Abstract
Low nephron number has been shown to be a risk factor for hypertension (HTN) in adulthood. Kidney volume may serve as a surrogate marker for nephron mass. The relationship between kidney volume and ambulatory blood pressure (BP) in the pediatric population is not known. A retrospective chart review of children younger than 21 years who were evaluated for HTN was performed. Twenty‐four‐hour BP and ultrasonography data were obtained. Multiple regression was used to examine associations between BP and kidney volume. Of 84 children (mean age 13.87 years, 72.6% males), 54 had HTN. Systolic BP index during the awake, sleep, and 24‐hour periods (all P≤.05) was found to be positively correlated with total kidney volume. Greater total kidney volume was found to be a positive predictor of 24‐hour and sleep systolic index (P≤.05). It failed to serve as a predictor of HTN, pre‐HTN, or white‐coat HTN. Contrary to expectation, total kidney volume was positively associated with systolic BP indices.
Keywords: ambulatory blood pressure, hypertension, kidney volume, pediatric
1. Introduction
By the 36th week of gestation, nephron development is usually complete in humans, with approximately 60% of development occurring during the third trimester.1 Upon completion of nephrogenesis, no new nephrons are formed.1, 2 Birth weight has been shown to be a strong determinant of nephron mass in the kidneys.2, 3 Previous literature has described a direct relationship between nephron number and birth weight. Manalich and colleagues4 found that infants with low birth weight had a 20% reduction in nephron number compared with normal controls.4 They also noted an inverse relationship between glomeruli number and glomerular volume, suggesting that low birth weight results in fewer, larger glomeruli.4
Reduced nephron endowment has been ascribed to be a risk factor for primary hypertension (HTN) and cardiovascular disease in adulthood as well as progressive renal disease.5, 6 Brenner and colleagues5 proposed an inverse relationship between nephron number and the risk of HTN, suggesting that a reduced filtration surface area in the kidneys impairs pressure natriuresis. Two recent autopsy studies have confirmed this relationship, discovering that patients with HTN were found to have fewer nephrons.7, 8 Populations with a high incidence of primary HTN have also been found to have smaller kidneys or enlarged glomeruli.9, 10, 11
Kidney volume can be used as a surrogate indicator for nephron number.12, 13, 14 Therefore, measuring renal volume through ultrasonography may serve as an indirect and noninvasive measure of nephron number. Although a number of studies have evaluated the relationship between birth weight and nephron number, research with regard to nephron number, birth weight, and ambulatory blood pressure (BP) in children is lacking. We sought to investigate whether there was an association between kidney volume and ambulatory BP in children with white‐coat HTN, pre‐HTN, and primary HTN. The aim of this study was to determine whether kidney volume could be identified as a risk factor for HTN in children and to evaluate the role of birth weight in this relationship.
2. Material and Methods
Children younger than 21 years followed by the pediatric nephrology division in a single tertiary center were examined in a retrospective chart review. Patients who were evaluated for HTN between the years of 2010 and 2014 were selected for review. Data were retrieved from 24‐hour ambulatory BP monitoring (ABPM) and kidney ultrasounds that were previously performed as part of the patient's clinical care. A renal ultrasound was performed in all patients who were studied by ABPM, as this is the standard practice within this HTN clinic. Only patients whose ultrasound and ambulatory BP studies were completed within a 3‐month period were included in the study. Patients who received a graft kidney or had cystic kidneys, hydronephrosis, a single kidney, dysplasia, agenesis, horseshoe kidney, asymmetrical kidneys, duplex kidneys, and/or pelvic kidneys were excluded from this study since these conditions may distort renal volume. Patients with a known secondary cause of HTN were also excluded. Medical records were abstracted for age, height, weight, body mass index (BMI), birth weight, medical history, and gestational age. For those whose birth weight and gestational age were not available from their medical record, their parents were contacted by phone to obtain this information. Oral consent was obtained before retrieving the patients' birth weight and gestational age from their parents. Overall, 84 children met the inclusion criteria stated above and were included in this study. This study was approved by the institutional review board of Northwell Health.
