Abstract
Sleep quality is an important aspect of sleep, but no meta‐analysis has elucidated its relationship with blood pressure (BP) and hypertension. A meta‐analysis was conducted in October 2016 using multiple databases, including Embase and Medline. Studies that assessed subjective sleep quality and BP or hypertension were included. Upon full‐text evaluation, 29 articles from 45 041 patients were selected, of which 22 articles were included in the meta‐analysis and seven were presented narratively. Poor sleep quality was significantly associated with a greater likelihood of hypertension (odds ratio, 1.48; P value = .01). Poor sleepers had higher average systolic BP (mean difference = 4.37, P value = .09) and diastolic BP (mean difference = 1.25, P value = .32) than normal sleepers without statistical significance. Patients with hypertension had significantly worse sleep quality scores (mean difference = 1.51, P value < .01), while BP dippers had significantly better scores (mean difference = −1.67, P value < .01). The findings highlight the relationship between sleep quality and hypertension.
Keywords: blood pressure, hypertension, meta‐analysis, sleep quality
1. INTRODUCTION
Patients with hypertension have been found to be more susceptible to various cardiovascular diseases including stroke and coronary heart diseases.1, 2 Adequate sleep duration not only maintains body function, but also prevents adverse cardiovascular outcomes. For instance, inadequate sleep has been associated with hypertension3 and mortality from cardiovascular diseases.4 Apart from sleep duration, sleep quality is another important aspect of sleep, but its relationship with cardiovascular outcomes has received little attention.
One possible reason underlying the paucity of studies on sleep quality concerns its complicated conceptualization. Sleep quality is a composite of sleep indexes, including sleep duration and presence of sleep problems, which can be measured objectively (eg, polysomnography or actigraphy) or subjectively (eg, sleep diaries or self‐reported surveys).5 Objective sleep quality is categorized into a set of indexes including awakenings, amount and percentage of sleep stages, rapid eye movement latency, number of apneas or hypopneas, and periodic movements of sleep.5 Despite their precision in measurement, the objective indexes are not consolidated into a global sleep‐quality index based on their relative importance.6
Although sleep measures resemble objective sleep indexes (eg, awakenings during sleep and sleep disturbances), the subjective sleep quality refers to the retrospective appraisal of the sleep experience as recalled by the individual, which can be summarized into a global sleep status.5 For instance, the Pittsburgh Sleep Quality Index (PSQI) encompasses sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction in the previous month.7 The PSQI is a popular and clinically accepted instrument of choice because of its high internal consistency (α = 0.83), test‐retest reliability (r = .85),7 and moderate structural validity identifying patients with poor sleep quality in both clinical and nonclinical populations (a global score >5 of 21).8 Several reviews have assessed the association between PSQI‐measured sleep quality and health outcomes including obesity9 and glycemic control.10
Studies conducted in Asia11 and Europe12, 13 have suggested that poor subjective sleep quality is associated with significantly higher odds ratios (ORs) of hypertension. Poor sleep has also been associated with significantly higher levels of systolic blood pressure (SBP) and/or diastolic blood pressure (DBP) in Asian14, 15 and European studies.16, 17 Nevertheless, the existing body of evidence is not entirely consistent, as insignificant associations between poor sleep and blood pressure (BP) have been found across different study regions, such as the American continent18, 19 and Africa.20 This observation indicates that the relationship between sleep quality and hypertension may vary across continents. In addition, several studies have revealed that patients with hypertension have higher global PSQI scores (indicative of poorer sleep quality) than patients with normotension.14, 20
Apart from its association with BP, poor sleep quality may be associated with the dipping pattern of BP. For a healthy patient, there is at least a 10% reduction in nocturnal BP as compared with daytime BP; such a patient is characterized as a “dipper.”21 “Nondippers” demonstrate an increased activity in the sympathetic nervous system and a decreased activity in the parasympathetic nervous system, which probably explains the higher nocturnal BP.22 Nondipping BP is associated with higher risk for cardiorenal organ damage such as left ventricular hypertrophy and cerebrovascular diseases.23 Poor sleep quality may be associated with nondipping patterns through their disruption of the circadian rhythm.24 This association is supported by previous research reporting higher PSQI global scores for nondippers than dippers.25, 26 However, no meta‐analysis has been conducted to summarize these findings.
Current evidence suggests a potential association between poor subjective sleep quality and hypertension, but it has not been verified through a systematic approach. The primary aims of the present systematic review and meta‐analysis are to summarize current evidence and to determine whether poor subjective sleep quality is associated with elevated BP. The secondary aim is to examine whether the associations differ by geographic regions.
2. METHODS
2.1. Search strategy
Multiple databases were used for the present review, namely Medline (1946 to present), Embase (1974 to 2016 week 44), Ovid Nursing Database (1946 to October week 3 2016), and PsycINFO (1806 to October week 4 2016). The search period was from inception to October 2016. Detailed keywords for literature search are outlined in the Supporting Information. Search terms included a combination of synonyms of “sleep quality,”9 “hypertension,”3 and “blood pressure”3 as adapted from relevant review articles.3, 9 All articles with English abstracts were assessed.
2.2. Study inclusion criteria
Primary studies with a cross‐sectional, prospective, or retrospective design
Studies that examined sleep quality with a self‐reported questionnaire
Studies that defined hypertension with criterion or guidelines or studies that assessed SBP and/or DBP
All human participants
2.3. Study exclusion criteria
Literature reviews, intervention studies, letters, or abstracts from conference proceedings
Articles without an abstract or full text in English.
2.4. Study selection
Initial screening on the titles and abstracts was independently conducted by two reviewers (KL and BW) using the aforementioned inclusion/exclusion criteria. Full texts of relevant articles were obtained, and their eligibility independently determined by the said reviewers. Discrepancies were solved by discussion and mediated by a third reviewer (WT).
2.5. Exposures and outcomes
The exposures and outcomes of the present review included subjective sleep quality, hypertension, and levels and dipping of BP, as both hypertension and sleep quality were designated as outcomes in previous studies. Studies entailing the use of self‐reported sleep quality tools or predefined categories of sleep quality were included in the review. Studies that included hypertension as an exposure/outcome and provided specific criteria or guidelines defining it were also included. For studies that assessed BP, objective measurement methods such as the use of sphygmomanometers had to be reported. For studies examining BP dipping, they would be included if clear definitions were given to differentiate between dippers and nondippers.
