Abstract
Obstructive sleep apnea causes blood pressure (BP) surges during sleep, which may lead to increased sleep‐onset cardiovascular events. The authors recently developed an oxygen‐triggered nocturnal BP monitoring system that initiates BP measurements when oxygen desaturation (SpO2) falls below a variable threshold. The association between nocturnal BP parameters obtained by nocturnal BP monitoring and simultaneously examined polysomnography‐derived sleep parameters in 116 patients with obstructive sleep apnea (mean age 57.9 years, 85.3% men) was studied. In multivariable analysis with independent factors of age, body mass index, sex, and polysomnography‐derived measures (apnea‐hypopnea index, apnea index, arousal index, lowest SpO2, and SpO2 < 90%), apnea‐hypopnea index (β = .26, P = .02) and lowest SpO2 (β = −.34, P < .001) were independent determinants of hypoxia‐peak systolic BP (SBP), defined as the maximum SBP value measured by nocturnal BP monitoring. Similarly, apnea‐hypopnea index (β = .21, P = .04) and lowest SpO2 (β = −.49, P < .001) were independent determinants of nocturnal SBP surge, defined as the difference between the hypoxia‐peak SBP and the average of the SBP values within 30 minutes before and after the hypoxia‐peak SBP, measured by the fixed‐interval function in the manner of conventional ambulatory BP monitoring. In conclusion, in polysomnography‐derived parameters, lowest SpO2, defined as the minimum SpO2 value during sleep, is the strongest independent determinant of hypoxia‐peak SBP and nocturnal SBP surge measured by nocturnal BP monitoring. Our findings suggest that the severity of the decrease in SpO2 and the frequency of such decreases would be important indicators to identify high‐risk patients who are likely to develop cardiovascular events specifically during sleep.
Keywords: hypertension, nocturnal blood pressure, nocturnal BP surge, obstructive sleep apnea, polysomnography, triggered nocturnal BP monitoring
1. INTRODUCTION
The nocturnal blood pressure (BP) obtained by ambulatory BP monitoring (ABPM) has been reported to be a stronger predictor of cardiovascular morbidity and mortality than either daytime ambulatory BP or clinic BP.1, 2, 3, 4 In addition to nocturnal BP level, nocturnal BP variability (BPV) expressed as the standard deviation (SD) of SBP values obtained by ABPM during sleep has been found to be an independent predictor of cardiovascular events and mortality.5
OSA, which is the most frequent cause of secondary hypertension and resistant hypertension,6 causes repetitive hypoxia, CO2 retention, consequent sympathetic activation, and repeated exaggerated BP surges during sleep.7, 8, 9 Thus, OSA can lead to increases in not only nocturnal BP level but also nocturnal BPV, each of which increases the cardiovascular risk independently of the other.10, 11 Although the detection of BP surges triggered by OSA episodes is clinically meaningful for the assessment of cardiovascular risks, conventional ABPM with a fixed interval (eg, 30 minutes) of BP measurements cannot detect such apnea‐triggered BP surges because it cannot measure BP synchronized with apnea episodes.
To solve this problem, we recently developed a triggered nocturnal BP monitoring (TNP) method based on an oxygen‐trigger function that initiates a BP measurement when the patient’s oxygen saturation (SpO2) falls below a variable threshold.12, 13, 14, 15, 16, 17, 18, 19 Our previous study19 demonstrated that hypoxia‐peak systolic BP (SBP) values measured by triggered nocturnal BP monitoring were markedly higher (by approximately 25 mm Hg) than mean nocturnal SBP values measured by conventional fixed‐interval BP monitoring, and the magnitude of hypoxia‐peak SBP was quite different even among patients with OSA with comparable levels of mean nocturnal SBP measured by conventional fixed‐interval BP monitoring. This difference might be partially explained by the patients’ demographics and the levels of sleep disorder or sleep quality. Therefore, an improved understanding of the factors affecting OSA‐related nocturnal BPs would be helpful for identifying patients with OSA at high risk for cardiovascular events.