2.1. BP monitoring
ABPM (Model 90207‐IQ; Spacelabs Medical, Redmond, WA, USA) was used to measure the patients' BP over a 24‐hour time period. BP was automatically recorded every 20 minutes during the day and every hour at night. Each patient was instructed to record the time he or she went to sleep and woke up, and the patients' awake and sleep periods were adjusted accordingly. The ABPM readings were averaged over the 24‐hour time period as well as for the awake and sleep periods. In agreement with the American Heart Association, only studies where 65% to 75% of the readings were valid were considered interpretable.15 Measured variables included the 24‐hour, awake, and sleep systolic and diastolic BPs and standard deviation. Ambulatory BP index was calculated by dividing the BP of the patient by the sex and height‐specific 95th percentiles for systolic and diastolic BP.15 BP loads were determined by calculating the percentage of BP readings that were above the 95th percentile for sex and height. Dipping status was calculated by dividing the difference between the mean awake and mean sleep BP by the awake BP, which was multiplied by a hundred. Patients were categorized as nondippers if their dipping status was <10%. American Heart Association criteria were used to classify patients by ambulatory BP data as hypertensive, pre‐hypertensive, and white‐coat hypertensive.15
2.2. Measurement of renal volume
Two radiologists from the department of radiology (one pediatric radiology board‐certified) independently performed measurements of the length, transverse diameter, and anterior‐posterior diameter of the left and right kidney blinded to BP status. The mean of the two measurements was used to calculate the renal volume for the left and right kidney. The ellipsoid formula was used to calculate renal volume: transverse diameter × anterior‐posterior diameter × length × 0.523.16 The total mean renal volume was found by taking the sum of the mean left kidney volume and the mean right kidney volume. The mean renal volume for each kidney was adjusted for body surface area (BSA) by dividing the renal volume measurement by BSA using the Mosteller formula ((height (cm) × weight (kg)/3600)(1/2)).17 The total renal volume adjusted for BSA was calculated by taking the sum of the mean BSA left kidney volume and the mean BSA right kidney volume. Inter‐rater reliability ranged from 0.996 to 0.999 for each set of measurements for the right and left kidney.
2.3. Statistical analysis
Descriptive statistics were used to characterize outcome measures. Means, standard deviations, and 95% confidence intervals were tabulated and reported for all continuous variables. Frequency counts and percentages were used for categorical variables. Differences in means between the white‐coat hypertensive, pre‐hypertensive, and hypertensive groups were assessed using one‐way analysis of variance, or the Kruskal‐Wallis test for data not normally distributed. Differences in proportions were assessed using chi‐square/Fisher test. Correlations between kidney volume and clinical measures (BP parameters and birth weight) were evaluated by Pearson correlation analysis or Spearman rank correlation for non‐normally distributed data. Associations between kidney volume, BP parameters, and birth weight were examined using linear regression models adjusted for age and sex. Sex‐ and age‐adjusted logistic regression models were used to determine whether kidney volume and birth weight were predictors of BP status. Statistical analyses were performed using the PASW 18 (SPSS Inc, Chicago, IL, USA) statistical package. A two‐tailed P value <.05 was the criterion for statistical significance.
3. Results
Of the 191 children who had a renal ultrasound and ABPM during the years 2010 to 2014, 84 were included in the analysis. There was no significant difference in the demographics of the children who were included and excluded from the study. Of those included in the study, the mean age was 13.87±3.32 years (range 4–20 years) and 61 (72.6%) were male. Twenty‐four (28.6%) children were classified into the white‐coat HTN group, six (7.1%) were in the pre‐HTN group, and 54 (64.3%) were in the HTN group. Demographic and BP data were summarized in Table 1. There were 11 children who were taking antihypertensive medication at the time of the ABPM study. Birth weight could not be obtained from 15 patients and gestational age could not be obtained from 11 patients.
Table 1.