2.6. Data extraction and quality assessments
For each included study, the study characteristics were independently extracted by two reviewers (KL and BW), namely the country where the study was conducted, study design, health status, age and number of participants, and references used to define hypertension or the dipping pattern of BP, by using a standard data extraction form created in Covidence (https://www.covidence.org/), an online platform that facilitates the preparation of systematic reviews and meta‐analyses. The effect measures, such as the mean difference, OR, and regression coefficients, and their standard errors were extracted from the articles. Where regression coefficients adjusted for covariates were provided by the articles, adjusted values were preferred. The types of adjusted variables were also extracted. Outcomes without sufficient information for conducting meta‐analysis were described in a narrative fashion.
The methodological quality of the included studies was assessed by critical appraisal tools proposed by the Joanna Briggs Institute.27 The tools were tailored for various study designs, and the checklists for cross‐sectional (8 items), case‐control (10 items), and cohort studies (11 items) were used in the present review. The checklists assessed the possibility of bias in the design, conduct, and analysis of each study type. The ratings of each item were “yes” (low risk of bias), “no” (high risk of bias), and “unclear” (information inadequate for judgment). The percentage of items rated as “yes” for each included study was computed as a measure of the study quality.
2.7. Statistical analysis
The statistical analysis was divided into three components: the associations between: (1) sleep quality and hypertension; (2) sleep quality and BP; and (3) hypertension and dipping BP on sleep quality. The OR and mean difference were, respectively, used as the effect measure for binary and continuous outcomes. If BP was measured at multiple time points within the same day, the average value over 24 hours was computed. Random effects models using the inversed variance approach were used to pool the estimates from individual studies because of the varying population and criteria used to define outcomes. The results were summarized using forest plots. I 2 was used to assess the heterogeneity, with an I 2 between 50% and 90% possibly representing substantial heterogeneity.28
Subgroup analysis was conducted to examine whether the heterogeneity of studies could be explained by the regional difference where the regions of studies were classified in terms of continents, such as America and Asia. Publication bias was examined by funnel plots and tested statistically by the Egger test if a particular outcome included at least 10 studies, as recommended by the Cochrane handbook.28 The “trim‐and‐fill” method was performed when publication bias (P value of the Egger test <0.1) was detected.29 The use of this method corrected the meta‐analysis parameters by trimming the studies that contributed to the asymmetry of the funnel plot.30 Sensitivity analysis was conducted by removing studies that either did not assess sleep quality by the PSQI or included patients with diagnosed diseases other than hypertension (eg, type 2 diabetes mellitus). All meta‐analyses, forest plots, and funnel plots were performed by Review Manager 5.2, while the Egger test and trim‐and‐fill method were conducted by STATA 11.0 (StataCorp).
3. RESULTS
3.1. Study selection
The literature search identified 5392 references, of which 1671 duplicates were removed. After screening the titles and abstracts, 41 articles were found to be potentially suitable, for which the full texts were retrieved. Finally, upon full‐text evaluation, 29 articles were included in this systematic review, of which 22 contained sufficient data for meta‐analysis and the remaining seven are presented narratively. Reasons for exclusion in each stage of the search are provided in Figure 1.
Figure 1.
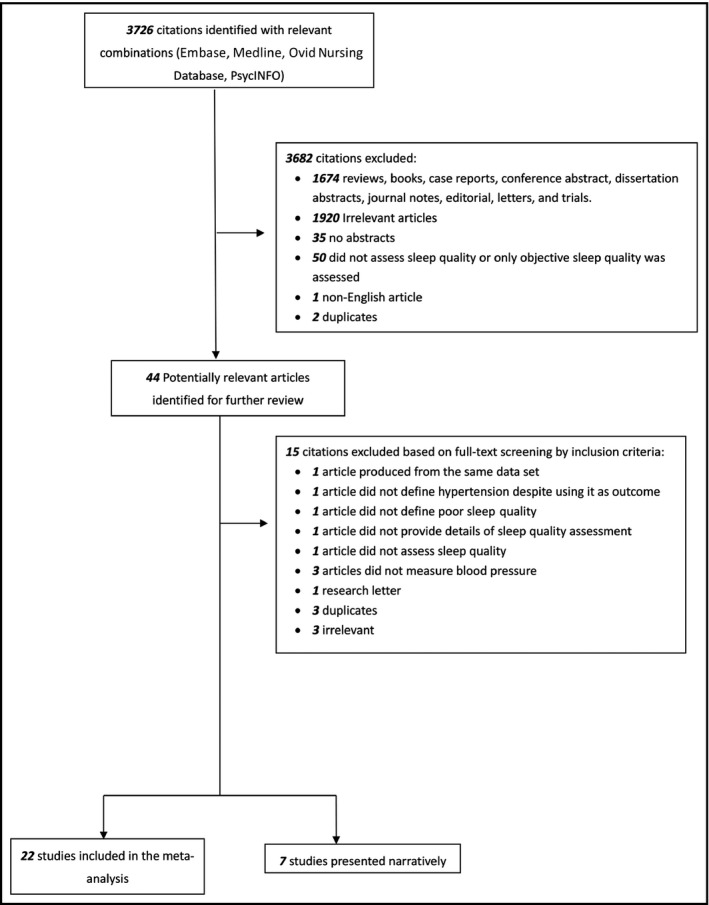
Study selection
3.2. Included articles
The characteristics and outcome definitions of the 29 studies from an aggregate of 45 041 patients are described in the Table. There were 26 cross‐sectional studies, one cohort study, and two case‐control studies, all published between 2007 and 2016. A total of seven studies were conducted in Asia (16 716 patients), 14 in Europe (13 663 patients), six in the American continent (14 202 patients), one in Australia (206 patients), and one in Africa (254 patients). The PSQI is the most popular measure for sleep quality among the included studies (25 of 29 articles). The methodological quality of the studies ranged from 37.5% to 100%. The adjusted variables of studies conducting regression analyses are also described in the Table. Commonly adjusted variables included sex, age, BMI, diabetic status, and/or smoking habits. However, some variables were adjusted in fewer studies, such as mental health status11, 12 and drug use.12, 31
Table 1.
