Abstract
Primary aldosteronism (PA) is a common form of secondary hypertension. Several guidelines recommend that patients with adrenal incidentaloma have a high probability of suffering from PA. We conducted a prospective study of 269 consecutive adults with adrenal incidentaloma to investigate the prevalence and clinical characteristics of PA. In total, 9 participants were detected with PA, suggesting a prevalence of 3.35% among the study population. PA participants had a higher blood pressure level by 14/20.8 mm Hg and a lower serum potassium level by 0.8 mmol/L (P < .05). Importantly, all patients with PA presented with concurrent indications (hypertension with or without hypokalemia) for screening of the disease, but they have not undergone relative screening by the referring physician, thus casting doubts about the appropriate implementation of current guidelines in real‐life practice. Intense efforts are needed to familiarize physicians with recommendations for PA to minimize undiagnosed cases and the detrimental sequelae of this endocrine form of hypertension.
Keywords: adrenal incidentaloma, hypertension, hypokalemia, primary aldosteronism, resistant hypertension
1. INTRODUCTION
Adrenal incidentaloma is an adrenal lesion identified at an imaging examination that was performed because of an extra‐adrenal disorder.1, 2, 3 The prevalence of adrenal incidentalomas (adrenal lesions ≥ 1 cm) varied between 1.9%‐10%, with an estimated prevalence of 3% in middle‐aged individuals that increased with age, reaching approximately 10% in the elderly.2, 3, 4, 5, 6, 7, 8, 9, 10 In the case of adrenal incidentaloma, the discrimination of a benign from a malignant adrenal mass is of crucial clinical importance. Fortunately, the vast majority of adrenal incidentalomas are nonfunctional benign tumors. Specifically, a benign mass was found in more than 80% of cases, whereas 3 out of 4 patients had a nonfunctional tumor.2, 3 In the rest of the cases with a hormone‐producing adrenal lesion, patients were more likely to suffer from Cushing syndrome, whereas pheochromocytoma and primary aldosteronism were more rarely detected.
Primary aldosteronism is the most common cause of secondary hypertension.11, 12 The Endocrine Society suggests that primary aldosteronism affects 6.1% of the hypertensive population, whereas it becomes more prevalent in the settings of moderate, severe, and resistant hypertension.13, 14, 15 Patients with primary aldosteronism have a higher cardiovascular risk and greater target organ damage compared with essential hypertensive patients with equal blood pressure levels.16, 17 Cause‐specific treatment (surgical or pharmacological) results in blood pressure reduction, reversal of target organ damage, and normalization of potassium levels.13, 18, 19, 20, 21, 22 Altogether, the aforementioned data emphasize the need for an early detection of patients with this endocrine form of hypertension.23
The Endocrine Society recommends the presence of adrenal incidentaloma as an independent cause that augments the clinical suspicion of primary aldosteronism, along with other conditions.13 In the recently published American Heart Association guidelines for the management of hypertension, it is stated that adrenal incidentaloma is a screening cause of primary aldosteronism among hypertensivepatients.24 Moreover, guidelines for the management of patients with adrenal incidentaloma note that there is no need for screening for primary aldosteronism, except if concurrent hypertension and/or hypokalemia are presented.2, 3 However, accumulating data suggest that primary aldosteronism has been uncommonly identified among individuals with adrenal incidentaloma, but these data derive mainly from retrospective surveys, most of them in Asian populations.9, 25, 26, 27, 28, 29, 30, 31, 32
The aim of the current study is to prospectively investigate the prevalence of primary aldosteronism among European patients who were referred to our center because of an adrenal incidentaloma, as well as to evaluate the clinical characteristics of such patients compared with participants with adrenal incidentaloma free of the disease.
2. METHODS
We conducted a prospective, observational, single‐center study of consecutive adults with an adrenal incidentaloma who were referred to the Hypertension Center of the Second Propedeutic Department of Internal Medicine, Thessaloniki, Greece for further evaluation, between January 2013 and August 2017. All the eligible patients gave written informed consent prior to inclusion in the trial. The current study was conducted in accordance with the principles of the Helsinki declaration, and the procedures used were in agreement with institutional guidelines and were approved by the institutional review board.