To our knowledge, however, the determinants of OSA‐related nocturnal BP parameters have not been clearly identified. The objective of our present study was to identify the determinants of OSA‐related nocturnal BP parameters measured by TNP in patients with OSA in order to determine which of the simultaneously conducted polysomnography (PSG)‐derived parameters (apnea‐hypopnea, arousal, or hypoxia [oxygen‐related] index) are associated with OSA‐related nocturnal BP parameters.
2. METHODS
2.1. Study protocol
A total of 147 outpatients were recruited between June 2009 and March 2013. The study participants met both of the following criteria: (1) subjective symptoms of sleep apnea syndrome such as heavy sleepiness during the day, and (2) 3% oxygen desaturation index >15 per hour in the screening test of sleep apnea syndrome with pulse oximetry. All patients underwent overnight full PSG and TNP simultaneously for two consecutive nights in the sleep laboratory of Washiya Hospital (Tochigi, Japan). Each patient’s PSG parameters, including SpO2, and findings obtained by electroencephalography, electrocardiology, electromyography, and electro‐oculography (L [left], R [right]), airflow, and thoracic and abdominal movement were assessed by the PS2 Plus Sleep Watcher system (Compumedics). The apnea‐hypopnea index (AHI) was defined as the mean number of apnea and hypopnea events per hour of sleep. Apnea was defined as the complete or almost complete cessation of airflow, and hypopnea was defined as a decrease in airflow or thoracoabdominal excursion of ≥50% of the baseline value for 10 seconds or longer, accompanied by a ≥3% decrease in SpO2. The arousal index (ArI) was defined as the mean number of arousal episodes per hour. Lowest SpO2 was defined as the minimum SpO2 value during the night, and SpO2 < 90% was defined as the percentage of time spent below 90%. These parameters were automatically calculated by the PSG system. Because 31 patients had no BP measured by TNP, the number of patients in the study analysis was 116. This study was approved by the institutional research board of Washiya Hospital, and informed consent for participation in the study was given by every participant.
2.2. Clinic BP measurements
Clinic BP was measured before bedtime (evening BP) and just after waking up (morning BP) on both measurement days. During each clinic BP measurement, triplicate BP measurements were taken by a nurse with an HEM‐780 validated cuff‐oscillometric BP monitor (Omron Healthcare)20 with the patient in the sitting position, with his or her back supported, without his or her legs crossed, and with both arms supported at the heart level after a 5‐minute rest. The average value of these three readings was used in the analysis.
2.3. TNP monitoring
The TNP system consists of a pulse oximeter, a cuff‐oscillometric BP monitor (HEM‐780), and a computer program including a BP measurement–triggering algorithm. SpO2 is continuously measured every 5 seconds by a pulse oximeter placed over the finger and is then transferred to the computer program run on a personal computer through the interface circuit.16 When the SpO2 value falls below the threshold level, the program sends a trigger signal to the BP monitor to initiate BP measurement. If the current SpO2 data have error‐flag information caused by movement (for example), the triggering algorithm ignores the data, which means it does not generate the signal to initiate BP measurement. The threshold to initiate BP measurement is initially set to the patient’s baseline SpO2 value (ie, the SpO2 value immediately before bedtime) minus 10% of the SpO2 value. Once triggered, the threshold is continuously decreased according to the current SpO2 value until a dip in SpO2 is reached, and, thereafter, the threshold is increased at a rate of 10% per hour from the values of the SpO2 dip. For example, if the baseline SpO2 value is 98% and the first dip of SpO2 after the start of the measurement is 80%, BP measurement is initiated when the SpO2 value falls below 88% and the threshold is renewed to 80%. After the threshold is renewed, the threshold level to initiate BP measurement is increased in proportion to the time until the threshold level reaches the initial threshold level (ie, the threshold at 30 minutes after the SpO2 dip is 85% and at 1 hour after the SpO2 dip is 88%). Once triggered, the BP monitor takes three measurements: SBP, diastolic BP, and pulse rate. The intervals between each BP measurement were set to 15 seconds. In addition to this oxygen‐triggered BP measurement function, TNP measures BP at a fixed interval (the same function as that of ABPM). In the present study, the nocturnal BP measurement was obtained by both the oxygen‐triggered function and the fixed‐interval function (every 30 minutes).
