Abstract
The authors determined the effect of high‐intensity aerobic interval training on arterial stiffness and microvascular dysfunction in patients with metabolic syndrome with hypertension. Applanation tonometry was used to measure arterial stiffness and laser Doppler flowmetry to assess microvascular dysfunction before and after 6 months of stationary cycling (training group; n = 23) in comparison to a group that remained sedentary (control group; n = 23). While no variable improved in controls, hypertension fell from 79% (59%–91%) to 41% (24%–61%) in the training group, resulting in lower systolic and diastolic pressures than controls (−12 ± 3 and −6 ± 2 mm Hg, P < .008). Arterial stiffness declined (−17% augmentation index, P = .048) and reactive hyperemia increased (20%, P = .028) posttreatment in the training group vs controls. Blood constituents associated with arterial stiffness and a prothrombotic state (high‐sensitivity C‐reactive protein, fibrinogen, platelets, and erythrocytes) remained unchanged in the training and control groups. In summary, 6 months of an intense aerobic exercise program reduced both arterial stiffness and microvascular dysfunction in patients with metabolic syndrome despite unchanged blood‐borne cardiovascular risk factors. Training lowers blood flow resistance in central and peripheral vascular beds in a coordinated fashion, resulting in clinically relevant reductions in hypertension.
Keywords: metabolic syndrome X, physical fitness, pulse wave velocity, reactive hyperemia, vascular stiffness
1. INTRODUCTION
Metabolic syndrome (MetS) is a cluster of disorders that increases the risk of developing cardiovascular disease (CVD) and diabetes mellitus.1 MetS is associated with poor blood pressure (BP) control2 which increases the risk of developing CVD and, thus, there is a growing interest in therapies that reduce BP in this population. Chronic hypertension has been shown to induce changes in the tunica media of arteries, resulting in arterial stiffness.3 Current evidence suggests that arterial stiffness precedes hypertension in older adults.4 Arterial stiffness can be assessed using applanation tonometry by assessing carotid‐femoral pulse wave velocity (PWV), measuring the shape of the radial pulse waveform, and calculating the augmentation index (AIx). In recent years, the use of these pulsatile measurements of pressure has gained popularity because they better predict the risk of coronary artery diseases than traditional measures of brachial BP using sphygmomanometry.5
Patients with MetS have increased arterial stiffness even when adjusting for associated factors such as age and sex.6 In fact, MetS accelerates the age‐associated increase in aortic stiffness in men and women.7 In patients with MetS, 8 weeks of intense aerobic exercise has been shown to lower arterial stiffness (PWV) but not brachial artery pressures.8 This suggests that pulsatile BP measures may be more sensitive than traditional brachial artery pressure measurements to readily identify the effects of exercise training on hemodynamics. Nevertheless, even when studies are based only on pulsatile measurements, there is no unanimous agreement on the effect of exercise training on BP. For instance, findings from a recent meta‐analysis suggest that continuous moderate‐intensity aerobic exercise training, without weight loss, does not improve arterial stiffness in obese adults.9 High‐intensity aerobic interval training (AIT) elicits superior peripheral adaptations in comparison with continuous moderate‐intensity training10 although its effect on arterial stiffness has not been explored.
Studies suggest that obesity and MetS are characterized by endothelial dysfunction in peripheral vascular beds, such as the subcutaneous microcirculation, which prevents adequate tissue perfusion and results in oxidative stress and inflammation.11, 12 Others argue that MetS has mainly a central effect that accelerates arterial stiffness13 while a third group supports that MetS affects both macrocirculation and microcirculation.14 To our knowledge, no study has systematically evaluated the central and peripheral cardiovascular effects of AIT in the same individuals with MetS using an intervention study rather than a cross‐sectional design. These data may help to reveal which vascular bed is more responsive and conversely which is more resistant to the effects of aerobic high‐intensity interval training on lowering BP.
The aim of this study was to determine the hemodynamic effects of prolonged (6 months) high‐intensity AIT in patients with MetS with a high prevalence of hypertension and thus increased risk of developing CVD. Importantly, we examined both central and peripheral vessel adaptations, with the hypothesis that exercise training would improve both vascular beds in a coordinated fashion. Our primary outcome was arterial stiffness measured using pulsatile indexes (ie, PWV and AIx).15
2. METHODS
2.1. Study design and population
A randomized pretest‐posttest control group design was used. Participants were recruited, clinically screened, and completed the treatments and testing in the order presented in Figure 1, while the study complied with the CONSORT (Consolidated Standards of Reporting Trials) statement.16 Fifty participants were randomly assigned to a training or control group, balancing the number of women allocated in each group. All participants were physically inactive (exercise <1 day per week) and weight stable (ie, ±2 kg) for at least 6 months prior to the study. Participants were enrolled based on fulfillment of three or more MetS criteria as per the harmonized definition using Europid waist circumference cut‐points (80 cm for women and 94 cm for men).1 Elevated BP for MetS is defined as systolic BP ≥130 mm Hg and/or diastolic BP ≥85 mm Hg measured at the brachial artery or taking antihypertensive drug treatment.1 Exclusion criteria included use of medications known to affect weight or appetite and/or any disease associated with exercise intolerance. Women were not taking hormone replacement therapy. Screening included physical examination to measure body mass index, resting BP, waist circumference (2 cm above the iliac crest), 12‐lead electrocardiography at rest and during an exercise stress test from which we obtained the maximal heart rate for each individual, and blood biochemistry. All patients provided written, witnessed, informed consent and the study was approved by the local hospital's ethics committee in accordance with the Declaration of Helsinki.