Patients were eligible for inclusion in the study if they were adults with an adrenal incidentaloma. Exclusion criteria were any type of documented malignancy and radiological indication of a malignant adrenal tumor and/or adrenal angiomyolipoma, because the management of these conditions differs significantly.2
On the screening visit, participant demographic and anthropometric characteristics, medical history, and medication were recorded; a thorough clinical examination was performed; and a sodium‐free diet was advised. Oral potassium supplementation was prescribed if needed. For participants who were on any antihypertensive agent other than a calcium channel blocker or an α‐adrenergic blocker, the treatment was substituted with either or both the aforementioned drug classes for at least 2 weeks or 6 weeks in the case of patients receiving a mineralocorticoid receptor antagonist. On the main visit, participants came to our center in the morning (07:00 am) after night sleeping (coffee and smoking were not allowed). Office blood pressure level was calculated as the average of 3 consecutive measurements with the patient in the seated position in rest for 5 minutes, using a mercury sphygmomanometer and an appropriate branch cuff, according to the guidelines.24 Blood samples from a peripheral vein were obtained (without barking) for the estimation of several biochemical parameters. Consequently, all participants underwent hormonal evaluation and suppression test with intravenous saline infusion. Furthermore, participants underwent the 1 mg dexamethasone suppression test and evaluation of other cortical adrenal hormones (dehydroepiandrosterone, catecholamine) according to clinical, laboratory, and radiological findings, but these data are not be discussed in the current study.
After 2 hours with the study participants in the supine position in a quiet room under steady conditions, plasma renin activity (PRA) and plasma aldosterone concentration (PAC) were assessed through peripheral venous sampling (9:30 am). The PAC to PRA ratio (aldosterone‐to‐renin ratio [ARR]) was calculated for each individual. Afterwards, all patients were administered an intravenous infusion of 2 L saline 0.9% while remaining in the supine position for 4 hours. PAC was measured via blood sampling from a peripheral vein, at the end of the intravenous saline suppression test (PACsuppressed). The diagnosis of primary aldosteronism was based on a PAC ≥ 6 ng/dL after the IV saline test and was later confirmed by the response to mineralocorticoid receptor antagonists on monotherapy or with minimal additional therapy.
Traditionally, ARR is used as the screening test for primary aldosteronism, and confirmatory testing is performed in patients with ARR >30 and PAC >15 ng/dL. For the purpose of this study, however, we performed confirmatory testing in all participants in order to overcome the false negative results of ARR and strengthen the validity of our study findings.
Potassium, creatinine, glucose, and lipids were measured from blood samples using fully automated instrumentation. Both PAC and PRA were measured with commercial radioimmunoassay kits (PRA: Angiotensin I RIAKit, with intra‐ and interassay coefficients of variability of up to 11.3% and 20.9%, respectively; PAC: Aldosterone RIA 100T, with intra‐ and interassay coefficient of variability of up to 7.7% and 8.4%, respectively) at the certificated radioimmunology laboratory of our department. Detection limits of the methods were below 1 ng/dL for PAC and 0.2 ng/mL/h for PRA. All blood samples for PRA calculation were collected in chilled anticoagulated glass tubes, immediately stored inice‐filled containers, and underwent refrigerated centrifuge.
Statistical analysis was performed with the Statistical Package for the Social Sciences version 25 for Windows. Results are expressed for continuous variables with a normal distribution as mean (SD), with nonnormal distributions as median (interquartile range [IQR]). Qualitative variables are expressed as frequencies. Kolmogorov‐Smirnov algorithm was performed to estimate whether a variable had a normal distribution. Independent student t test or Mann‐Whitney U test was used to estimate differences between mean values of 2 independent groups. The comparison for repeated measurements of the same variable were performed with paired student's t test (between 2 time points) or 1‐way analysis of variance (ANOVA) (between 3 or more time points) as parametric alternatives, and the Wilcoxon signed‐rank test (between 2 time points) or the Friedman test (between 3 or more timepoints), Pearson chi‐square test with the Yates correction where appropriate was used for comparison of frequencies of nonparametric alternatives (continuity correction was used to improve the fit to the exact probability). A P value of less than .05 was considered statistically significant.