2.4. Definition of BP parameters
Nocturnal BP was defined as in our previous studies.14, 19 Regarding nocturnal BP parameters, we defined the “hypoxia‐peak SBP” as the maximum SBP value measured by the oxygen‐triggered function, the “maximum nocturnal SBP” as the maximum SBP value measured only by the fixed‐interval function, the “mean nocturnal SBP” as the average of the nocturnal SBP values measured only by the fixed‐interval function, and the “minimum nocturnal SBP” as the lowest SBP value among all of the nocturnal SBP values measured by either the oxygen‐triggered or fixed‐interval functions. Regarding the nocturnal BPV parameters, we defined the “nocturnal SBP surge” as the difference between the hypoxia‐peak SBP and the average of the SBP values measured by the fixed‐interval function within 30 minutes before and after the hypoxia‐peak SBP and the “SD of nocturnal SBP” as the SD of SBP measured only by the fixed‐interval function.
2.5. Statistical analysis
PSG‐derived parameters (ie, the AHI, apnea index, ArI, lowest SpO2, and SpO2 < 90%) and BP and pulse rate levels were calculated as the average value of each parameter on two consecutive days in 116 patients with OSA and were expressed as mean ± SD. In the univariable analysis of nocturnal BP parameters and nocturnal BPV parameters, the individual values for each night (116 patients × 2 nights = 232 data points) were used in the analysis. Pearson correlation analysis was used for the analysis of all variables other than sex and Spearman correlation was used for the analysis of sex. Multivariate linear regression analyses of nocturnal BP parameters and nocturnal BPV parameters were conducted by the forcible loading method to assess independent determinants of nocturnal BP parameters. All statistical analyses were performed using SPSS software, version 24 (IBM). A P value of <.05 was considered statistically significant.
3. RESULTS
The clinical characteristics of the 116 patients with OSA are shown in Table 1. Their average age was 57.9 ± 13.7 years (mean ± SD). There were 99 men and 17 women. Most patients were taking hypertensive drugs. Their average AHI value was 39.6 ± 17.5 events per hour (mean ± SD). AHI had a close relationship with BMI in the univariable analysis (r = .47, P < .001).
Table 1.
Clinical characteristics of the study patients with obstructive sleep apnea (n = 116)
| Age, y | 57.9 ± 13.7 |
| Men/women, No. (%) | 99/17 (85.3/14.7) |
| BMI, kg/m2 | 29.2 ± 5.5 |
| Hypertension, % | 70.7 |
| Hyperlipidemia, % | 25.9 |
| Diabetes mellitus, % | 16.4 |
| History of angina, % | 6.9 |
| History of myocardial infarction, % | 5.2 |
| History of stroke, % | 4.3 |
| Antihypertensive drugs, % | 66.4 |
| Calcium channel blockers, % | 43.1 |
| ACEIs/ARBs, % | 56.0 |
| Diuretics, % | 30.2 |
| Other, % | 17.2 |
| PSG‐derived parameters | |
| Apnea‐hypopnea index, per h | 39.6 ± 17.5 |
| Apnea index, per h | 19.0 ± 16.1 |
| Arousal index, per h | 35.7 ± 16.5 |
| Lowest SpO2, % | 74.8 ± 8.3 |
| SpO2 < 90%, % | 16.8 ± 17.2 |
ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; SpO2, oxygen saturation.
Data are expressed as mean ± standard deviation or percentage. Polysomnography (PSG)‐derived parameters are the average value of each parameter on two consecutive days.
Table 2 shows BP and pulse rate levels calculated as the average value of each parameter over two consecutive days in 116 patients with OSA. The hypoxia‐peak SBP measured by the oxygen‐triggered function was higher by 25.1 mm Hg than the mean nocturnal SBP measured by the fixed‐interval function. The hypoxia‐peak SBP was significantly correlated with the maximum nocturnal SBP (r = .83, P < .001). Although the hypoxia‐peak SBP was not significantly higher than the maximum nocturnal SBP in all patients, it was significantly higher than the maximum nocturnal SBP in patients in the moderate and severe OSA (AHI > 20) group (149.4 ± 21.0 mm Hg for hypoxia‐peak SBP and 146.3 ± 16.6 mm Hg for maximum nocturnal SBP, P < .01 by paired t test; data not shown).