Figure 1.
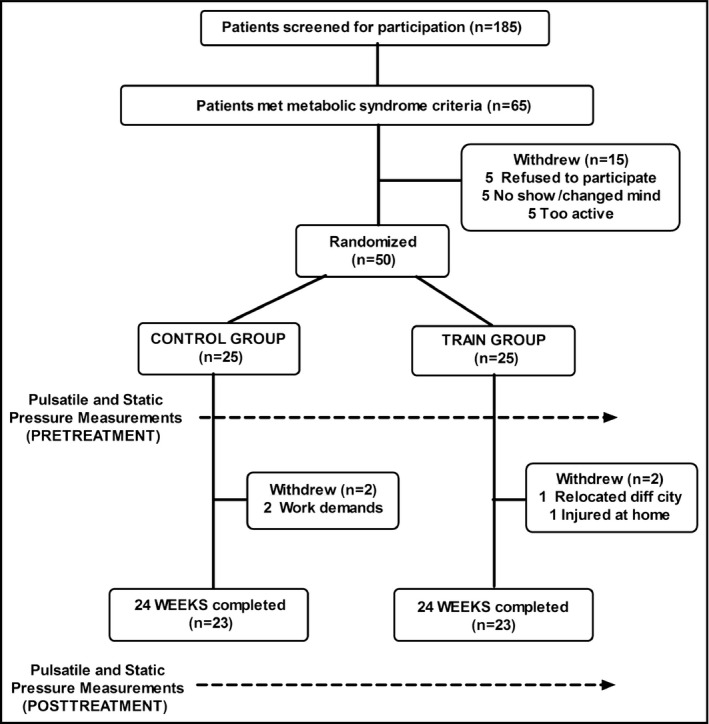
CONSORT schematic representation of the study procedures
2.2. Intervention program
Participants in the control group were instructed to remain sedentary during the 6 months of study. Individuals were instructed to maintain a steady dietary pattern, which was analyzed monthly using a 3‐day meal diary (CESNID). The supervised training program consisted of 45‐minute sessions of pedaling exercise, 3 days per week, for a total of 6 months. Attendance to at least 85% of the sessions was required. In every exercise session, participants wore a heart rate monitor and workloads were self‐adjusted to reach the target heart rate. Each exercise session consisted of a 10‐minute warm‐up, followed by four bouts of 4 minutes of pedaling at an intensity that elicited 90% of maximal heart rate (ie, HRMAX) interspersed with 3‐minute active recovery periods at 70% maximal heart rate. The average oxygen consumption rate during each exercise session in the training group was 26.5 ± 4.7 mLO2 kg−1 min−1, which corresponded to 7.6 ± 1.3 metabolic equivalents. Maximal heart rate was reevaluated monthly during a maximal cycling bout to exhaustion and workloads were adjusted accordingly to maintain training stimulus constant. This intermittent exercise protocol was previously shown to improve cardiorespiratory fitness and be tolerable in the MetS population.10, 17, 18
2.3. Clinical investigation
Before and after the 6 months of intervention (exercise or no exercise), BP and pulse‐wave contour measurements were assessed at rest in the morning after an overnight fast. For the training group, post‐training measurements were scheduled at least 48 hours after the last exercise training session to examine the chronic effects of exercise training rather than the acute most recent exercise session. On a different day, postocclusion reactive hyperemia was assessed in the cutaneous circulation. In addition, percentage of body fat was determined by dual energy x‐ray absorptiometry scan (DXA Hologic Serie Discovery Wi QDR). Finally, patients were referred to a clinic where blood was drawn in the morning after a 10‐hour overnight fast preintervention and postintervention. Participants were instructed to complete the three visits (two to the laboratory and one to the clinic) within the same week.
2.4. Central arterial stiffness measurements
After 20 minutes of undisturbed supine rest on a gurney, brachial BPs (systolic and diastolic BPs) were measured in triplicate on the left arm with a calibrated electrocardiography‐gated electrosphygmomanometer (Tango, Sun Tech Medical). The first reading was discarded and the mean of the two following readings with a coefficient of variation <10% was used, with additional readings if required.19 Aortic systolic and diastolic pressures were then calculated by applanation tonometry in the radial artery using high‐fidelity pressure contours (SphygmoCor, AtCor Medical). The SphygmoCor system synthetizes a central (ascending aortic) pressure waveform from the radial pressure waveform that has been validated against the intra‐arterial recorded wave.20 AIx was calculated as the maximal systolic pulse wave peak minus the pressure at the inflection point expressed as a percentage of pulse pressure. Because AIx is influenced by heart rate,21 AIx data were normalized to a heart rate of 75 beats·min−1 before analysis. Only measurements within the default specifications, which were average pulse height >80 units, pulse height and diastolic variation <5%, and quality index >80%, were averaged.