Description of the included studies
| Authors, y | Country | Study design | Sample size | Age range or mean ± SD (SE), y | Health status | Definition of sleep quality | Definition of morbidity | Men, No. (%) | Qualitya %a |
|---|---|---|---|---|---|---|---|---|---|
| Alebiosu et al (2009)20 | Nigeria | Cross‐sectional | 254 | Hypertension: 58.2 ± 9.65 Nonhypertension: 58.7 ± 10.8 | Patients with hypertension and normal patients without known disease | PSQI | JNC 7: SBP ≥140 mm Hg and DBP ≥90 mm Hg or receiving treatment | 123 (48.4) | 50.0 |
| Bansil et al (2011)18, b | United States | Cross‐sectional | 10 308 | 18–39: 38.9%, 40–59: 38.3%, ≥60: 22.7% | General | Answered “often” or “almost always” to any of the 6 items on sleeping habits | JNC 7: SBP ≥140 mmHg and DBP ≥90 mmHg or receiving treatment | 5061 (49.1) | 87.5 |
| Berentzen et al (2014)37 | Netherlands | Cohort | 1481 | Baseline: 11.4 ± 0.3; follow‐up: 12.7 ± 0.4 | General | One variable on nighttime awakenings and three variables on daytime outcomes | None | 722 (48.8) | 63.6 |
| Bruno et al (2013)12, b | Italy | Cross‐sectional | 222 | 56.6 ± 12.5 | Essential arterial hypertension | PSQI | Office BP >140/90 mm Hg with ≥3 antihypertensive drugs or controlled BP with ≥4 drugs, including a diuretic | 113 (50.9) | 100 |
| Colbay et al (2015)16 | Turkey | Cross‐sectional | 94 | Diabetes mellitus: 51.4 ± 8.3; no diabetes mellitus: 50.5 ± 9.8 | With or without type 2 diabetes mellitus | PSQI | None | 37 (39.4) | 62.5 |
| Elliott and Lal (2016)40 | Australia | Cross‐sectional | 206 | 31.63 ± 8.51 | General | PSQI | None | 140 (68.0) | 100 |
| Erden et al (2010)24 | Turkey | Cross‐sectional | 133 | Dipper: 43.3 ± 6.3; nondipper: 44.3 ± 5.3 | Newly diagnosed stage I hypertension | PSQI | Seated SBP ≥140 mm Hg and seated DBP ≥90 mm Hg; dippers: decrease in nighttime systolic BP was at least 10% of the mean daytime values | 60 (45.1) | 100 |
| Henskens et al (2011)36 | Netherlands | Cross‐sectional | 203 | 52.1 ± 12.5 | Hypertension without antihypertensive treatment for ≥2 wk | GSQS | Untreated office SBP ≥140 mm Hg and/or DBP ≥90 mm Hg | 104 (51.2) | 75.0 |
| Huang et al (2011)25 | China | Cross‐sectional | 307 | 18–70; good sleeper: 54.3 ± 7.9; poor sleeper: 55.5 ± 9.5 | Hypertension with lifestyle modification for at least 6 mo and stable antihypertensive medication for >8 wk | PSQI | JNC 7: office SBP ≥140 mm Hg and/or DBP ≥90 mm Hg | 160 (52.1) | 100 |
| Jackowska et al (2016)35 | United Kingdom | Cross‐sectional | 119 | <45; 26 ± 4.9 | General | PSQI | None | 0 (0) | 50.0 |
| Kani et al (2016)49 | Turkey | Case‐control | 95 | 18–60; dipper: 46.4 ± 12.4; nondipper: 47.1 ± 11.9; control: unknown | Dipper and nondipper groups: hypertension; Control: healthy | PSQI | SBP ≥140 mm Hg and DBP ≥90 mm Hg with/without antihypertensive medication; dipping hypertension: >10% decrease in SBP and DBP | Dipper and nondipper group: 33; control: unknown | 50.0 |
| Kaya et al (2014)13, b | Turkey | Case‐control | 74 | Case: 43.7 ± 12.9; control: 45 ± 11.8 | Case: WCH; control: normotension | PSQI | WCH | 32 (43.2) | 80.0 |
| Liu et al (2016)14 | China | Cross‐sectional | 9404 | 20–93; 52.11 ± 14.10 | General | PSQI | JNC 7: SBP ≥140 mm Hg, DBP ≥90 mm Hg, or receiving treatment | 4770 (50.7) | 75.0 |
| Lu (2015)11, b | China | Cross‐sectional | 5461 | Men <45: 39.76 ± 5.08: men >45: 51.43 ± 8.03; women <45: 40.53 ± 3.74; women >45: 49.91 ± 4.46 | General without secondary hypertension, pregnancy hypertension, OSAS, or RLS | PSQI | JNC 7: SBP ≥140 mm Hg, DBP ≥90 mm Hg, or receiving treatment | 4076 (74.6) | 100 |
| Mahmood et al (2013)17 | Ireland | Cross‐sectional | 114 | Good sleeper: 64.0 ± 11.32; poor sleeper: 66.0 ± 10.98 | Type 2 diabetes mellitus | PSQI | None | 62 (54.4) | 62.5 |
| Mesas et al (2014)31, b | Spain | Cross‐sectional | 10342 | No difficulty in falling asleep: 45.2 (0.3); difficulty in falling asleep: 51.1 (0.5) | General | Difficulties in falling asleep | SBP ≥130 mm Hg or DBP ≥85 mm Hg or receiving treatment | 5185 (50.1) | 87.5 |
| Narang et al (2012)34 | Canada | Cross‐sectional | 3372 | 14.6 ± 0.5 | General | PSQI | Prehypertension: >90th to <95th percentile; hypertension: ≥99th percentile | 1650 (48.9) | 87.5 |
| Osonoi et al (2015)15 | Japan | Cross‐sectional | 724 | 25–69; 57.8 ± 8.6 | Type 2 diabetes mellitus | PSQI | None | 456 (62.9) | 87.5 |
| Sekercioglu et al (2015)19 | Canada | Cross‐sectional | 303 | 62.7 ± 14.5 | Chronic kidney disease | PSQI | None | 178 (58.7) | 62.5 |
| Senthil and Krishnadasa (2016)39 | India | Cross‐sectional | 84 | 18–23; 19.69 ± 0.878 | At least one known risk factor of hypertension | PSQI | JNC 7: prehypertension SBP 120–139 mm Hg; DBP 80–89 mm Hg | 40 (47.6) | 37.5 |
| Sforza et al (2014)41, b | France | Cross‐sectional | 500 | 72.0 ± 1.1 | General without insomnia | PSQI | SBP > 140 mm Hg or DBP > 90 mm Hg, receiving treatment, or 24‐h monitoring revealing a diurnal mean SBP > 135 mm Hg or DBP > 85 mm Hg; dippers: nocturnal SBP fell > 10% from daytime values | 220 (44.0) | 100 |