3. RESULTS
In total, 269 consecutive patients (122 male) with adrenal incidentaloma were included in the study. The characteristics of patients with and without primary aldosteronism as well as of the total sample are summarized in Table 1. All of them were Caucasians with a median age of 65 years and 23.2% were smokers. The mean systolic and median diastolic blood pressure levels for all study participants were 138.7 ± 16.5 and 80 (72‐86) mm Hg, respectively, whereas 51.3% of the study sample had normal blood pressure levels. Study participants had a median serum potassium level of 4.3 (4.0‐4.6) mg/dL, and eGFR of 83.3 (67.3‐98.6) mL/min/1.73 m2. Radiological characteristics of adrenal incidentaloma are shown for the total sample, as well as for each subgroup separately in Table 2. Cholecystitis and abdominal pain (diffuse or localized) were the main cause (79%) of imaging evaluation that led to the identification of adrenal incidentaloma. Adrenal incidentalomas had a mean size of 1.8 ± 0.8 cm and were located on the left gland in 139 individuals and was unilateral in more than 81% of participants (n = 219), whereas hypertrophy of the contralateral gland was observed in 41 patients and a contralateral adrenal mass in 9 individuals.
Table 1.
Clinical, biochemical, and hormonal characteristics of participants
| Characteristics | Total sample (n = 269) | Primary aldosteronism (n = 9) | Without primary aldosteronism (n = 260) |
|---|---|---|---|
| Sex (males) | 45% | 44.4% | 45% |
| Age (y) | 65 (56.5‐71.0) | 58.8 ± 10 | 65 (57‐71) |
| BMI (kg/m2) | 30.1 (26.5‐33.3) | 31.1 ± 5.9 | 30.1 (26.4‐33.0) |
| Waist Circumference (cm) | 108 (100‐115) | 110 ± 12.5 | 108 (100‐115) |
| Smoking | 23.2% | 11.1% | 23.5% |
| Hypertensiona | 48.7% | 100% | 46.9% |
| SBP (mm Hg)b | 138.7 ± 16.5 | 152.7 ± 11.2 | 138.2 ± 16.5 |
| DBP (mm Hg)a | 80 (72‐86) | 100.8 ± 5.2 | 78.9 ± 9.8 |
| HR (bytes/min) | 74 (64‐82) | 72.8 ± 10.4 | 75 (67‐82) |
| eGFR (mL/min/1.73 m2) | 83.3 (67.3‐98.6) | 71.1 (49.7‐91.9) | 83.6 (67.4‐98.8) |
| Potassium (mmol/L)a | 4.3 (4.0‐4.6) | 3.6 ± 0.5 | 4.3 (4.0‐4.7) |
| Sodium (mmol/L)a | 142.5 ± 7.8 | 145.0 ± 1.4 | 142.5 ± 7.8 |
| Glucose (mg/dL) | 145 (123‐183.5) | 139 (130‐235) | 145 (122.5‐182.0) |
| Total cholesterol (mg/dL) | 185.4 ± 43.9 | 189 ± 35.7 | 185.3 ± 44.3 |
| Triglycerides (mg/dL) | 132 (101‐180) | 161 ± 62.5 | 132 (101‐179) |
| LDL (mg/dL) | 100 (78‐125) | 98.4 ± 25.4 | 105.3 ± 36.3 |
| HDL (mg/dL) | 49 (42‐58) | 54.4 ± 11.9 | 49 (42‐58) |
| PAC (ng/dL)a | 7.0 (5.2‐9.3) | 46 ± 24.6 | 6.9 (5.2‐9.0) |
| PRA (ng/mL/h)a | 0.7 (0.4‐1.1) | 0.3 ± 0.1 | 0.8 (0.4‐1.2) |
| ARRa | 10.3 (5.7‐18.1) | 176 ± 83.6 | 10.3 (5.5‐17.2) |
| PACsuppressed (ng/dL)a | 3.5 (2.8‐4.2) | 42.1 ± 27.8 | 3.4 (2.8‐4.1) |
ARR: aldosterone to plasma renin activity ratio; BMI: body mass index; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; HDL: high‐density lipoprotein; HR: Heart rate; LDL: low‐density lipoprotein; PAC: plasma aldosterone concentration; PACsuppressed: plasma aldosterone concentration after saline infusion test; PRA: plasma renin activity; SBP: systolic blood pressure.