Carotid‐femoral PWV was measured by applanation tonometry (AtCor Medical) as an index of central arterial stiffness. Electrocardiography‐gated waveforms were recorded and time delay was calculated from the foot of the wave. Aortic distance was calculated from the carotid to the suprasternal notch and from the suprasternal notch to the femoral artery at the groin. All measures (AIx and PWV) were taken in duplicate and the average of those two readings were reported. However, when differences between the two readings were present, additional readings were performed averaging two consecutives readings with a coefficient of variation <12%. The same trained researcher performed all pulsatile measurements. The intrasubject day‐to‐day reliability of the pressure wave contour measurement in our laboratory was established in five patients at the same time of day, resulting in a mean coefficient of variation of 8.1% for PWV and 10.1% for AIx.
2.5. Peripheral postocclusion reactive hyperemia
On a different day, patients arrived to the laboratory after an overnight fast having abstained from drinking tea or coffee in the 12 hours prior to attendance. Patients were instrumented with a deflated sphygmomanometer cuff around their mid arm and a laser Doppler fluximeter probe (DRT4, Moor Instruments) was affixed with a flat holder and adhesive tape to the ventral right forearm (12 cm proximal from the wrist crease). After 20 minutes of lying supine with the arm resting on a gurney, the sphygmomanometer cuff was inflated 30 mm Hg above resting systolic BP for 3 minutes.22, 23 A satisfactory blood flow occlusion was evidenced by the loss of cutaneous pulsatile flow and a steadily low blood flow display.22 After 3 minutes, the cuff was quickly deflated, inducing reactive hyperemia. Participants were instructed to remain as still as possible during cuff deflation. The peak flux value above baseline and the time to reach it were recorded as indexes of microvascular reactivity.24 Intrapatient reproducibility for this technique in our laboratory results in a coefficient of variation <15%.
2.6. Blood analyses
Before and after the intervention and following an overnight fast, a 6‐mL blood sample was drawn into a blood collection tube containing EDTA (Vacutainer) and analyzed for erythrocytes, leukocytes, fibrinogen and platelets (BC 5800 Mindray, Bio‐Medical Electronics Ltd), high‐sensitivity C‐reactive protein (hs‐CRP) using immunoturbidimetry, high‐density lipoprotein cholesterol using accelerator selective detergent method, blood triglycerides with glycerol‐3‐phosphate oxidize method, and total cholesterol by an enzymatic method with a single aqueous reagent. Low‐density lipoprotein cholesterol was calculated as proposed by Friedewald.25 Plasma glucose was analyzed using the glucose oxidase‐peroxidase method. All of the above analyses were run in an automated Mindray BS 400 Chemistry Analyzer (Mindray Medical Instrumentation). Insulin concentration was measured in duplicate using chemiluminescent microparticle immunoassay (Architect ci4100, Abbott Laboratories).
2.7. Statistical analysis
Normality was evaluated by the Shapiro‐Wilk test. Sample size calculation revealed that 18 patients per group were sufficient to detect a moderate (Cohen's effect size [ES]) group×time interaction effect for PWV, assuming a power of 0.8 and an α error probability of .05. Differences between groups at baseline were analyzed using a t test for independent samples. The effects of the intervention were tested using a two‐way (time×group), mixed‐model analysis of variance. If an interaction existed, test for simple effects were explored using Bonferroni post hoc test. Effect size (ES26) of time‐group interaction effect were calculated using partial Eta square, based on the following criteria: >0.14 large effect, 0.14 to 0.06 moderate effect, and <0.06 small effect. All analyses were performed with SPSS version 21 (IBM). Data are presented as mean ± standard deviation. Statistical significance level was set at P ≤ .05.
3. RESULTS
3.1. Participant characteristics
Patients reported unchanged dietary pattern (amount and composition, CESNID) during the study. Attendance to the exercise sessions averaged 95% (85%–100%). The age of the patients was 53.5 ± 8.9 years and there were eight women (17%) among the 46 individuals who composed the entire sample (Figure 1). Sex and age did not have significant interactions with time in any of the main outcome measures (ie, AIx normalized to a heart rate of 75 beats·min−1 [AIx@75HR], PWV, and reactive hyperemia). While anthropometric data did not vary in the control group after 6 months, there were modest yet significant reductions in the training group in body weight (91.0 ± 13.6 vs 89.7 ± 12.4 kg, P = .019), BMI (32.8 ± 3.3 vs 32.3 ± 3.1 kg·m−2, P = .014), and percentage of body fat (36.0 ± 6.6 vs 35.4 ± 6.6%, P = .001) following 6 months of AIT. Six months of AIT in the training group also resulted in significant reductions in two MetS parameters, –waist circumference and BP (Table 1). Other parameters associated with arterial stiffness did not significantly change in either group (ie, total cholesterol, low‐density lipoprotein cholesterol, hs‐CRP, and insulin concentrations) (Table 2). Furthermore, indexes of blood cellular content and prothrombotic state did not vary in training or controls (Table 2).
Table 1.