| Sherwood et al (2011)38 | United States | Cross‐sectional | 128 | 40–60; 45.7 ± 8.4 | Untreated hypertension | PSQI | JNC 7: SBP 130–159 mm Hg and/or DBP 85–99 mmHg | 76 (59.4) | 87.5 |
| Suh (2013)51 | United States | Cross‐sectional | 30 | 25–60; 40.82 ± 6.69 | General | PSQI | Nondippers were defined as those whose average nighttime SBP dropped <10% from the average daytime SBP | 0 (0) | 62.5 |
| Tavasoli et al (2015)32 | Iran | Cross‐sectional | 76 | Age range of all participants: 5–15; good sleepers: 7.4 ± 2.7; poor sleepers: 7.7 ± 2.6 | Urinary tract infection | PSQI | SBP or DBP ≥95 percentile for age, sex, and height. A <10% SBP drop during sleep was considered a nondipper state. | 19 (25.0) | 62.5 |
| Ulmer et al (2013)33 | United States | Cross‐sectional | 61 | 38.28 ± 12.08 | PTSD (either with or without depression), women with depression only (no PTSD), and a comparison group of women without PTSD or depression | PSQI | Dippers were defined as those having a day vs night difference in BP ≥10% of daytime BP | 0 (0) | 75.0 |
| Ulu et al (2013)36 | Turkey | Cross‐sectional | 100 | Dipper: 47.6 ± 14; nondipper: 55.2 ± 15 | Normotension | PSQI | None | 45 (45.0) | 75.0 |
| Yilmaz et al (2007)50 | Turkey | Cross‐sectional | 73 | 19–64 dipper: 48.5 ± 12.8; nondipper: 47.5 ± 11.9 | Stage I hypertension | PSQI | SBP 141–159 mm Hg and DBP 91–99 mmHg | 45 (61.6) | 50.0 |
| Yue et al (2012)42, b | China | Cross‐sectional | 660 | 93.5 ± 3.4 | General without secondary hypertension | PSQI | JNC 7: SBP ≥140 mmHg, DBP ≥90 mmHg, or receiving treatment | 216 (32.7) | 100 |
| Yuksel (2014)52 | Turkey | Cross‐sectional | 113 | 41.0 ± 10.7 | Normotension and healthy | PSQI | None | 81 (71.7) | 62.5 |
DBP, diastolic blood pressure; GSQS, Groningen Sleep Quality Scale; JNC 7, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; OSAS, obstructive sleep apnea syndrome; PSQI, Pittsburgh sleep quality index; PTSD, posttraumatic stress disorder; RLS, restless legs syndrome; SBP, systolic blood pressure; SD, standard deviation; SE, standard error; WCH, white‐coat hypertension.
Measured by the Joanna Briggs Institute tool.
Adjusted covariates among studies that examined the associations between poor sleep quality and the risk of hypertension using regression analysis.
Bansil et al: sex, age, body mass index, race, poverty status, diabetes mellitus, and smoking; Bruno et al: age, sex, previous cardiovascular events, diabetes mellitus, obesity, depressive symptoms, trait anxiety, and psychiatric drug use; Kaya et al: age, body mass index, sex, and diabetes mellitus; Lu et al: age, body mass index, total cholesterol, triglycerides, fasting blood glucose, exercise habit, status of smoking, status of drinking, and scores on the Generalized Anxiety Disorder 7‐Item Questionnaire and Patient Health Questionnaire 9; Mesas et al: sex, age, educational level, occupation‐based social class, smoking, alcohol intake, binge drinking, coffee intake, comorbidities, sleep duration, total energy intake and Mediterranean Diet Adherence Screener score, physical activity, time watching TV, and antihypertensive or lipid‐lowering drug treatment; Sforza et al: sex, body mass index, glycemia, dyslipidemia, current smoking, Hypnotic Intake, and Epworth Sleepiness Scale; Yue et al: age, blood pressure (BP), body mass index, blood glucose level, smoking habit, alcohol consumption, tea consumption, and exercise.
3.3. Sleep quality on hypertension
In eight of the included articles (Figure 2), poor sleep quality was significantly associated with a greater likelihood of hypertension (OR, 1.48; 95% confidence interval [CI], 1.13–1.95 [I 2 = 87%]).
Figure 2.
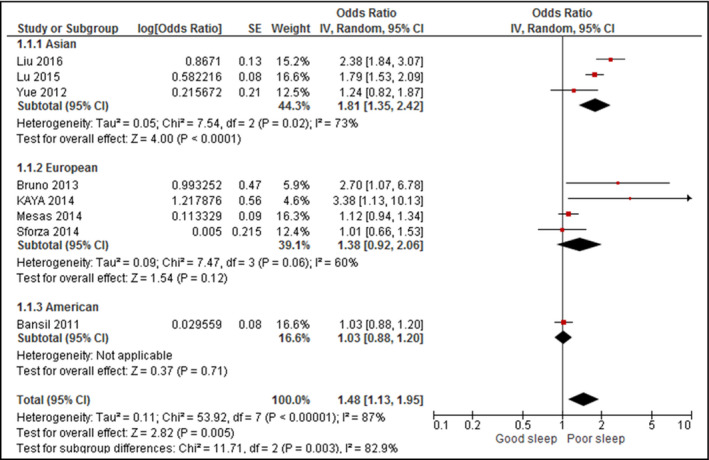
Forest plot for sleep quality and hypertension. CI indicates confidence interval; SD, standard deviation
When stratified by continents, a significant positive association was discerned between poor sleep quality and hypertension in Asia (OR, 1.81; 95% CI, 1.35–2.42) but not Europe (OR, 1.38; 95% CI, 0.92–2.06).
Heterogeneity decreased in all continents (I 2 = 73% in Asia and 60% and Europe), but remained substantially present (between 50% and 90%).28
Upon exclusion of studies that did not assess sleep quality by the PSQI,18, 31 the significant association persisted between poor sleep quality and hypertension (OR, 1.72; 95% CI, 1.29–2.31 [I 2 = 71%]). None of the included studies involved patients with medical diagnoses apart from hypertension.
3.4. Sleep quality on BP
A total of 11 studies assessed the association between sleep quality and SBP (Figure 3), while 10 studies assessed DBP (Figure 4). Although poor sleepers had higher SBP (mean difference, 4.37; 95% CI, −0.69 to 9.42 [I 2 = 96%]) and DBP (mean difference, 1.25; 95% CI, −1.20 to 3.70 [I 2 = 82%]), the difference was not statistically significant.
Figure 3.