Demographic and BP Data
| White‐Coat HTN | Pre‐HTN | HTN | P Value | |
|---|---|---|---|---|
| No. | 24 | 6 | 54 | |
| Men, No. (%) | 16 (67) | 6 (100) | 39(72) | .26 |
| Age, y | 12.75±4.29 | 15.50±2.07 | 14.19±2.82 | .10 |
| Height, cm | 157.50±22.78 | 178.67±6.71 | 165.17±17.01 | .04 |
| Weight, kg | 64.54±24.38 | 73.50±11.61 | 70.94±24.18 | .49 |
| BMI z score | 1.53±.69 | 0.66±0.80 | 1.05±1.18 | .09 |
| Birth weight, g | 3044±777N=20 | 3172±616N=6 | 3462±2832N=44 | .79 |
| Preterm, No. (%) | 2 (9.1) | 1 (16.7) | 6 (11.1) | .74 |
| 24‐h systolic BP index | 0.91±0.06 | 0.98±0.05 | 1.01±0.08 | <.0001 |
| 24‐h diastolic BP index | 0.84±0.08 | 0.87±0.04 | 0.96±0.12 | <.0001 |
| Awake systolic BP index | 0.85±0.07 | 0.95±0.03 | 0.96±0.07 | <.0001 |
| Awake diastolic BP index | 0.78±0.09 | 0.83±0.09 | 0.88±0.11 | .001 |
| Sleep systolic BP index | 0.89±0.06 | 0.96±0.02 | 1.02±0.09 | <.0001 |
| Sleep diastolic BP index | 0.82±0.09 | 0.87±0.05 | 0.97±0.14 | <.0001 |
| Systolic BP load, % | 5.58±5.58 | 22.45±8.21 | 37.65±24.55 | <.0001 |
| Diastolic BP load, % | 6.78±6.36 | 13.65±9.71 | 28.84±24.21 | <.0001 |
| Systolic nondipper, No. (%) | 18 (75) | 3 (50) | 36 (66.7) | .48 |
| Diastolic nondipper, No. (%) | 7 (29.2) | 3 (50) | 24 (44.4) | .40 |
Abbreviations: BMI, body mass index; BP, blood pressure; HTN, hypertension.
Kidney measurements and volumes are reported in Tables 2 and 3. The total BSA‐adjusted renal volume was not significantly different among the BP groups (P=.36 [Table 3]). Twnety‐four‐hour systolic BP index was found to be positively correlated with the BSA‐adjusted total renal volume (r=0.25, P=.03). Similarly, the BSA‐adjusted total kidney volume was found to be positively and significantly correlated with the mean systolic BP index during the awake (r=0.24, P=.03) and sleep (r=0.23, P=.03) periods. Diastolic BP index and systolic/diastolic BP load were not significantly correlated with renal volume.
Table 2.
BSA‐Adjusted Total Renal Volume Linear Regression Analysis
| BSA‐Adjusted Total Renal Volume | β | 95% CI | P Value |
|---|---|---|---|
| 24‐h systolic index | 108.4 | 9.4–207.4 | .03 |
| Awake systolic index | 96.9 | −6.4–200.2 | .07 |
| Sleep systolic index | 90.7 | 1.17–180.2 | .047 |
| 24‐h systolic BP load | 0.30 | −0.05–0.65 | .10 |
Abbreviations: BP, blood pressure; BSA, body surface area; CI, confidence interval.
Models adjusted for age and sex.
Table 3.
Kidney Volumes
| White‐Coat HTN | Pre‐HTN | HTN | P Value | |
|---|---|---|---|---|
| Mean right kidney volume, cm3 | 125.4±46.7 | 144.2±27.8 | 142.9±57.2 | .39 |
| Mean left kidney volume, cm3 | 126.6±56.1 | 117.2±28.2 | 140.7±52.1 | .38 |
| BSA‐adjusted mean right kidney volume, cm3/m2 | 74.2±15.0 | 75.8±14.3 | 79.0±24.9 | .65 |
| BSA‐adjusted mean left kidney volume, cm3/m2 | 74.3±15.0 | 60.9±14.3 | 78.7±24.9 | .18 |
| Total volume, cm3 | 252.0±94.9 | 261.4±49.4 | 283.6±102.6 | .41 |
| BSA‐adjusted total volume, cm3/m2 | 148.5±30.1 | 136.6±18.8 | 157.74±44.80 | .36 |
Abbreviations: BSA, body surface area; HTN, hypertension.