P < .001 for comparison between patients with and without primary aldosteronism.;
P < .05 for comparison between patients with and without primary aldosteronism. Values are expressed as mean ± SD or median (interquartile range).
Table 2.
Radiologic findings of adrenal incidentaloma
| Characteristics | Total sample (n = 269) | Primary aldosteronism (n = 9) | Without primary aldosteronism (n = 260) |
|---|---|---|---|
| Size (cm) | 1.6 (1.2‐1.9) | 1.6 ± 0.5 | 1.6 (1.2‐1.9) |
| Localization | |||
| Unilaterala | 81.3% | 33.3% | 83% |
| Left adrenal gland | 41.6% | 22.2% | 42.3% |
| Contralateral adrenal gland lesiona | 18.5% | 66.7% | 16.9% |
| Contralateral hyperplasia | 15.2% | 55.6% | 13.8% |
| Contralateral mass | 3.3% | 11.1% | 3.1% |
P < .05 for comparison between patients with and without primary aldosteronism. Values in parenthesis are mean (±SD) or median (±IQR).
Overall, primary aldosteronism was identified in 9 individuals (4 male), equaling a prevalence of 3.35% among white adults with adrenal incidentaloma. Participants with primary aldosteronism had a mean age of 58.8 years, body mass index of 31.1 ± 5.9 kg/m2, and serum potassium level of 3.6 ± 0.5 mmol/L. All patients with primary aldosteronism suffered from hypertension with a mean blood pressure level of 152.7/100.8 mm Hg. The mean size of the adrenal masses was 1.6 ± 0.5 cm and most of them (n = 5) were found with hyperplasia of the contralateral adrenal gland. A unilateral adrenal mass was identified in 3 participants with primary aldosteronism (2 in the left gland) and 1 patient had a contralateral tumor as well. In individuals with this endocrine form of hypertension PAC and PRA were 46 ng/dL and 0.3 ng/mL/h, respectively. PACsuppressed was 42.1 ng/dL, featuring a reduction of 3.9 ng/dL in PAC after the saline infusion test (P = NS).
Compared with participants free of the disease, patients with primary aldosteronism were more likely to be hypertensive (P < .001), had higher systolic and diastolic blood pressure levels by 14 and 20.8 mm Hg (P < .05 and P < .001, respectively), and lower serum potassium levels by 0.8 mg/dL (P < .05). Furthermore, stage 2 hypertension (blood pressure ≥ 160/100 mm Hg) was more prevalent in patients with primary aldosteronism (66.7% vs 13.1%; P < .001). The between‐group differences in the rest of the characteristics did not reach statistical significance (Table 1). Both groups had equal adrenal incidentaloma size (1.6 vs 1.6 cm; P = .32). However, participants without primary aldosteronism were more likely to be found with a unilateral lesion (83% vs 33%), whereas patients with primary aldosteronism were more likely to be detected with a lesion in the contralateral adrenal gland as well (P < .05; 5 out of 9 had contralateral hypertrophy and 1 a contralateral mass). In total, 6 patients were diagnosed with bilateral hyperplasia and 3 patients with unilateral adenoma.
As expected, the hormonal evaluation revealed that patients with primary aldosteronism had a higher PAC and PACsuppressed by 39 and 38.6 ng/dL, respectively, more suppressed PRA by 0.4 ng/mL/h, and a substantially elevated ARR compared with participants without primary aldosteronism (P < .001 for all comparisons). The latter group exhibited a more pronounced suppression in PAC level after the saline infusion test (approximately 51% reduction; P < .001) compared with participants with primary aldosteronism (approximately 11%; reduction; P = .1). All PAC values in both subgroups are summarized in Figure 1.
Figure 1.