Table 2.
Blood pressure and pulse rate levels in all patients
| All patients (N = 116) | Nonmedicated patients with hypertension (n = 39) | Medicated patients with hypertension (n = 77) | |
|---|---|---|---|
| Evening | |||
| SBP, mm Hg | 127.7 ± 14.0 | 127.8 ± 15.0 | 127.6 ± 13.6 |
| DBP, mm Hg | 80.6 ± 11.3 | 84.6 ± 12.6 | 78.6 ± 10.1* |
| PR, beats per min | 70.3 ± 9.6 | 74.3 ± 10.9*** | 68.2 ± 8.2* |
| Nighttime | |||
| Fixed‐interval function | |||
| Maximum SBP, mm Hg | 147.1 ± 16.4 | 148.6 ± 17.6 | 146.3 ± 15.9 |
| Mean SBP, mm Hg | 123.7 ± 14.0 | 124.4 ± 14.8 | 123.4 ± 13.7 |
| SD of SBP, mm Hg | 11.7 ± 3.0 | 11.5 ± 3.0 | 11.8 ± 3.0 |
| SBP at 2 am, mm Hg | 121.6 ± 17.8 | 121.9 ± 18.3 | 121.4 ± 17.7 |
| Mean SBP at 2 am and 3 am, mm Hg | 121.4 ± 16.4 | 121.5 ± 17.9 | 121.4 ± 15.6 |
| Mean SBP at 2 am, 3 am, and 4 am, mm Hg | 121.7 ± 16.1 | 122.1 ± 17.1 | 121.5 ± 15.7 |
| Mean DBP, mm Hg | 76.4 ± 10.8 | 79.9 ± 11.7 | 74.6 ± 9.9** |
| Mean PR, beats per min | 60.1 ± 7.5 | 62.7 ± 9.4 | 58.8 ± 6.0* |
| Oxygen‐triggered function | |||
| Hypoxia––peak SBP, mm Hg | 148.8 ± 20.5 | 150.4 ± 22.5 | 148.0 ± 19.5 |
| Hypoxia––mean SBP, mm Hg | 125.3 ± 15.1 | 125.2 ± 14.5 | 125.3 ± 15.5 |
| Hypoxia––SD SBP, mm Hg | 10.5 ± 3.6 | 10.4 ± 4.2 | 10.6 ± 3.2 |
| Hypoxia––mean DBP, mm Hg | 76.7 ± 10.7 | 79.5 ± 10.7 | 75.3 ± 10.4** |
| Hypoxia––mean PR, beats per min | 59.8 ± 7.2 | 61.8 ± 8.7 | 58.8 ± 6.2** |
| Nocturnal SBP surge, mm Hg | 25.9 ± 12.7 | 27.6 ± 15.6 | 24.9 ± 10.9 |
| Minimum nocturnal SBP, mm Hg | 96.3 ± 14.2 | 96.9 ± 15.1 | 96.0 ± 13.8 |
| Morning | |||
| SBP, mm Hg | 136.6 ± 15.2 | 134.3 ± 17.1 | 137.7 ± 14.1 |
| DBP, mm Hg | 83.2 ± 11.1 | 86.5 ± 12.1 | 81.5 ± 10.3** |
| PR, beats per min | 64.0 ± 9.5 | 67.9 ± 12.3*** | 62.0 ± 6.9* |
DBP, diastolic blood pressure; PR, pulse rate; SBP, systolic blood pressure.
Calculated as the average value of each parameter on two consecutive days in all 116 patients: 39 nonmedicated patients with hypertension and 77 medicated patients with hypertension and obstructive sleep apnea. Data are expressed as mean ± standard deviation (SD). The individual values of each parameter were calculated as the average of two consecutive nights.
*P < .01 and **P < .05 vs nonmedicated patients with hypertension by t test; ***P < .05 vs all patients by t test.
In the univariable analysis of nocturnal BP parameters (Table S1), young age, high body mass index (BMI), high AHI, high apnea index, low lowest SpO2, and high SpO2 < 90% were associated with increased hypoxia‐peak SBP. Maximum nocturnal SBP was associated with high BMI, low lowest SpO2, and high SpO2 < 90%. Mean nocturnal SBP was associated with only high SpO2 < 90%. Minimum nocturnal SBP was associated with low BMI and high lowest SpO2.