Using linear regression for further evaluation, BSA‐corrected total renal volume adjusted for age and sex was found to be a significant predictor of both the 24‐hour systolic index and the sleep systolic index (all P<.05 [Table 2]). However, it did not prove to be a significant predictor for the systolic index during the awake period and the 24‐hour systolic BP load (all P>.05 [see Figures 1 and 2]).
Figure 1.
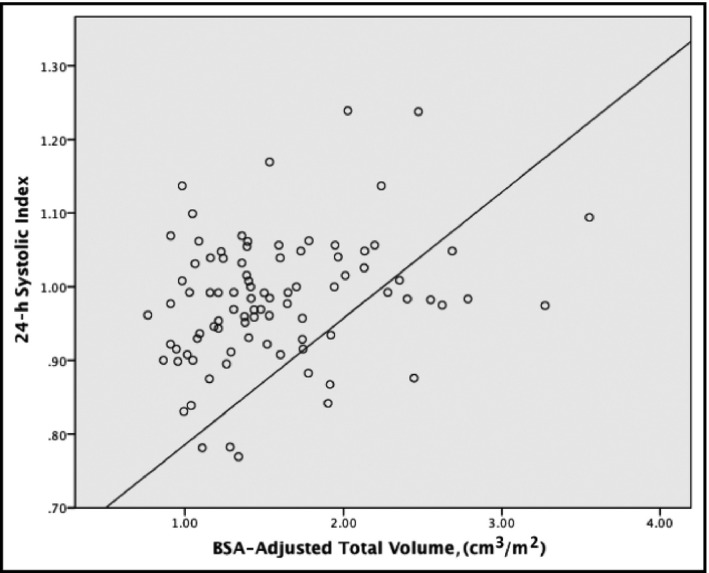
Scatterplot of total renal volume and 24‐hour systolic blood pressure index. BSA indicates body surface area
Figure 2.
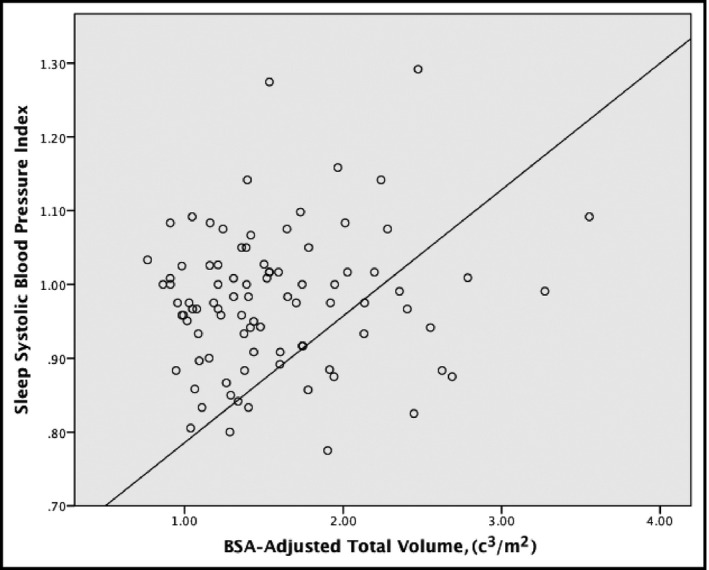
Scatterplot of total renal volume and sleep systolic blood pressure index. BSA indicates body surface area
Birth weight was not found to significantly differ among those who had white‐coat HTN, pre‐HTN, and HTN (P=.79). There was no significant correlation found between birth weight and BP index and BP load parameters (all P>.05). BSA‐adjusted total renal volume was also not correlated with birth weight (r=−.026, P=.83).
