Quality Initiative (K/DOQI) . K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1‐S290. [PubMed] [Google Scholar]
- 25. Thorp ML, Ditmer DG, Nash MK, et al. A study of the prevalence of significant increases in serum creatinine following angiotension‐converting enzyme inhibitor administration. J Hum Hypertens. 2005;19:389‐392. [DOI] [PubMed] [Google Scholar]
- 26. Hirsch S, Hirsch J, Bhatt U, Rovin BH. Tolerating increases in the serum creatinine following aggressive treatment of chronic kidney disease, hypertension and proteinuria: pre‐renal success. Am J Nephrol. 2012;36:430‐437. [DOI] [PubMed] [Google Scholar]
- 27. Ruggenenti P, Remuzzi G. Dealing with renin‐angiotensin inhibitors, don't mind serum creatinine. Am J Nephrol. 2012;36:427‐429. [DOI] [PubMed] [Google Scholar]
- 28. Mangrum AJ, Bakris GL. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol. 2004;24:168‐175. [DOI] [PubMed] [Google Scholar]
- 29. Handley A, Lloyd E, Roberts A, Barger B. Safety and tolerability of azilsartan medoxomil in subjects with essential hypertension: a one‐year, phase 3, open‐label study. Clin Exp Hypertens. 2016;38:180‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kipnes MS, Handley A, Lloyd E, Barger B, Roberts A. Safety, tolerability, and efficacy of azilsartan medoxomil with or without chlorthalidone during and after 8 months of treatment for hypertension. J Clin Hypertens (Greenwich). 2015;17:183‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. White WB, Weber MA, Sica D, et al. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension. 2011;57:413‐420. [DOI] [PubMed] [Google Scholar]
- 32. White WB, Cuadra RH, Lloyd E, Bakris GL, Kupfer S. Effects of azilsartan medoxomil compared with olmesartan and valsartan on ambulatory and clinic blood pressure in patients with type 2 diabetes and prediabetes. J Hypertens. 2016;34:788‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gitt AK, Bramlage P, Potthoff SA, et al. Azilsartan compared to ACE inhibitors in anti‐hypertensive therapy: one‐year outcomes of the observational EARLY registry. BMC Cardiovasc Disord. 2016;16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palmer BF. Metabolic complications associated with use of diuretics. Semin Nephrol. 2011;31:542‐552. [DOI] [PubMed] [Google Scholar]
- 35. The SPRINT research group . A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