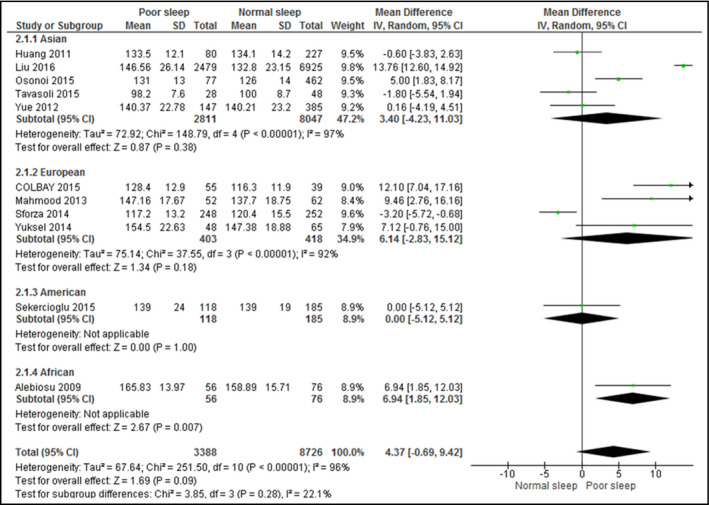
Forest plot for sleep quality and systolic blood pressure. CI indicates confidence interval; SD, standard deviation
Figure 4.
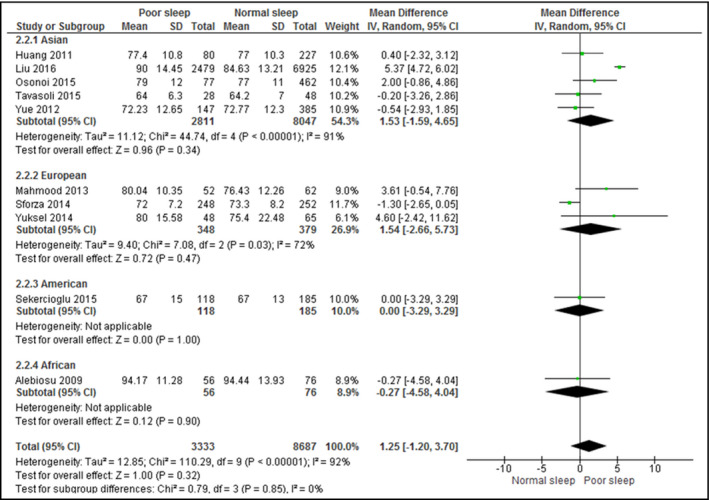
Forest plot for sleep quality and diastolic blood pressure. CI indicates confidence interval; SD, standard deviation
When stratified by continents, the association between poor sleep quality and SBP or DBP remained statistically insignificant for all continents. Heterogeneity remained high (over 70%) in studies from all continents for both outcomes and did not demonstrate any significant difference. All analyzed studies assessed the sleep quality by the PSQI.
Upon exclusion of studies that included patients with diabetes mellitus,15, 16, 17 urinary tract infections,32 and chronic kidney diseases,19 the association between poor sleep quality and SBP (mean difference, 3.99; 95% CI, −3.88 to 11.86 [I 2 = 98%]) or DBP (mean difference, 1.23; 95% CI, −2.28 to 4.74 [I 2 = 95%]) remained statistically insignificant and highly heterogeneous.
3.5. Hypertension/dipping on sleep quality
The difference in sleep quality scores between patients with hypertension and those with normotension was examined in five studies (Figure 5), while that between dippers and nondippers was examined in seven studies (Figure 6). The PSQI was the only scale used for sleep quality assessment. Patients with hypertension attained significantly higher PSQI scores (mean difference, 1.51; 95% CI, 1.00–2.02 [I 2 = 64%]), indicating poorer sleep quality, whereas dippers attained significantly lower scores (mean difference, −1.67; 95% CI, −2.43 to −0.91 [I 2 = 41%]), indicating better sleep quality.
Figure 5.
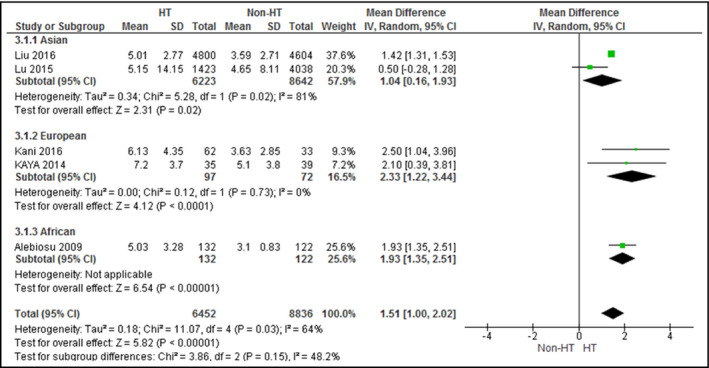
Forest plot for sleep quality scores between patients with and without hypertension. CI indicates confidence interval; SD, standard deviation
Figure 6.
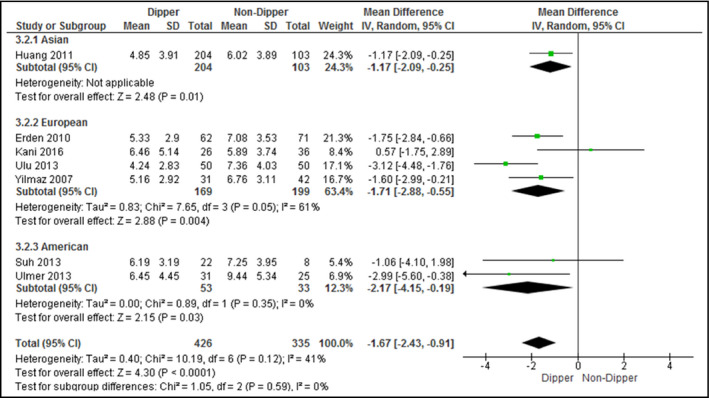
Forest plot for sleep quality scores between dippers and nondippers. CI indicates confidence interval; SD, standard deviation
When stratified by continents, Asian (mean difference, 1.04; 95% CI, 0.16–1.93) and European studies (mean difference, 2.33; 95% CI, 1.22–3.44) revealed poorer sleep quality for patients with hypertension than those with normotension. In European (mean difference, −1.71; 95% CI, −2.88 to −0.55) and American (mean difference, −2.17; 95% CI, −4.15 to −0.19) studies, dippers demonstrated better sleep quality than nondippers. No heterogeneity (I 2 = 0%) was found among European studies that examined the influence of hypertension on sleep quality and American studies that assessed the influence of BP dipping on sleep quality. All analyzed studies assessed sleep quality by the PSQI.
Upon exclusion of studies that recruited patients with posttraumatic stress disorder and/or depression,33 dippers were still found to have better sleep quality than nondippers with low heterogeneity (mean difference, −1.57; 95% CI, −2.37 to −0.77 [I 2 = 45%]). No studies were included for the analysis on the difference in sleep quality between patients with hypertension and those with normotension.