Among the 84 children we examined, logistic regression was used to ascertain whether BSA total renal volume could serve as a predictor for white‐coat HTN, pre‐HTN, and HTN, which was not the case (all P<.05). Similarly, birth weight was not found to be a predictor of white‐coat HTN, pre‐HTN, or HTN (all P<.05).
4. Discussion
Contrary to expectation, greater BSA‐adjusted total renal volume was found to be significantly associated with higher systolic BP indices for the 24‐hour, awake, and sleep periods. BSA‐adjusted total renal volume was found to be positively and significantly correlated with systolic BP load as well. Children who were diagnosed with HTN had the largest renal volume compared with those who had pre‐HTN and white‐coat HTN, although differences in renal volume among all three groups were not significant. BSA‐adjusted total renal volume failed to serve as a predictor of white‐coat HTN, pre‐HTN, or HTN in this study.
Previous literature has found an inverse relationship between glomerular number and BP, where hypertensive patients were found to have fewer glomeruli than their normotensive counterparts.7, 8 It has been proposed that adults with lower birth weights have fewer nephrons, which may lead to primary HTN later in life. In this study, we sought to determine whether a similar relationship could be detected in children. Previous studies have examined the relationship among renal volume and ambulatory BP parameters in children with autosomal recessive polycystic kidney disease.18 However, to our knowledge, the association between these factors has not been described in children without polycystic kidneys. We have also investigated how birth weight affects renal volume and HTN in children. As previously mentioned, Brenner and colleagues5, 6 proposed an inverse relationship between nephron number and HTN due to the inability of the kidneys to maintain proper pressure natriuresis. Therefore, we hypothesized that children who had HTN would have smaller kidneys compared with those who had white‐coat HTN and pre‐HTN.
Other studies also reported contrary findings where an association between renal volume and BP was not detected in children and adults. Bakker and colleagues19 did not detect an association between renal volume and BP in their cohort study of 6397 children. Similarly, Laganović and colleagues20 showed that the relationship between BP and birth weight was not mediated by renal volume among their patients.
Alternatively, kidney volume may not be a reliable marker for nephron number, and a more sensitive approach is needed. Currently, only invasive measures based on histology and pathology are used to determine nephron number in humans. Autopsy studies have also confirmed this inverse relationship where patients with documented primary HTN were found to have fewer and larger glomeruli per kidney than normotensive controls.7, 8 Given the increased risk of cardiovascular and renal disease in patients with fewer nephrons, a noninvasive measure is needed. Therefore, further investigation to elucidate these findings is warranted.
Lower birth weight has been shown to be a risk factor for HTN and cardiovascular disease in adulthood.21, 22, 23 In particular, this relationship has been strongly exhibited in those who were born small‐for‐gestational age (SGA).21, 24 However, our findings did not demonstrate a negative relationship between BP indices and birth weight. Low birth weight was not found to be a predictor of HTN, pre‐HTN, or white‐coat HTN. It may be too early to determine whether low birth weight has a significant effect on BP in children. As Moore and colleagues25 describe, low birth weight has a greater impact on BP with increasing age. Additionally, in a study by Doyle and colleagues,26 children with very low birth weights (<1500 g) were found to have higher ambulatory systolic BP than children with normal birth weight.26 Therefore, higher BPs may only be detected in very low birth weight cohorts.
In addition, Johansson and colleagues24 reported that SGA was significantly associated with a higher risk of elevated systolic BP in men. However, this relationship was only found to be true in men who had a gestational age >32 weeks.24 However, whether these differences are due to in utero growth restriction or prematurity are unclear. Due to the lack of available data, this relationship could not be thoroughly examined in our study.