In the univariable analysis of nocturnal BP surge and variability (Table S2), young age, high BMI, high AHI, high apnea index, high ArI, low lowest SpO2, and high SpO2 < 90% were associated with increased nocturnal SBP surge. High BMI, low lowest SpO2, and high SpO2 < 90% were associated with increased SD of nocturnal SBP. SD of nocturnal SBP was also associated with nocturnal SBP surge (r = .21, P < .01), hypoxia‐peak SBP (r = .19, P < .01) and minimum nocturnal SBP (r = −.30, P < .001) (data not shown).
Figure S1 shows scatterplots of the linear relationships between nocturnal BP parameters and PSG‐derived parameters and Figure S2 shows that in nonmedicated patients with hypertension (39 patients × 2 nights = 78 data points). FigureA shows the slope of the regression line (absolute value) between hypoxia‐peak SBP and lowest SpO2 (=slope A) and FigureB shows that between nocturnal SBP surge and lowest SpO2 (=slope B) in different subgroups of different classes of hypertensive drugs. Slope A in nonmedicated patients with hypertension (−1.25) was significantly greater than that in all patients (−1.02) and that in each class of drugs. Slope A in patients who were administrated calcium channel blockers (−0.58) was the lowest of all subgroups. Similarly, slope B in nonmedicated patients with hypertension (−1.02) was significantly greater than that in all patients (−0.88) and that for each class of drugs. Slope B in patients who were administrated α‐adrenergic or β‐adrenergic blockers (−0.64) was the lowest of all subgroups. Similar trends were seen in the analysis of the correlation coefficient between hypoxia‐peak SBP and lowest SpO2 (Figure S3A) and that between nocturnal SBP surge and lowest SpO2 (Figure S3B).
Figure 1.
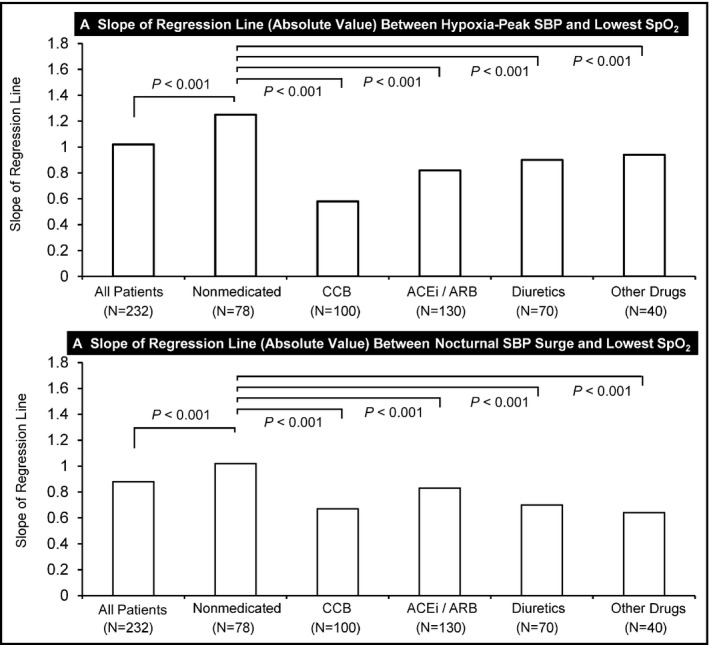
Slope of the regression line (absolute value) between hypoxia‐peak systolic blood pressure (SBP) and lowest oxygen saturation (SpO2) and between nocturnal SBP surge and lowest SpO2 in subgroups treated with different classes of hypertensive medication. ARB, angiotensin receptor blocker; ACEi, angiotensin‐converting enzyme inhibitor; CCB, calcium channel blocker; SBP, systolic blood pressure
Table 3 shows the multiple linear regression analysis of nocturnal BP parameters with demographics and PSG‐derived parameters. When the independent factors were age, BMI, sex, and PSG‐derived parameters (AHI, apnea index, ArI, lowest SpO2, and SpO2 < 90%), high AHI and low lowest SpO2 were independent determinants of increased hypoxia‐peak SBP. Maximum nocturnal SBP and mean nocturnal SBP had no independent determinants. BMI was an independent determinant of minimum nocturnal SBP. When we defined minimum nocturnal SBP as the minimum SBP measured by only a fixed‐interval function, minimum nocturnal SBP had no significant independent determinant in the multivariate analysis (data not shown). In the subgroup of nonmedicated patients with hypertension, old age, high BMI, and low lowest SpO2 were independent determinants of increased hypoxia‐peak SBP. Here, β means standardized partial regression coefficient. Old age was an independent determinant of maximum nocturnal SBP, high BMI was an independent determinant of mean nocturnal SBP, and minimum nocturnal SBP had no independent determinant in the nonmedicated group. High AHI and low lowest SpO2 were independent determinants of increased nocturnal SBP surge (Table 4). Old age and high BMI were independent determinants of increased SD of nocturnal SBP. In the subgroup of nonmedicated patients with hypertension, low lowest SpO2 was an independent determinant of increased nocturnal SBP surge. Old age was an independent determinant of increased SD of nocturnal SBP in the nonmedicated group.