3.6. Publication bias
Since only the analysis on the difference of SBP (Figure 3) and DBP (Figure 4) between poor and normal/good sleepers included at least 10 studies, publication bias was assessed for these two outcomes. The funnel plots were not perfectly symmetrical, which suggests publication bias (Figures S1 and S2). The Egger test demonstrated potential publication bias for the meta‐analysis of SBP (P = .06) and DBP (P = .07). However, after performing the trim‐and‐fill method, no nonsymmetric study was identified, so the results of meta‐analysis remained the same.
3.7. Studies not included in meta‐analysis
Of the 29 eligible articles, seven were not included in the meta‐analysis, whose results are presented herein narratively. Narang and colleagues34 assessed sleep quality using a self‐developed sleep disturbance scale, with higher scores reflecting worse sleep quality. The results showed that patients with the highest tertile of sleep disturbance scores had significantly higher odds of hypertension (OR, 1.44; 95% CI, 1.00–1.64) than those with the lowest tertile.
As for the relationship between sleep quality and BP, Jackowska and associates35 conducted correlation analysis between BP and PSQI scores among 119 healthy women in the United Kingdom, but neither SBP nor DBP demonstrated any significant correlation with sleep quality. Henskens and reasearchers36 investigated whether subjective sleep disturbances during overnight BP monitoring resulted in higher nighttime BP readings of patients with untreated hypertension. The results showed that participants whose subjective sleep quality was lower on the second ambulatory BP monitoring (ABPM) than the first ABPM exhibited higher nocturnal BP levels than their counterparts with a similar sleep quality for both ABPM measurements. From the regression analysis performed by Berentzen and colleagues,37, 38 sleep quality did not have any significant prospective association with SBP and DBP in both sexes but was associated with SBP and DBP dipping.
Senthil and coworkers39, 40 studied the potential mechanism through which elevated BP could interfere with PSQI scores. Senthil and colleagues39 did not find significant differences in sleep quality between patients with systolic prehypertension and those with normotension, but those with diastolic prehypertension exhibited significantly lower PSQI scores. Moreover, Elliott and associates40 ascertained that poor sleep quality was significantly correlated with higher SBP and DBP values.
4. DISCUSSION
In the present review, a positive association between poor sleep quality and the presence of hypertension is observed. The average values of SBP and DBP are higher for poor sleepers, although the difference does not reach statistical significance. Two reasons can potentially explain the insignificant association between sleep quality and BP. First, only two studies examined both BP and the likelihood of hypertension.41, 42 Although the outcomes were similar, the studies were not identical in the meta‐analyses. Second, the studies examining the risk of hypertension involved 36 971 participants, whereas those assessing the SBP and DBP involved only 8726 and 8687 participants, respectively. The smaller sample size might have been insufficient for the detection of statistically significant results.
Despite the insignificant difference in BP between normal and poor sleepers, the results from the meta‐analysis are generally in agreement with those in the literature. Some measurements of objective sleep quality have been positively associated with the likelihood of hypertension, such as lower sleep efficiency (being asleep for less than 85% of the time in bed),43 higher beta power during nonrapid eye movement sleep,44 and decreased slow‐wave sleep.45 Some studies not included in the meta‐analysis demonstrated the significant influence exerted by poor sleep quality on the presence of hypertension34 and levels of BP,40 and the effect of hypertension on sleep quality.39 The above results imply that poor sleep quality leads to significant influence on the presence of hypertension. Apart from longer sleep duration, greater sleep hygiene and better sleep quality warrant the attention of scholars and health promotion practitioners.
In addition to the significant association between poor sleep and hypertension, patients with hypertension and nondippers have been found to exhibit worse sleep quality. The relationship between sleep‐disordered breathing, hypertension, and dipping in BP may account for the observation. Two large prospective cohort studies demonstrated that sleep‐disordered breathing increased the risk of hypertension after adjusting for anthropometric measurements and lifestyle factors.46, 47 Meanwhile, obstructive sleep apnea as a prevalent sleep disorder might increase BP variability and lessen nocturnal dipping by altering neuroendocrine control.48 As for poor sleep quality and nondipping BP, the direction of association is unlikely to be reversed. Physiologically, poor sleep quality may disrupt the circadian rhythm, leading to nondipping BP.24
As demonstrated by the sensitivity analysis, the magnitude of the effect and heterogeneity of the results remained the same upon removal of studies that did not assess sleep quality by the PSQI and of those that included patients with diagnosed diseases other than hypertension. Although some studies included did not assess sleep quality by the PSQI or the participants were not recruited from the general population, the overall findings of the present review were not altered.
Heterogeneity is high in the majority of the analyzed outcomes, which can be explained partly by regional differences in some outcomes, including the association between poor sleep and hypertension and the sleep quality scores of patients with and those without hypertension. However, other outcomes remained highly heterogeneous after subgroup analysis by regions. The numbers of participants included in Asia, Europe, and America were similar, so it is unlikely that the heterogeneity originated from large‐scale studies in any one particular continent. The varieties of the clinical characteristics of the included studies and of the sleep assessment tools might account for the heterogeneity. However, in the present study, the meta‐analysis that included only the general population or used the PSQI as an assessment tool remained far too heterogeneous. The high I‐square value implies the varieties of the clinical characteristics and of the assessment methods. Another possible reason is the differences in adjustments of regression analysis across studies. However, as previously described, many of the adjusted variables were similar across studies, including sex, age, body mass index, diabetic status, and/or smoking habits. Accordingly, it was unlikely for the heterogeneity to have originated from the difference in the adjustment methods.
The PSQI was the prominent sleep quality measurement in the present review, as is similar to the observation in previous reviews on sleep quality.9, 10 Only four of the included studies did not assess sleep quality by the PSQI.18, 31, 36, 37 Among these, three studies acquired the frequency of sleep problems and/or daytime outcomes, including short sleep,18 difficulties in falling asleep31 and difficulties in awakening.37 The remaining one study by Henskens and colleagues36 assessed sleep quality by the Groningen Sleep Quality Scale, which transformed the frequency of sleep disturbance into scores and provided definite cutoff points for poor sleep quality. Although different assessment tools were used, sleep disturbance, sleep duration, and daytime outcomes were the common constructs of sleep quality, which match with the seven components of the PSQI.
As mentioned, the PSQI is a reliable and valid instrument, the results of which can be interpreted easily. As revealed in previous studies, the PSQI global score had moderate associations with some objective sleep‐quality indexes including Polysomnography sleep maintenance, sleep efficiency, and microarousal index.8 Although it entails some subjectivity, the PSQI is a more convenient method to identify poor sleep quality in practice than the objective but prohibitively expensive methods such as polysomnography. The finding of our study lends evidence to its potential as a screening tool in both self‐assessments and clinical settings. Poor sleep quality and the presence of cardiovascular events can be further confirmed by sophisticated assessments.