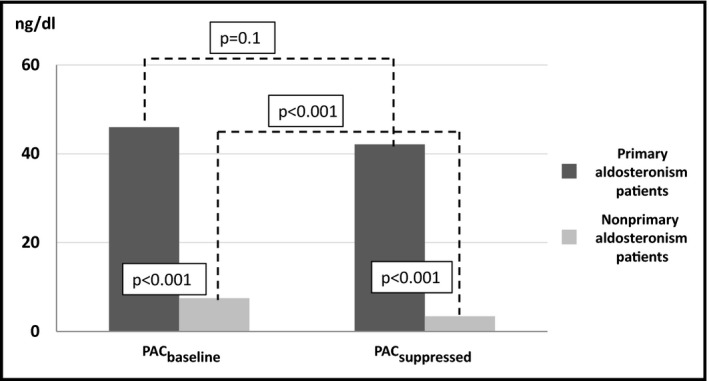
Plasma aldosterone concentration of patients with or without primary aldosteronism before and after the saline infusion test
Comparison between patients with primary aldosteronism and patients with hypertension but free of the disease showed that patients with primary aldosteronism had similar systolic blood pressure levels with disease‐free patients (152.7 ± 16.5 vs 151.9 ± 10.2 mm Hg respectively; P = .8). On the contrary, diastolic blood pressure levels were significantly higher in patients with the disease compared with hypertensive patients free of the disease (100.8 ± 5,2 vs 82.2 ± 9.9 mm Hg, respectively; P < .001). Potassium levels and PRA were lower in patients with primary aldosteronism compared with disease‐free hypertensive patients [potassium levels: 3.6 ± 0.5 vs 4.4 ± 0.5 mg/dL, P < .001; PRA: 0.3 ± 0.1 vs 0.7 (0.4‐1.2) ng/mL/h, P < .05, respectively]. In addition, primary aldosteronism patients had substantially greater PAC and PACsuppressed values (PAC: 46 vs 7.2 ng/dL, P < .001; PACsuppressed: 42.1 vs 3.6 ng/dL, P < .001, respectively).
4. DISCUSSION
Our study is the first prospective clinical trial that has evaluated the prevalence and clinical characteristics of primary aldosteronism among patients with adrenal incidentaloma in a European population. In a large sample of 269 consecutive adults with adrenal incidentaloma, we found 9 cases of primary aldosteronism, suggesting a prevalence of 3.35% among this group of patients. Of great interest, all 9 participants detected with primary aldosteronism had an additional clinical or laboratory finding that raised the clinical suspicion of the disease. Specifically, 4 participants had concurrent hypokalemia along with hypertension, 4 presented with resistant hypertension (uncontrolled hypertension despite of the use of at least 3 different antihypertensive agents including a diuretic), whereas 1 suffered from both hypokalemia and resistant hypertension. Therefore, our study has 2 important findings.
First, all patients diagnosed with primary aldosteronism in our sample should have been screened for the disease because of strong suspicion prior to the incidentally discovered adrenal mass; thus, our findings highlight the necessity of improved knowledge about primary aldosteronism by primary care physicians for the better implementation of guideline recommendations about primary aldosteronism. Indeed, a recent survey of 500 general practitioners in Italy and Germany (2 wealthy European countries with good healthcare systems) revealed that only half of general practitioners claimed to be aware of the guidelines, and 19%‐36% of them did not have even 1 patient with primary aldosteronism; therefore, the prevalence of aldosteronism was very low (1%‐2%), which is not surprising because even the simplest test (serum potassium) was measured in only half of hypertensive patients.33 Second, there were no cases of primary aldosteronism without a concurrent clinical or laboratory sign of primary aldosteronism, providing further credence on recent guidelines that recommend screening for primary aldosteronism in patients with adrenal incidentaloma only in the case of concurrent hypertension and/or hypokalemia.2, 3, 13, 24
Our study presents a similar, but slightly higher prevalence of primary aldosteronism between patients with adrenal incidentaloma than the median of 2.0% by the International Endocrine Society13 and of 2.5% by the European Society of Endocrinology reports.3 However, several observations might explain this discrepancy. First, we excluded patients with radiological indications of adrenal angiomyolipoma and carcinoma. Second, ours is the only study that evaluated with a confirmatory test all participants, minimizing the risk of false exclusion of a primary aldosteronism diagnosis. Third, patients with potassium levels < 3.5 mmol/L were not included in 4 studies referenced by the European Society of Endocrinology.9, 29, 31, 34 Finally, several studies either had not documented the diagnosis of primary aldosteronism with a confirmatory test30, 32, 35 or partially used the postural stimulation test that has questionable utility for the confirmation of the disease.25, 27, 28