Table 3.
Multiple linear regression analysis of nocturnal blood pressure parameters with demographics and PSG‐derived parameters
| Variable | All patients (N = 232) | Nonmedicated with patients hypertension (n = 78) | Medicated patients with hypertension (n = 154) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | β | SE | P value | |
| Hypoxia‐peak SBP | |||||||||
| Age | .00 | 0.12 | .99 | .31 | 0.25 | .02 | −.18 | 0.18 | .06 |
| Sex (0: women, 1: men) | .07 | 4.17 | .29 | .07 | 10.8 | .47 | .06 | 4.57 | .45 |
| BMI | −.06 | 0.38 | .55 | .41 | 0.66 | .03 | −.25 | 0.49 | .02 |
| Apnea‐hypopnea index | .26 | 0.13 | .02 | .03 | 0.24 | .88 | .30 | 0.17 | .01 |
| Apnea index | −.19 | 0.13 | .054 | .14 | 0.27 | .51 | −.24 | 0.15 | .03 |
| Arousal index | −.01 | 0.09 | .87 | .12 | 0.17 | .32 | −.08 | 0.11 | .40 |
| Lowest SpO2 | −.34 | 0.24 | <.001 | −.57 | 0.42 | <.01 | −.18 | 0.29 | .09 |
| SpO2 < 90% | .09 | 0.15 | .42 | −.42 | 0.25 | .07 | .24 | 0.19 | .049 |
| R 2 | 19.4% | <.001 | 38.5% | <.001 | 19.1% | <.001 | |||
| Maximum nocturnal SBP | |||||||||
| Age | .08 | 0.11 | .34 | .38 | 0.23 | .02 | −.03 | 0.16 | .80 |
| Sex (0: women, 1: men) | .13 | 3.63 | .07 | .11 | 10.1 | .35 | .12 | 4.05 | .18 |
| BMI | .03 | 0.33 | .74 | .31 | 0.61 | .15 | −.05 | 0.43 | .67 |
| Apnea‐hypopnea index | .13 | 0.12 | .28 | .17 | 0.22 | .51 | .12 | 0.15 | .37 |
| Apnea index | −.10 | 0.11 | .35 | −.08 | 0.25 | .76 | −.10 | 0.13 | .38 |
| Arousal index | −.13 | 0.08 | .11 | −.05 | 0.16 | .74 | −.15 | 0.09 | .14 |
| Lowest SpO2 | −.08 | 0.21 | .42 | −.04 | 0.39 | .86 | −.06 | 0.25 | .57 |
| SpO2 < 90% | .15 | 0.13 | .22 | .09 | 0.23 | .74 | .14 | 0.17 | .30 |
| R 2 | 6.9% | .04 | 15.3% | .15 | 5.8% | .36 | |||
| Mean nocturnal SBP | |||||||||
| Age | .02 | 0.09 | .81 | .29 | 0.19 | .06 | −.13 | 0.13 | .19 |
| Sex (0: women, 1: men) | .10 | 2.99 | .16 | .21 | 8.02 | .08 | .05 | 3.35 | .58 |
| BMI | −.01 | 0.27 | .94 | .44 | 0.49 | .045 | −.2 | 0.36 | .14 |
| Apnea‐hypopnea index | .13 | 0.09 | .28 | −.002 | 0.18 | .99 | .16 | 0.12 | .22 |
| Apnea index | −.20 | 0.09 | .06 | −.08 | 0.20 | .74 | −.18 | 0.11 | .11 |
| Arousal index | −.08 | 0.06 | .30 | −.004 | 0.13 | .98 | −.12 | 0.08 | .26 |
| Lowest SpO2 | .03 | 0.17 | .74 | −.06 | 0.31 | .78 | .12 | 0.21 | .28 |
| SpO2 < 90% | .24 | 0.10 | .054 | −.06 | 0.18 | .82 | .29 | 0.14 | .03 |
| R 2 | 5.6% | .11 | 14.8% | .17 | 7.8% | .15 | |||
| Minimum nocturnal SBP | |||||||||
| Age | −.06 | 0.099 | .44 | .11 | 0.21 | .50 | −.17 | 0.14 | .09 |
| Sex (0: women, 1: men) | .03 | 3.33 | .65 | .21 | 8.86 | .09 | −.06 | 3.68 | .54 |
| BMI | −.20 | 0.30 | .046 | .14 | 0.54 | .55 | −.33 | 0.39 | <.01 |
| Apnea‐hypopnea index | .21 | 0.11 | .07 | .09 | 0.19 | .72 | .21 | 0.13 | .10 |
| Apnea index | −.20 | 0.10 | .06 | −.05 | 0.22 | .85 | −.20 | 0.12 | .08 |