The quality of the methodologies for the included studies varies, but only a few of them (5 of 29) scored ≤50%.20, 35, 39, 49, 50 The study with the lowest rating (37.5%) was not included in the meta‐analysis, while the number of participants of the other four studies was 541, which accounted for only 1.20% of the total patients included in the present review only. Therefore, the quality of the methodologies does not have a significant impact on the validity of the results.
5. STUDY STRENGTHS AND LIMITATIONS
The major strength of the present meta‐analysis lies in quantifying the influence of poor sleep quality on the presence of hypertension and the levels and dipping pattern of BP, which covers various populations in the world with reasonably large sample sizes. Nonetheless, several limitations should be addressed. First of all, the present study included only articles written in English, wherein eligible studies published in other languages might have been overlooked. Nevertheless, the geographical regions of interest in the included articles were evenly distributed throughout different continents. The language restriction is not a major flaw in the present literature search. In addition, the present review did not include studies that assessed objective sleep quality, so the utility of subjective sleep quality in assessing the risk of hypertension cannot be compared with that of objective sleep quality. Moreover, despite the predominance of the PSQI in assessing subjective sleep quality, only two of the 29 included articles reported how the PSQI subscales might associate with hypertension.11, 14 Last, the heterogeneity of the analyzed outcomes was high, despite the use of consistent methods of measurement for sleep quality and BP for most studies. Although the Egger test found the evidence of publication bias, the main findings were not altered by asymmetrical studies as shown in the trim‐and‐fill analysis.
In the present meta‐analysis, 26 of the 29 included studies were cross‐sectional; only the data of the cohort studies could not be included in the meta‐analysis.37 Although cohort studies have some protection from bias related to reverse causation, the cross‐sectional studies do not have such protection. Although the significant association found between poor sleep quality on BP has been supported by physiological evidence, the direction of the association remains to be confirmed. Accordingly, prospective cohort studies should be conducted to elucidate the temporal relationships. The PSQI is a recommended instrument because of its reliability and validity in sleep quality assessment.
6. CONCLUSIONS
Although it is not significantly associated with BP, poor subjective sleep quality has a significant association with an elevated risk of hypertension. Patients with hypertension and nondippers demonstrated significantly poorer subjective sleep quality. The findings suggest a relationship between sleep quality and the prevalence of hypertension, instead of one between sleep duration alone and its prevalence. The direction of the association between sleep quality and hypertension needs to be verified by larger‐scale cohort studies, and the PSQI is a recommended tool for sleep quality assessment. The general population can use the PSQI as a self‐assessment tool for identifying the presence of adverse BP levels.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Supporting information
Lo K, Woo B, Wong M, Tam W. Subjective sleep quality, blood pressure, and hypertension: a meta‐analysis. J Clin Hypertens. 2018;20:592–605. 10.1111/jch.13220
REFERENCES
- 1. van den Hoogen PCW, Feskens EJM, Nagelkerke NJD, et al. The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world. New Engl J Med. 2000;342:1‐8. [DOI] [PubMed] [Google Scholar]
- 2. Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang QJ, Xi B, Liu M, Zhang YQ, Fu MS. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta‐analysis. Hypertens Res. 2012;35:1012‐1018. [DOI] [PubMed] [Google Scholar]
- 4. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta‐analysis of prospective studies. Eur Heart J. 2011;32:1484‐1492. [DOI] [PubMed] [Google Scholar]
- 5. Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9:S10‐S17. [DOI] [PubMed] [Google Scholar]
- 6. Rosipal R, Lewandowski A, Dorffner G. In search of objective components for sleep quality indexing in normal sleep. Biol Psychol. 2013;94:210‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiat Res. 1989;28:193‐213. [DOI] [PubMed] [Google Scholar]
- 8. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non‐clinical samples: a systematic review and meta‐analysis. Sleep Med Rev. 2016;25:52‐73. [DOI] [PubMed] [Google Scholar]
- 9. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta‐analysis. Obes Rev. 2016;17:1154‐1166. [DOI] [PubMed] [Google Scholar]
- 10. Lee SW, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta‐analysis. Sleep Med Rev. 2017;31:91‐101. [DOI] [PubMed] [Google Scholar]
- 11. Lu K, Ding RJ, Tang Q, et al. Association between self‐reported global sleep status and prevalence of hypertension in Chinese adults: data from the Kailuan community. Int J Environ Res Public Health. 2015;12:488‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bruno RM, Palagini L, Gemignani A, et al. Poor sleep quality and resistant hypertension. Sleep Med. 2013;14:1157‐1163. [DOI] [PubMed] [Google Scholar]
- 13. Kaya Z, Kayrak M, Demir K, et al. The relationship between white coat hypertension and sleep quality. Sleep Biol Rhythms. 2014;12:203‐211. [Google Scholar]
- 14. Liu RQ, Qian ZM, Trevathan E, et al. Poor sleep quality associated with high risk of hypertension and elevated blood pressure in China: results from a large population‐based study. Hypertens Res. 2016;39:54‐59. [DOI] [PubMed] [Google Scholar]
- 15. Osonoi Y, Mita T, Osonoi T, et al. Poor sleep quality is associated with increased arterial stiffness in Japanese patients with type 2 diabetes mellitus. BMC Endocr Disord. 2015;15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colbay G, Cetin M, Colbay M, Berker D, Guler S. Type 2 diabetes affects sleep quality by disrupting the respiratory function 2. J Diabetes. 2015;7:664‐671. [DOI] [PubMed] [Google Scholar]
- 17. Mahmood WA, Yusoff MS, Behan LA, et al. Association between sleep disruption and levels of lipids in Caucasians with type 2 diabetes. Int J Endocrinol. 2013;2013:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bansil P, Kuklina EV, Merritt RK, Yoon PW. Associations between sleep disorders, sleep duration, quality of sleep, and hypertension: results from the National Health and Nutrition Examination Survey, 2005 to 2008. J Clin Hypertens (Greenwich). 2011;13:739‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sekercioglu N, Curtis B, Murphy S, Barrett B. Sleep quality and its correlates in patients with chronic kidney disease: a cross‐sectional design. Ren Fail. 2015;37:757‐762. [DOI] [PubMed] [Google Scholar]