Evolution of metabolic syndrome factors and prevalence of hypertension in the training and control groups
| Training group (n = 23) | Control group (n = 23) | Training vs control groups at baseline | |||
|---|---|---|---|---|---|
| Baseline | Follow‐up | Baseline | Follow‐up | P value | |
| Waist circumference, cm | 106 ± 7 | 104 ± 6*,** | 108 ± 6 | 109 ± 5 | .191 |
| HDL‐C, mmol·L−1 | 0.93 ± 0.18 | 0.98 ± 0.24 | 0.89 ± 0.15 | 0.90 ± 0.21 | .205 |
| Glucose, mmol·L−1 | 6.42 ± 1.36 | 6.35 ± 1.60 | 6.11 ± 0.75 | 6.18 ± 0.69 | .332 |
| Triglycerides, mmol·L−1 | 1.42 ± 0.86 | 1.44 ± 0.98 | 1.44 ± 0.98 | 1.34 ± 0.63 | .938 |
| Systolic BP, mm Hg | 136 ± 17 | 127 ± 12*,** | 138 ± 16 | 140 ± 12 | .709 |
| Diastolic BP, mm Hg | 84 ± 10 | 77 ± 6*,** | 84 ± 11 | 83 ± 8 | .777 |
| Prevalence of hypertension,% (95% CI) | 79.3 (59.2–91.0) | 41.4 (23.9–61.3) | 76.5 (56.2–89.2) | 82.4 (62.6–92.9) | |
Abbreviations: CI, confidence interval; HDL‐C, high‐density lipoprotein cholesterol.
Data are presented as mean ± standard deviation for 46 patients with metabolic syndrome divided into training and control groups. Systolic and diastolic blood pressures (BPs) measured by electrocardiography‐gated sphygmomanometer at the brachial artery.
*Significantly different from baseline within that group.
**Significantly different from controls at that time point.
Table 2.
Blood parameters associated with viscosity, prothrombotic state, and arterial stiffness
| Training group (n = 23) | Control group (n = 23) | Training vs control groups at baseline | |||
|---|---|---|---|---|---|
| Baseline | Follow‐up | Baseline | Follow‐up | P value | |
| Erythrocytes, 106·μL−1 | 4.88 ± 0.4 | 4.84 ± 0.4 | 5.03 ± 0.3 | 4.94 ± 0.3 | .191 |
| Platelets, 103·μL−1 | 242 ± 52 | 238 ± 44 | 228 ± 39 | 223 ± 28 | .228 |
| Leukocytes, 103·μL−1 | 5.9 ± 1.3 | 6.5 ± 1.7 | 5.5 ± 1.1 | 5.9 ± 2.3 | .312 |
| Fibrinogen, μmol·L−1 | 8.4 ± 2.0 | 8.2 ± 1.3 | 8.3 ± 2.3 | 8.1 ± 1.1 | .906 |
| hs‐CRP, ηmol·L−1 | 28 ± 21 | 28 ± 21 | 22 ± 18 | 25 ± 22 | .278 |
| Insulin, ρmol·L−1 | 87 ± 32 | 82 ± 36 | 91 ± 36 | 93 ± 38 | .725 |
| Total cholesterol, mmol·L−1 | 4.8 ± 0.8 | 4.7 ± 1.0 | 4.7 ± 0.8 | 4.9 ± 0.7 | .758 |
| LDL‐C, mmol·L−1 | 3.2 ± 0.8 | 3.0 ± 0.9 | 3.1 ± 0.8 | 3.2 ± 0.5 | .831 |
Abbreviations: hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol.
Data are presented as mean ± standard deviation for 46 patients with metabolic syndrome divided into training and control groups.
3.2. Hemodynamic measurements
While no changes were detected in the control group, after 6 months of AIT, participants in the training group had reduced brachial artery systolic and diastolic BPs (Table 1), lowering the prevalence of hypertension from 79% (95% confidence interval, 59%–91%) to 41% (95% confidence interval, 24%–61%) according to MetS elevated BP thresholds.1 A significant group‐time interaction was found for systolic and diastolic BP (F = 6.23, P = .016 [ES = 0.124] and F = 4.31, P = .044 [ES = 0.089], respectively). Thus, after 6 months, arterial pressures declined by 6% to 9% with exercise training in comparison to controls, resulting in post‐treatment lower systolic (−12 ± 3 mm Hg, P = .001) and diastolic (−6 ± 2 mm Hg, P = .007) pressures (Table 1). Aortic pressures were also reduced after training by 7.8% in systolic (127 ± 17 to 117 ± 12 mm Hg, P = .002) and 8% in diastolic (85 ± 10 to 78 ± 6 mm Hg, P = .001) resulting in post‐treatment lower systolic (−13 ± 4 mm Hg, P = .030) and diastolic (−6 ± 2 mm Hg, P = .020) pressures than controls. A significant group‐time interaction was found for PWV and AIx (F = 4.02, P = .048 [ES = 0.082] and F = 3.81, P = .023 [ES = 0.096], respectively). PWV decreased in the training group from 8.5 ± 2.1 to 7.8 ± 2.3 m·s−1 (P = .05 [Figure 2A]), while it remained at baseline level in the control group. However, post‐treatment, PWV was not significantly lower in the training group compared with the control group. AIx@75HR was reduced after training from 24.7 ± 11.6% to 21.9 ± 9.2% (P = .038) (Figure 2B), while it remained unchanged in the control group. Therefore, after 6 months (ie, follow‐up), AIx@75HR was lower in the training group than the control group (17% lower, P = .048) (Figure 2B). A significant group‐time interaction was found for postocclusive reactive hyperemia flux (F = 4.71, P = .035 [ES = 0.074]). Postocclusive reactive hyperemia after 3 minutes of occlusion of the cutaneous forearm vessels was unchanged in the control group but increased after AIT in the training group (303 ± 129 vs 377 ± 147%, P = .015) (Figure 3A). Therefore, after 6 months (ie, follow‐up), postocclusive reactive hyperemia was higher in the training group than in the control group (20% higher, P = .028) (Figure 3A). However, the time to peak flow was not affected by treatment in any group (11.6 ± 2.3 vs 11.9 ± 2.2 seconds, P = .59) (Figure 3B).