In the present sample, all patients with primary aldosteronism had concurrent hypertension, which is in accordance with the findings by Vierhapper.36 In this study, 100 normotensive and 169 hypertensive individuals with adrenal incidentaloma underwent PAC, PRA, and ARR evaluation and if needed (PAC > 15 ng/dL and ARR > 50) underwent oral sodium loading test to determine the prevalence of primary aldosteronism. None of the normotensive patients were detected with the disease, whereas all 6 patients with primary aldosteronism had concurrent hypertension. In another study of 125 normokalemic patients with adrenal incidentaloma (90 with arterial hypertension), it was found that all 5 patients with primary aldosteronism were hypertensives.34 Another study that was conducted among normokalemic individuals with adrenal incidentaloma observed that all 16 patients with primary aldosteronism had high blood pressure as well.9 These findings were confirmed in a Korean study, in which 8 out of 79 participants who were detected with primary aldosteronism were hypertensives.28 Only in a retrospective study of 150 Japanese with adrenal incidentaloma, it was stated that 2 out of 16 patients with primary aldosteronism were normotensives; but it is not clarified whether such a diagnosis was based on a positive postural stimulation test.27
We found a mean potassium level of 3.6 mmol/L for the primary aldosteronism subgroup, with 4 individuals expressing hypokalemia. These outcomes are in accordance with data on primary aldosteronism that suggest normokalemia as common as 60% among patients with primary aldosteronism.37 We found a statistically significant lower potassium level of 0.8 mmol/L among participants with primary aldosteronism. Three previously published studies have described the serum potassium status among patients with primary aldosteronism and adrenal incidentaloma. Tobuchi and colleagues27 found serum potassium levels of 3.8 ± 0.5 mmol/L in patients with primary aldosteronism, but insignificant difference was observed in comparison with the rest of patients with adrenal incidentaloma. Mantero and colleagues reported that 60% of such patients had serum potassium levels between 3.5 and 3.8 mmol/L (no patients with hypokalemia included),9 whereas Vierhapper found that 4 out of 5 patients with adrenal incidentaloma and primary aldosteronism had hypokalemia.36
Moreover, we found that patients with primary aldosteronism were more likely to present a lesion in the contralateral gland than participants without this disorder. Accumulating data suggest that adrenal incidentalomas are unilateral in more than 80% of cases,2, 3, 38 similar to our results. Regarding the lateralization of adrenal incidentalomas among individuals with primary aldosteronism, existing data are quite restricted. All of patients with primary aldosteronism had a unilateral adrenal mass in both a Korean (n = 8) and a Swedish (n = 9) study.28, 32 Similarly, in a Chinese population of 1941 individuals with adrenal incidentaloma, primary aldosteronism was detected in 78 participants.25 Patients with primary aldosteronism showed a tendency to suffer from unilateral adrenal lesions compared with bilateral abnormalities (4.3% vs 3.6%; P = .686).
The prospective design and the relatively large sample of study population appear as significant strengths of our study. More important, we used an appropriate algorithm for the diagnosis of primary aldosteronism, following all the recommendations for the preparation and conduction of tests and evaluation of hormonal outcomes. Moreover, the diagnosis of primary aldosteronism was further confirmed by the response to mineralocorticoid receptor antagonists. Finally, we performed a confirmation test in all study participants, overcoming the possibility of false negative screening with ARR. In contrast, the small sample of participants with primary aldosteronism restricts the generalization of study findings. However, this limitation is due to the low prevalence of primary aldosteronism among patients with adrenal incidentalomas.