| Arousal index | −.04 | 0.07 | .66 | −.18 | 0.14 | .20 | .03 | 0.09 | .75 |
| Lowest SpO2 | .12 | 0.19 | .22 | −.06 | 0.34 | .79 | .21 | 0.23 | .051 |
| SpO2 < 90% | .08 | 0.12 | .51 | −.22 | 0.20 | .43 | .13 | 0.15 | .31 |
| R 2 | 5.5% | .12 | 10.1% | .47 | 12.0% | .02 | |||
β, standardized partial regression coefficient; PSG, polysomnography; SE, standard error; SBP, systolic blood pressure.
Variables included in multiple linear regression analysis were age, apnea‐hypopnea index, apnea index, arousal index, body mass index (BMI), lowest oxygen saturation (SpO2), sex, and SpO2 < 90%. The individual values for each night (116 patients × 2 nights for all patients, 39 patients × 2 nights for nonmedicated patients with hypertension and 77 patients × 2 nights for medicated patients with hypertension) were used in the analysis.
Table 4.
Multiple linear regression analysis of nocturnal blood pressure variability with demographics and PSG‐derived parameters
| Variable | All patients (N = 232) | Nonmedicated patients with hypertension (n = 78) | Medicated patients with hypertension (n = 154) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | β | SE | P value | |
| Nocturnal SBP surge | |||||||||
| Age | −.06 | 0.08 | .41 | .11 | 0.18 | .42 | −.15 | 0.11 | .13 |
| Sex (0: women, 1: men) | .05 | 2.68 | .45 | −.08 | 7.49 | .42 | .10 | 2.87 | .27 |
| BMI | −.098 | 0.25 | .29 | .09 | 0.47 | .63 | −.17 | 0.31 | .13 |
| Apnea‐hypopnea index | .21 | 0.09 | .045 | −.01 | 0.17 | .98 | .31 | 0.10 | .01 |
| Apnea index | .01 | 0.08 | .88 | .27 | 0.19 | .22 | −.06 | 0.09 | .60 |
| Arousal index | .04 | 0.06 | .53 | .15 | 0.12 | .20 | −.05 | 0.07 | .59 |
| Lowest SpO2 | −.49 | 0.15 | <.001 | −.69 | 0.29 | <.001 | −.37 | 0.18 | <.001 |
| SpO2 < 90% | −.098 | 0.09 | .38 | −.39 | 0.17 | .10 | −.01 | 0.12 | .97 |
| R 2 | 26.6% | <.001 | 40.4% | <.001 | 22.4% | <.001 | |||
| SD of nocturnal SBP | |||||||||
| Age | .31 | 0.02 | <.001 | .41 | 0.04 | <.01 | .32 | 0.03 | <.01 |
| Sex (0: women, 1: men) | .09 | 0.66 | .18 | −.20 | 1.59 | .06 | .20 | 0.75 | .02 |
| BMI | .25 | 0.06 | .01 | .11 | 0.10 | .58 | .30 | 0.08 | .01 |
| Apnea‐hypopnea index | −.04 | 0.02 | .72 | .37 | 0.04 | .12 | −.15 | 0.03 | .20 |
| Apnea index | .08 | 0.02 | .46 | −.15 | 0.04 | .50 | .07 | 0.02 | .53 |
| Arousal index | −.12 | 0.01 | .12 | .02 | 0.03 | .89 | −.15 | 0.02 | .12 |
| Lowest SpO2 | −.17 | 0.04 | .08 | −.12 | 0.06 | .53 | −.20 | 0.05 | .06 |
| SpO2 < 90% | .06 | 0.02 | .64 | .24 | 0.04 | .32 | .02 | 0.03 | .85 |
| R 2 | 14.3% | <.001 | 30.7% | <.01 | 17.1% | <.01 | |||
β, standardized partial regression coefficient; PSG, polysomnography; SBP, systolic blood pressure; SD, standard deviation; SE, standard error.