Figure 2.
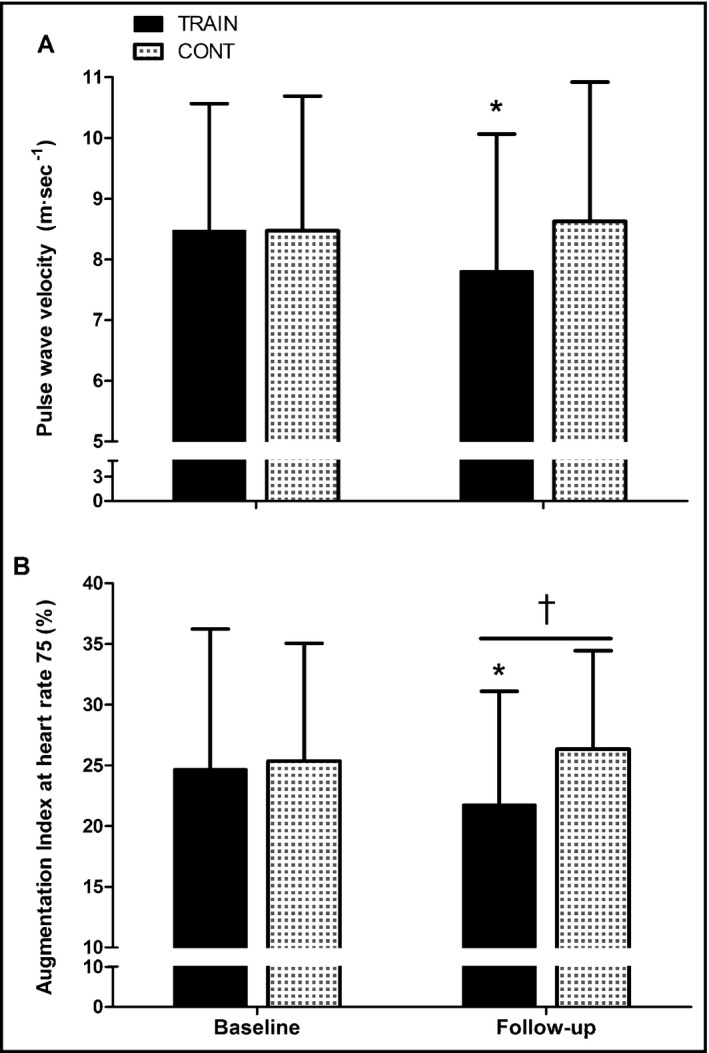
Changes in (A) pulse wave velocity from the carotid to the femoral artery and (B) augmentation index normalized to a heart rate of 75 beats·min−1 in the training (n = 23) and control(n = 23) groups before and after 6 months of treatment. Data are expressed as mean±standard deviation. *Significant difference from baseline within that group. †Significantly different from controls at that time point (all P < .05)
Figure 3.
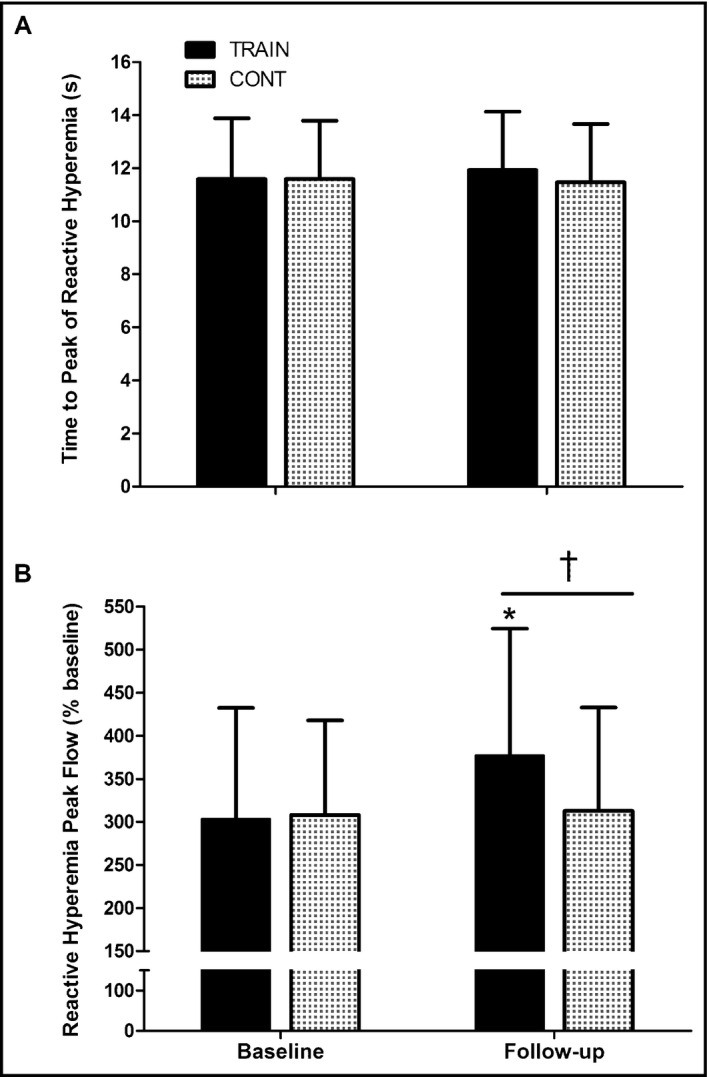
Changes in (A) the time to peak flow and (B) peak forearm reactive hyperemia flow (percentage of baseline) in the training (n = 23) and control (n = 23) groups before and after 6 months of treatment. Data are expressed as mean±standard deviation. *Significant difference from baseline within that group. †Significantly different from controls at that time point (all P < .05)
4. DISCUSSION
The aim of the present study was to determine the BP response to 6 months of an intense AIT program in a sample of participants with MetS with a high prevalence of hypertension (77%–79%) (Table 1) and thus elevated risk for CVD.13 After AIT, we observed a marked reduction in brachial artery systolic and diastolic BPs (Table 1) reducing by half the prevalence of hypertension in the training group. Our data reveal that the effect of AIT on lowering BP was mirrored by reductions in central artery stiffness (ie, PWV and AIx) (Figure 2) and improvements in peripheral vessel vasodilation capacity (ie, reactive hyperemia) (Figure 3). Previous studies support that both macrocirculation and microcirculation resistances to flow contribute to hypertension in MetS14, 27 and we currently report that both are blunted by AIT training (Figures 2B and 3A), likely allowing half of our sample to regain BP control.
Epidemiological studies reveal that from early to late adulthood (ie, 20–90 years), systolic BP increases approximately 14%, while AIx increases fivefold and PWV twofold.28 PWV has been shown to be an independent determinant of the longitudinal increase in systolic BP,29 and thus arterial pulse‐wave contour measurements are regarded as more sensitive than traditional brachial BP measures to detect the alterations in vascular structure and function that occur with aging or disease status such as MetS. However, we observed similar central vasculature effects of exercise training with both measurements. Namely, brachial systolic pressure (Table 1) and pulsatile PWV and AIx@75HR (Figure 2) both were reduced by approximately 8% after 6 months of training. Thus, although central artery stiffness is better assessed by PWV and AIx, the reduction in brachial systolic BP after exercise training seems to reflect the reductions in arterial stiffness facilitating the evaluation of the effects of exercise training.