5. CONCLUSION
Primary aldosteronism is relatively uncommon among patients with adrenal incidentaloma. Our study suggests a prevalence of 3.35% for primary aldosteronism among such patients. A closer look at our data reveals all individuals with primary aldosteronism presented with a concurrent sign (clinical or laboratory) mandating screening for the disease (hypertension with or without hypokalemia). Therefore, our findings highlight the poor implementation of guideline recommendations in real‐life practice. Not all patients diagnosed with primary aldosteronism were screened for the disease by the referring physician, despite the clear‐cut indications for such screening. Intense efforts are needed to familiarize primary care physicians with current recommendations for primary aldosteronism in order to minimize undiagnosed cases and the detrimental sequelae of primary aldosteronism.
On the other hand, our findings support current guideline recommendations that patients with adrenal incidentaloma should be investigated for primary aldosteronism only in the case of concurrent hypertension and/or hypokalemia. Given that the diagnosis of primary aldosteronism is time consuming, costly, and tricky and necessitates specialized clinicians, laboratories, and centers, a more appropriate selection of candidates for screening should be preferred. Moreover, the high prevalence of adrenal incidentalomas combined with the wider use of radiologic modalities render our findings clinically meaningful, because of the ever‐increasing absolute number of individuals with incidentally discovered adrenal masses.
CONFLICT OF INTEREST
None to declare.
Stavropoulos K, Imprialos KP, Katsiki N, et al. Primary aldosteronism in patients with adrenal incidentaloma: Is screening appropriate for everyone?. J Clin Hypertens. 2018;20:942–948. 10.1111/jch.13291
REFERENCES
- 1. Young JF Jr. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:601‐610. [DOI] [PubMed] [Google Scholar]
- 2. Terzolo M, Stigliano A, Chiodini I, et al. AME position statement on adrenal incidentaloma. Eur J Endocrinol. 2011;164:851‐870. [DOI] [PubMed] [Google Scholar]
- 3. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175:G1‐G34. [DOI] [PubMed] [Google Scholar]
- 4. Barzon L, Sonino N, Fallo F, et al. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149:273‐285. [DOI] [PubMed] [Google Scholar]
- 5. Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann Intern Med. 2003;138:424‐429. [DOI] [PubMed] [Google Scholar]
- 6. Cawood TJ, Hunt PJ, O'Shea D, et al. Recommended evaluation of adrenal incidentalomas is costly, has high false‐positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur J Endocrinol. 2009;161:513‐527. [DOI] [PubMed] [Google Scholar]
- 7. Kloos RT, Gross MD, Francis IR, et al. Incidentally discovered adrenal masses. Endocr Rev. 1995;16:460‐484. [DOI] [PubMed] [Google Scholar]
- 8. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309‐340. [DOI] [PubMed] [Google Scholar]
- 9. Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85:637‐644. [DOI] [PubMed] [Google Scholar]
- 10. Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29:298‐302. [DOI] [PubMed] [Google Scholar]
- 11. Fuller PJ. Adrenal diagnostics: an endocrinologist's perspective focused on hyperaldosteronism. Clin Biochem Rev. 2013;34:111‐116. [PMC free article] [PubMed] [Google Scholar]
- 12. Faselis C, Doumas M, Papademetriou V. Common secondary causes of resistant hypertension and rational [sic] for treatment. Int J Hypertens. 2011;2011:236239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:1889‐1916. [DOI] [PubMed] [Google Scholar]
- 14. Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293‐2300. [DOI] [PubMed] [Google Scholar]
- 15. Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371:1921‐1926. [DOI] [PubMed] [Google Scholar]
- 16. Savard S, Amar L, Plouin PF, et al. Cardiovascular complications associated with primary aldosteronism: a controlled cross‐sectional study. Hypertension. 2013;62:331‐336. [DOI] [PubMed] [Google Scholar]
- 17. Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2018;6:41‐50. [DOI] [PubMed] [Google Scholar]