5. Study Limitations and Strengths
The main limitation of this study is its single‐center, retrospective design, which may introduce selection bias. Other limitations include its sample size and the lack of available data on SGA children and on children who have low birth weights. Of the 84 children studied, only six met the World Health Organization's definition of low birth weight (<2500 g).27 Previous literature has found the inverse relationship between birth weight and nephron number to be true in low birth weight cohorts. Singh and Hoy28 found that renal volume adjusted for sex, age, and BSA was inversely correlated with systolic BP in Australian Aboriginal children and adults. A 10‐mL increase in kidney volume was associated with a 1.1‐mm Hg decrease in systolic BP in children.28 However, it is important to note that the Australian Aborigines have high rates of low birth weight and, of the individuals studied, 34.4% of the adults and 20.4% of the children had a birth weight <2.5 kg.28 Therefore, our findings may imply that a stronger association between renal volume and BP may be detected in children with a birth weight <2500 g and it may be useful to investigate this relationship in a low birth weight cohort.
Another limitation is the usage of renal volume as a surrogate marker for nephron mass. However, due to the lack of noninvasive methodologies to count nephron number, renal volume was used. A strength of this study is the usage of ABPM to determine the association between renal volume and BP in children. ABPM allows for the continuous monitoring of an individual's BP throughout the day, outside of the physician's office, and allows for the determination of BP parameters including BP load and dipping status.
6. Conclusions
Nephron endowment arises from a complex interplay between one's genetic and environmental background.29 Fewer nephrons have been shown to increase susceptibility to cardiovascular and renal disease. Brenner and colleagues5 proposed that fewer nephrons may lead to HTN in adulthood. This study sought to determine whether renal volume could be identified as a risk factor in children. Contrary to our expectation, total BSA‐adjusted renal volume was found to be positively and significantly correlated with systolic BP indices during awake, sleep, and 24‐hour time periods as well as with systolic BP load. Additionally, renal volume was not found to be a predictor of HTN in children. However, the single‐center, retrospective nature of this study did not allow us to extensively investigate the relationship between renal volume and ambulatory BP, as we did not obtain sufficient data to do so. Therefore, further investigation to elucidate these findings is warranted since oligonephropathy has been shown to increase susceptibility to cardiovascular and renal disease.
Conflicts of Interest
No conflicts of interest to disclose.
Gurusinghe S, Palvanov A, Bittman ME, et al. Kidney volume and ambulatory blood pressure in children. J Clin Hypertens. 2017;19:498–503. 10.1111/jch.12954
References
- 1. Haycock G. Development of glomerular filtration and tubular sodium reabsorption in the human fetus and newborn. BJU Int. 1998;81:33–38. [DOI] [PubMed] [Google Scholar]
- 2. Bertram JF, Douglas‐Denton RN, Diouf B, et al. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26:1529–1533. [DOI] [PubMed] [Google Scholar]
- 3. Hughson M, Farris AB 3rd, Douglas‐Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63:2113–2122. [DOI] [PubMed] [Google Scholar]
- 4. Manalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58:770–773. [DOI] [PubMed] [Google Scholar]
- 5. Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure: less of one, more the other? Am J Hypertens. 1988;1:335–347. [DOI] [PubMed] [Google Scholar]
- 6. Brenner BM, Mackenzie HS. Nephron mass as a risk factor for progression of renal disease. Kidney Int Suppl. 1997;63:S124–S127. [PubMed] [Google Scholar]
- 7. Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. [DOI] [PubMed] [Google Scholar]