Increased central arterial stiffness results in most of the pulsatile energy of the forward pressure wave transferred to the vasculature, potentially damaging vessel structures.30 We measured central arterial stiffness and peripheral vessel reactive hyperemia and detected that both improved with intense AIT. Epidemiological studies have shown an association between increased arterial stiffness and reduced microvascular response to ischemia with aging and CVD risk factors.31 Exercise training of the duration and intensity used in this study simultaneously lowers the resistances to flow in central and peripheral vessels in patients with MetS. Individuals with MetS are an important population to study since they have not yet transitioned to more advanced stages of CVD (ie, heart failure, coronary artery diseases, stroke, and peripheral vascular disease) and seem to be still responsive to lifestyle therapies that include intense aerobic exercise.
It is tempting to speculate that the simultaneous improvements in peripheral vascular reactivity and arterial stiffness are driven by an external dominant factor that improves along with exercise training. We observed modest yet significant reduction in body mass and percentage of fat mass following 6 months of AIT (ie, −1.7%). Indeed, we32 and others33 have shown that losses of weight and fat are associated with reductions in BP in this population. However, it is unlikely that the reductions in BP (6–13 mm Hg comparing training and control groups at the end of intervention) (Table 1) were the result of the modest weight loss in the 6 months of training (1.3 kg). Furthermore, other factors associated with hypertension such as elevated blood lipids and hyperglycemia were not affected by the 6 months of training (Tables 1 and 2). Therefore, the improvement in arterial stiffness and microvascular response to ischemia seem to pertain to the direct effects in the vasculature of the repeated bouts of interval exercise and not by lowering of blood glucose, lipids, or body fat.
Skin circulation is of prime interest because its dysfunction is associated with the pathogenesis of many diseases such as diabetes mellitus, hypertension, and obesity34 and because its vasodilation capacity has been shown to diminish with age. A decreased ability of the endothelium to induce vasodilation in response to occlusion (ie, reactive hyperemia) is linked to classic risk factors for MetS and CVD risk factors including dyslipidemia, hypertension, inactivity, and obesity.35 While there is no clear consensus on the acute endothelial response to a single bout of exercise, aerobic training seems to consistently improve endothelial function. Furthermore, more profound improvements in vasodilation are observed with high‐intensity rather than continuous, lower‐intensity exercise training.10 In agreement with the cited literature, we observed a significant increase (ie, 20%) (Figure 3B) in cutaneous microcirculation reactive flow in patients after 6 months of intense training when compared with patients with MetS who remained sedentary.