- 18. Karagiannis A, Tziomalos K, Kakafika AI, et al. Medical treatment as an alternative to adrenalectomy in patients with aldosterone‐producing adenomas. Endocr Relat Cancer. 2008;15:693‐700. [DOI] [PubMed] [Google Scholar]
- 19. Karagiannis A, Tziomalos K, Papageorgiou A, et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother. 2008;9:509‐515. [DOI] [PubMed] [Google Scholar]
- 20. Karagiannis A. Treatment of primary aldosteronism: where are we now? Rev Endocr Metab Disord. 2011;12:15‐20. [DOI] [PubMed] [Google Scholar]
- 21. Stavropoulos K, Imprialos KP, Doumas M. Bypass of confirmatory tests of primary aldosteronism in leaner patients? J Clin Hypertens (Greenwich). 2017;19:798‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jonsdottir G, Gudmundsson J, Birgisson G, et al. Primary aldosteronism: from case detection to histopathology with up to 6 years of follow‐up. J Clin Hypertens (Greenwich). 2017;19:424‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doumas M, Athyros V, Papademetriou V. Screening for primary aldosteronism: whom and how? J Clin Hypertens (Greenwich). 2015;17:547‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017; pii: HYP.0000000000000065. 10.1161/hyp.0000000000000065. [Epub ahead of print]. [DOI] [Google Scholar]
- 25. Li L, Yang G, Zhao L, et al. Baseline demographic and clinical characteristics of patients with adrenal incidentaloma from a single center in China: a survey. Int J Endocrinol. 2017;2017:3093290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsvetov G, Shimon I, Benbassat C. Adrenal incidentaloma: clinical characteristics and comparison between patients with and without extraadrenal malignancy. J Endocrinol Invest. 2007;30:647‐652. [DOI] [PubMed] [Google Scholar]
- 27. Tabuchi Y, Otsuki M, Kasayama S, et al. Clinical and endocrinological characteristics of adrenal incidentaloma in Osaka region, Japan. Endocr J. 2016;63:29‐35. [DOI] [PubMed] [Google Scholar]
- 28. Kim HY, Kim SG, Lee KW, et al. Clinical study of adrenal incidentaloma in Korea. Korean J Intern Med. 2005;20:303‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cho YY, Suh S, Joung JY, et al. Clinical characteristics and follow‐up of Korean patients with adrenal incidentalomas. Korean J Intern Med. 2013;28:557‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goh Z, Phillips I, Hunt PJ, et al. Characteristics of adrenal incidentalomas in a New Zealand centre. Intern Med J. 2018;48:173‐178. 10.1111/imj.13651. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31. Mantero F, Masini AM, Opocher G, et al. Adrenal incidentaloma: an overview of hormonal data from the National Italian Study Group. Horm Res. 1997;47:284‐289. [DOI] [PubMed] [Google Scholar]
- 32. Patrova J, Jarocka I, Wahrenberg H, et al. Clinical outcomes in adrenal incidentaloma: experience from one center. Endocr Pract. 2015;21:870‐877. [DOI] [PubMed] [Google Scholar]
- 33. Mulatero P, Monticone S, Burrello J, et al. Guidelines for primary aldosteronism: uptake by primary care physicians in Europe. J Hypertens. 2016;34:2253‐2257. [DOI] [PubMed] [Google Scholar]
- 34. Bernini G, Moretti A, Argenio G, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523‐529. [DOI] [PubMed] [Google Scholar]
- 35. Tzanela M, Effraimidis G, Vassiliadi D, et al. The aldosterone to renin ratio in the evaluation of patients with incidentally detected adrenal masses. Endocrine. 2007;32:136‐142. [DOI] [PubMed] [Google Scholar]
- 36. Vierhapper H. Determination of the aldosterone/renin ratio in 269 patients with adrenal incidentaloma. Exp Clin Endocrinol Diabetes. 2007;115:518‐521. [DOI] [PubMed] [Google Scholar]
- 37. Rossi E, Regolisti G, Negro A, et al. High prevalence of primary aldosteronism using postcaptopril plasma aldosterone to renin ratio as a screening test among Italian hypertensives. Am J Hypertens. 2002;15:896‐902. [DOI] [PubMed] [Google Scholar]
- 38. Pasternak JD, Seib CD, Seiser N, et al. Differences between bilateral adrenal incidentalomas and unilateral lesions. JAMA Surg. 2015;150:974‐978. [DOI] [PubMed] [Google Scholar]