- 8. Samuel T, Hoy WE, Douglas‐Denton R, Hughson MD, Bertram R. Determinants of glomerular volume in different cortical zones of the kidney. J Am Soc Nephrol. 2005;16:3102–3109. [DOI] [PubMed] [Google Scholar]
- 9. Kikkawa R, Araki S, Haneda M, Kajiwara N, Hidaka H, Shigeta Y. Hypertension and development of complications in patients with non‐insulin dependent diabetes mellitus in Japan. J Am Soc Nephrol. 1992;3(Suppl 4):S120–S125. [DOI] [PubMed] [Google Scholar]
- 10. Weder AB, Schork NJ. Adaptation, allometry and hypertension. Hypertension. 1994;24:145–156. [DOI] [PubMed] [Google Scholar]
- 11. Hoy WE, Hughson MD, Singh GR, Douglas‐Denton R, Bertram JF. Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int. 2006;70:104–110. [DOI] [PubMed] [Google Scholar]
- 12. Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney and body weight in normal man. Anat Rec. 1992;232:194–268. [DOI] [PubMed] [Google Scholar]
- 13. Abitbol CL, Ingelfinger JR. Nephron mass and cardiovascular and renal disease risks. Semin Nephrol. 2009;29:445–454. [DOI] [PubMed] [Google Scholar]
- 14. Silver LE, Decamps PJ, Korst LM, Platt LD, Castro L. Intrauterine growth restriction is accompanied by decreased renal volume in the human fetus. Am J Obstet Gynecol. 2003;188:1320–1325. [DOI] [PubMed] [Google Scholar]
- 15. Flynn JT, Daniels SR, Hayman LL, et al. American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63:1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dinkel E, Ertel M, Dittrich M, Peters H, Berres M, Schulte Wissermann H. Kidney size in childhood. Sonographical growth charts for kidney length and volume. Pediatr Radiol. 1985;15:38–43. [DOI] [PubMed] [Google Scholar]
- 17. Mosteller RD. Simplified calculation of body‐surface area. N Engl J Med. 1987;317:1098. [DOI] [PubMed] [Google Scholar]
- 18. Seeman T, Dusek J, Vondrichova H, et al. Ambulatory blood pressure correlates with renal volume and number of renal cysts in children with autosomal dominant polycystic kidney disease. Blood Press Monit. 2003;8:107–110. [DOI] [PubMed] [Google Scholar]
- 19. Bakker H1, Kooijman MN, van der Heijden AJ, et al. Kidney size and function in a multi‐ethnic population‐based cohort of school‐age children. Pediatr Nephrol. 2014;9:1589–1598. [DOI] [PubMed] [Google Scholar]
- 20. Laganović M, Kuzmanić D, Zeljković‐Vrkić T, Pećin I, Dika Z, Jelaković B. Kidney volume and albuminuria as markers of birth weight‐blood pressure relationship in essential hypertension. Kidney Blood Press Res. 2009;32:399–404. [DOI] [PubMed] [Google Scholar]
- 21. Jarvelin MR, Sovio U, King V, et al. Early life factors and blood pressure at age 31 years in the 1966 northern Finland birth cohort. Hypertension. 2004;44:838–846. [DOI] [PubMed] [Google Scholar]
- 22. Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14:935–941. [PubMed] [Google Scholar]
- 23. Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch‐up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–883. [DOI] [PubMed] [Google Scholar]
- 24. Johansson S, Iliadou A, Bergvall N, Tuvemo T, Norman M, Cnattingius S. Risk of high blood pressure among young men increases with degree of immaturity at birth. Circulation. 2005;112:3430–3436. [DOI] [PubMed] [Google Scholar]
- 25. Moore VM, Cockington RA, Ryan P, Robinson JS. The relationship between birth weight and blood pressure amplifies from childhood to adulthood. J Hypertens. 1999;17:883–888. [DOI] [PubMed] [Google Scholar]
- 26. Doyle LW, Faber B, Callanan C, Morley R. Blood pressure in late adolescence and very low birth weight. Pediatrics. 2003;111:252–257. [DOI] [PubMed] [Google Scholar]
- 27. UNICEF , WHO . Low birthweight: country, regional and global estimates. Geneva: United Nations Children's Fund and World Health Organization; 2004. [Google Scholar]
- 28. Singh GR, Hoy WE. Kidney volume, blood pressure, and albuminuria: findings in an Australian aboriginal community. Am J Kidney Dis. 2004;43:254–259. [DOI] [PubMed] [Google Scholar]
- 29. Charlton JR, Springsteen CH, Carmody JB. Nephron number and its determinants in early life: a primer. Pediatr Nephrol. 2014;29:2299–2308. [DOI] [PubMed] [Google Scholar]