Blunted microvascular reactivity, assessed by postocclusion reactive hyperemia flow, has been related to CVD risk factors36 such as increased total cholesterol,37 reduced high‐density lipoprotein cholesterol,31 high blood triglycerides,38 hyperinsulinemia, and low insulin sensitivity.39 We presently report that after 6 months of exercise training, the increase in postocclusion reactive hyperemia flow (Figure 3B) occurred despite unchanged blood cholesterol (high‐density lipoprotein and total), triglycerides (Table 1), or insulin (Table 2). On the other hand, hs‐CRP in patients with MetS has been regarded as a predictor of coronary heart diseases and thus a contributor of the elevated aortic stiffness in this population.40 Increases in hs‐CRP could provoke platelet hyperreactivity, promote fibrinogen biosynthesis, and increase erythrocyte aggregability resulting in a prothrombotic state.41 Conversely, all of these events seem to be reversed by aerobic exercise training. We did not find reductions in hs‐CRP with 6 months of exercise training (Table 2) or patients’ fibrinogen, platelets, leukocytes, or erythrocyte count. Our results suggest a minor role for this unspecific inflammation marker (ie, hs‐CRP) and other markers of prothrombotic state in the reductions in aortic stiffness observed with exercise training.
5. STUDY LIMITATIONS
Our study is not free of limitations. Our patients were all diagnosed with MetS and most were taking medications that were neither withdrawn nor lowered during the study. We deemed it inappropriate to withhold antihypertensive medication during the study in patients with increased CVD risk. It is currently unclear whether exercise training lowers BP through mechanisms different to medication; therefore, a possible interaction between medication and exercise training could have existed in our data resulting in an overestimation of the effects of exercise in individual cases. Other limitations of this study were the lack of patient familiarization prior to obtaining measurements and no control over tobacco usage. Furthermore, there is no gold standard measurement for microvascular function, and several other indices besides postocclusion reactive hyperemia flux exist that were not assessed in the present study.
6. CONCLUSIONS
Six months of AIT at a frequency of three times per week reduced the prevalence of hypertension from 79% (59–91%) to 41% (24–61%) in patients with MetS upon a 6‐ to 13‐mm Hg reduction in diastolic and systolic BP. The mechanism of the normalization of BP involved a simultaneous reduction in central arterial stiffness and an augmented capacity of the peripheral microvasculature to vasodilate. These responses seemed to be directly mediated by the training stimulus since we observed minimal changes in other risks factors associated with arterial stiffness (dyslipidemia, hyperglycemia, or body fat) or with blood prothrombotic state.
DISCLOSURES
The authors report no conflicts of interest. This work was partly funded by a grant from the Spanish Ministry of Economy and Competivity (DEP‐2014‐52930‐R).
Mora‐Rodriguez R, Ramirez‐Jimenez M, Fernandez‐Elias VE, et al. Effects of aerobic interval training on arterial stiffness and microvascular function in patients with metabolic syndrome. J Clin Hypertens. 2018;20:11–18. 10.1111/jch.13130
ClinicalTrials.gov identifier: NCT03019796.
REFERENCES
- 1. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640‐1645. [DOI] [PubMed] [Google Scholar]
- 2. Kjeldsen SE, Naditch‐Brule L, Perlini S, Zidek W, Farsang C. Increased prevalence of metabolic syndrome in uncontrolled hypertension across Europe: the global cardiometabolic risk profile in patients with hypertension disease survey. J Hypertens. 2008;26:2064‐2070. [DOI] [PubMed] [Google Scholar]
- 3. Safar ME, O'Rourke MF. Arterial Stiffness in Hypertension. Vol 23. Edinburgh, United Kingdom: Elsevier; 2006. [Google Scholar]
- 4. Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension. 2014;64:210‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weber T, Auer J, Lamm G, O'Rourke MF, Eber B. Arterial stiffness, central blood pressures, and wave reflections in cardiomyopathy‐implications for risk stratification. J Card Fail. 2007;13:353‐359. [DOI] [PubMed] [Google Scholar]
- 6. Safar ME, Thomas F, Blacher J, et al. Metabolic syndrome and age‐related progression of aortic stiffness. J Am Coll Cardiol. 2006;47:72‐75. [DOI] [PubMed] [Google Scholar]
- 7. Scuteri A, Cunha PG, Rosei EA, et al. Arterial stiffness and influences of the metabolic syndrome: a cross‐countries study. Atherosclerosis. 2014;233:654‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donley DA, Fournier SB, Reger BL, et al. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J Appl Physiol. 2014;116:1396‐1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montero D, Roberts CK, Vinet A. Effect of aerobic exercise training on arterial stiffness in obese populations: a systematic review and meta‐analysis. Sports Med. 2014;44:833‐843. [DOI] [PubMed] [Google Scholar]
- 10. Tjonna AE, Lee SJ, Rognmo O, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grassi G, Seravalle G, Brambilla G, et al. Impact of the metabolic syndrome on subcutaneous microcirculation in obese patients. J Hypertens. 2010;28:1708‐1714. [DOI] [PubMed] [Google Scholar]