- 20. Alebiosu OC, Ogunsemi OO, Familoni OB, Adebayo PB, Ayodele OE. Original research: quality of sleep among hypertensive patients in a semi‐urban Nigerian community: a prospective study. Postgrad Med. 2009;121:166‐172. [DOI] [PubMed] [Google Scholar]
- 21. Routledge FS, McFetridge‐Durdle JA, Dean CR. Stress, menopausal status and nocturnal blood pressure dipping patterns among hypertensive women. Can J Cardiol. 2009;25:E157‐E163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakano Y, Oshima T, Ozono R, et al. Non‐dipper phenomenon in essential hypertension is related to blunted nocturnal rise and fall of sympatho‐vagal nervous activity and progress in retinopathy. Auton Neurosci‐Basic. 2001;88:181‐186. [DOI] [PubMed] [Google Scholar]
- 23. Birkenhager AM, van den Meiracker AH. Causes and consequences of a non‐dipping blood pressure profile. Neth J Med. 2007;65:127‐131. [PubMed] [Google Scholar]
- 24. Erden I, Erden EC, Ozhan H, et al. Poor‐quality sleep score is an independent predictor of nondipping hypertension. Blood Press Monit. 2010;15:184‐187. [DOI] [PubMed] [Google Scholar]
- 25. Huang YL, Mai WY, Hu YZ, et al. Poor sleep quality, stress status, and sympathetic nervous system activation in nondipping hypertension. Blood Press Monit. 2011;16:117‐123. [DOI] [PubMed] [Google Scholar]
- 26. Ulu SM, Ulu S, Ulasli SS, et al. Is impaired sleep quality responsible for a nondipping pattern even in normotensive individuals? Blood Press Monit. 2013;18:183‐187. [DOI] [PubMed] [Google Scholar]
- 27. JBI Institute . Joanna Briggs Institute reviewers’ manual: 2011 ed. Adelaide, South Australia: Joanna Briggs Institute; 2011. [Google Scholar]
- 28. Cochrane Collaboration . Cochrane handbook for systematic reviews of interventions, version 5.1.0. London, UK: Cochrane Collaboration; 2011. [Google Scholar]
- 29. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119‐1129. [DOI] [PubMed] [Google Scholar]
- 30. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455‐463. [DOI] [PubMed] [Google Scholar]
- 31. Mesas AE, Guallar‐Castillon P, Lopez‐Garcia E, et al. Sleep quality and the metabolic syndrome: the role of sleep duration and lifestyle. Diabetes‐Metab Res. 2014;30:222‐231. [DOI] [PubMed] [Google Scholar]
- 32. Tavasoli A, Saeidi M, Hooman N. Correlation between sleep quality and blood pressure changes in Iranian children. J Compr Ped. 2015;6:ee24805. [Google Scholar]
- 33. Ulmer CS, Calhoun PS, Bosworth HB, Dennis MF, Beckham JC. Nocturnal blood pressure non‐dipping, posttraumatic stress disorder, and sleep quality in women. Behav Med. 2013;39:111‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Narang I, Manlhiot C, Davies‐Shaw J, et al. Sleep disturbance and cardiovascular risk in adolescents. Can Med Assoc J. 2012;184:E913‐E920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jackowska M, Ronaldson A, Brown J, Steptoe A. Biological and psychological correlates of self‐reported and objective sleep measures. J Psychosom Res. 2016;84:52‐55. [DOI] [PubMed] [Google Scholar]
- 36. Henskens LHG, van Boxtel MPJ, Kroon AA, van Oostenbrugge RJ, Lodder J, de Leeuw PW. Subjective sleep disturbance increases the nocturnal blood pressure level and attenuates the correlation with target‐organ damage. J Hypertens. 2011;29:242‐250. [DOI] [PubMed] [Google Scholar]
- 37. Berentzen NE, Smit HA, Bekkers MB, et al. Time in bed, sleep quality and associations with cardiometabolic markers in children: the Prevention and Incidence of Asthma and Mite Allergy birth cohort study. J Sleep Res. 2014;23:3‐12. [DOI] [PubMed] [Google Scholar]
- 38. Sherwood A, Routledge FS, Wohlgemuth WK, Hinderliter AL, Kuhn CM, Blumentnal JA. Blood pressure dipping: ethnicity, sleep quality, and sympathetic nervous system activity. Am J Hypertens. 2011;24:982‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Senthil S, Krishnadasa SN. Prehypertension and its determinants in apparently healthy young adults. J Clin Diagn Res. 2016;10:CC05‐CC08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elliott JL, Lal S. Blood pressure, sleep quality and fatigue in shift working police officers: effects of a twelve hour roster system on cardiovascular and sleep health. Int J Environ Res Public Health. 2016;13:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sforza E, Saint Martin M, Barthelemy JC, Roche F. Association of self‐reported sleep and hypertension in non‐insomniac elderly subjects. J Clin Sleep Med. 2014;10:965‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yue JR, Wang H, Huang CQ, Dong BR. Association between sleep quality and arterial blood pressure among Chinese nonagenarians/centenarians. Med Sci Monitor. 2012;18:Ph36‐Ph42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Javaheri S, Storfer‐Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matthews KA, Chang YF, Kravitz HM, et al. Sleep and risk for high blood pressure and hypertension in midlife women: the SWAN (Study of Women's Health Across the Nation) Sleep Study. Sleep Med. 2014;15:203‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fung MM, Peters K, Redline S, et al. Decreased slow wave sleep increases risk of developing hypertension in elderly men. Hypertension. 2011;58:596‐U159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hu FB, Willett WC, Colditz GA, et al. Prospective study of snoring and risk of hypertension in women. Am J Epidemiol. 1999;150:806‐816. [DOI] [PubMed] [Google Scholar]
- 47. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. New Engl J Med. 2000;342:1378‐1384. [DOI] [PubMed] [Google Scholar]
- 48. Horne RS, Yang JS, Walter LM, et al. Nocturnal dipping is preserved in children with sleep disordered breathing regardless of its severity. Pediatr Pulm. 2013;48:1127‐1134. [DOI] [PubMed] [Google Scholar]
- 49. Kani AS, Sunbul M, Kani HT, Yanartas O, Tezcan N, Emul M. Dream anxiety, chronotype and dipping pattern in hypertensive patients assessed with 24 h ambulatory blood pressure monitoring. Sleep Biol Rhythms. 2016;14:23‐30. [Google Scholar]
- 50. Yilmaz MB, Yalta K, Turgut OO, et al. Sleep quality among relatively younger patients with initial diagnosis of hypertension: dippers versus non‐dippers. Blood Press. 2007;16:101‐105. [DOI] [PubMed] [Google Scholar]
- 51. Suh M, Barksdale DJ, Logan J. Relationships among acculturative stress, sleep, and nondipping blood pressure in Korean American women. Clin Nurs Res. 2013;22:112‐129. [DOI] [PubMed] [Google Scholar]
- 52. Yuksel M, Yildiz A, Demir M, et al. Effect of sleep quality on hemodynamic response to exercise and heart rate recovery in apparently healthy individuals. Clin Invest Med. 2014;37:352‐362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