- 12. Rizzoni D, De Ciuceis C, Porteri E, Semeraro F, Rosei EA. Structural alterations in small resistance arteries in obesity. Basic Clin Pharmacol Toxicol. 2012;110:56‐62. [DOI] [PubMed] [Google Scholar]
- 13. Ingelsson E, Sullivan LM, Murabito JM, et al. Prevalence and prognostic impact of subclinical cardiovascular disease in individuals with the metabolic syndrome and diabetes. Diabetes. 2007;56:1718‐1726. [DOI] [PubMed] [Google Scholar]
- 14. Edgell H, Petrella RJ, Hodges GJ, Shoemaker JK. Central versus peripheral cardiovascular risk in metabolic syndrome. Front Physiol. 2012;3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Safar ME, Balkau B, Lange C, et al. Hypertension and vascular dynamics in men and women with metabolic syndrome. J Am Coll Cardiol. 2013;61:12‐19. [DOI] [PubMed] [Google Scholar]
- 16. Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637‐639. [DOI] [PubMed] [Google Scholar]
- 17. Mora‐Rodriguez R, Ortega JF, Hamouti N, et al. Time‐course effects of aerobic interval training and detraining in patients with metabolic syndrome. Nutr Metab Cardiovasc Dis. 2014;24:792‐798. [DOI] [PubMed] [Google Scholar]
- 18. Stensvold D, Tjonna AE, Skaug EA, et al. Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J Appl Physiol. 2010;108:804‐810. [DOI] [PubMed] [Google Scholar]
- 19. Schjerve IE, Tyldum GA, Tjonna AE, et al. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin Sci (Lond). 2008;115:283‐293. [DOI] [PubMed] [Google Scholar]
- 20. Chen CH, Ting CT, Nussbacher A, et al. Validation of carotid artery tonometry as a means of estimating augmentation index of ascending aortic pressure. Hypertension. 1996;27:168‐175. [DOI] [PubMed] [Google Scholar]
- 21. Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(pt 1):263‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keymel S, Sichwardt J, Balzer J, et al. Characterization of the non‐invasive assessment of the cutaneous microcirculation by laser Doppler perfusion scanner. Microcirculation. 2010;17:358‐366. [DOI] [PubMed] [Google Scholar]
- 23. Yvonne‐Tee GB, Rasool AH, Halim AS, Rahman AR. Reproducibility of different laser Doppler fluximetry parameters of postocclusive reactive hyperemia in human forearm skin. J Pharmacol Toxicol Methods. 2005;52:286‐292. [DOI] [PubMed] [Google Scholar]
- 24. Roustit M, Cracowski JL. Non‐invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation. 2012;19:47‐64. [DOI] [PubMed] [Google Scholar]
- 25. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499‐502. [PubMed] [Google Scholar]
- 26. Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988:569. [Google Scholar]
- 27. Czernichow S, Greenfield JR, Galan P, et al. Macrovascular and microvascular dysfunction in the metabolic syndrome. Hypertens Res. 2010;33:293‐297. [DOI] [PubMed] [Google Scholar]
- 28. Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88(4 pt 1):1456‐1462. [DOI] [PubMed] [Google Scholar]
- 29. Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore longitudinal study of aging. J Am Coll Cardiol. 2008;51:1377‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end‐organ damage. J Appl Physiol. 2008;105:1652‐1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mitchell GF, Vita JA, Larson MG, et al. Cross‐sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722‐3728. [DOI] [PubMed] [Google Scholar]
- 32. Mora‐Rodriguez R, Ortega JF, Guio de Prada V, et al. Effects of simultaneous or sequential weight loss diet and aerobic interval training on metabolic syndrome. Int J Sports Med. 2016;37:274‐281. [DOI] [PubMed] [Google Scholar]
- 33. Stevens VJ, Obarzanek E, Cook NR, et al. Long‐term weight loss and changes in blood pressure: results of the trials of hypertension prevention, phase II. Ann Intern Med. 2001;134:1‐11. [DOI] [PubMed] [Google Scholar]
- 34. Jonk AM, Houben AJ, de Jongh RT, Serne EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity‐associated insulin resistance and hypertension. Physiology (Bethesda). 2007;22:252‐260. [DOI] [PubMed] [Google Scholar]
- 35. Currie KD, McKelvie RS, Macdonald MJ. Flow‐mediated dilation is acutely improved after high‐intensity interval exercise. Med Sci Sports Exerc. 2012;44:2057‐2064. [DOI] [PubMed] [Google Scholar]
- 36. Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow‐mediated dilation: the Framingham heart study. Hypertension. 2004;44:134‐139. [DOI] [PubMed] [Google Scholar]
- 37. Binggeli C, Spieker LE, Corti R, et al. Statins enhance postischemic hyperemia in the skin circulation of hypercholesterolemic patients: a monitoring test of endothelial dysfunction for clinical practice? J Am Coll Cardiol. 2003;42:71‐77. [DOI] [PubMed] [Google Scholar]
- 38. Gokce N, Duffy SJ, Hunter LM, Keaney JF, Vita JA. Acute hypertriglyceridemia is associated with peripheral vasodilation and increased basal flow in healthy young adults. Am J Cardiol. 2001;88:153‐159. [DOI] [PubMed] [Google Scholar]
- 39. Serne EH, Stehouwer CD, ter Maaten JC, et al. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation. 1999;99:896‐902. [DOI] [PubMed] [Google Scholar]
- 40. Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C‐reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland coronary prevention study. Circulation. 2003;108:414‐419. [DOI] [PubMed] [Google Scholar]
- 41. Chen YW, Apostolakis S, Lip GY. Exercise‐induced changes in inflammatory processes: implications for thrombogenesis in cardiovascular disease. Ann Med. 2014;46:439‐455. [DOI] [PubMed] [Google Scholar]