Variables included in the multiple linear regression analysis were age, apnea‐hypopnea index, apnea index, arousal index, body mass index (BMI), lowest oxygen saturation (SpO2), sex, and SpO2 < 90%. The individual values for each night (116 patients × 2 nights for all patients, 39 patients × 2 nights for nonmedicated patients with hypertension, and 77 patients × 2 nights for medicated patients with hypertension) were used in the analysis.
4. DISCUSSION
The present study using TNP was the first to demonstrate that hypoxia‐peak SBP and the nocturnal SBP surge (measured by an oxygen‐triggered function) were much more closely associated with OSA‐related PSG parameters than maximum nocturnal SBP and mean nocturnal SBP (measured by a fixed‐interval function in the manner of conventional ABPM) in patients with OSA. Lowest SpO2, defined as the minimum SpO2 value during sleep, was the strongest independent determinant of hypoxia‐peak SBP and also nocturnal SBP surge.
4.1. Hypoxia‐peak SBP: the maximum SBP value measured by the oxygen‐triggered function
In the present study, the hypoxia‐peak SBP was significantly correlated with the maximum nocturnal SBP. However, the associations of these two parameters with the PSG‐derived sleep parameters were quite different. Whereas the maximum nocturnal SBP had no significant relationship with the PSG‐derived sleep parameters, the hypoxia‐peak SBP had a strong relationship with those parameters, which means that the hypoxia‐peak SBP represents a specific component of nocturnal BP affected by OSA. In the present study, lowest SpO2 was the strongest independent determinant of hypoxia‐peak SBP. This can be attributed to the long OSA duration. The patients’ long OSA episodes may have induced a severe drop in oxygen saturation, stronger transient sympathetic activation, and a stronger Valsalva effect, resulting in an exaggerated sleep BP surge. Clinically, the severity of OSA is now determined by the number of apnea‐hypopnea episodes from the viewpoint of sleep disturbance, but from the viewpoint of cardiovascular risk, the severity of the drop in oxygen saturation during apnea episodes and the frequency of these episodes would be more important indicators to identify the high‐risk patients who are likely to develop cardiovascular events specifically during sleep. Some studies have demonstrated that hypoxia‐peak SBP could be reduced by suppressing sympathetic nerve activity. Catheter‐based renal denervation, which ablates the afferent and efferent sympathetic nerves around the renal artery, significantly reduced peak nocturnal SBP values measured by ABPM21, 22 and hypoxia‐peak SBP detected by TNP by 10 mm Hg23 in patients with drug‐resistant hypertension who had OSA. Another study showed that bedtime dosing of 20 mg of carvedilol significantly reduced hypoxia‐peak SBP by 23 mm Hg in patients with hypertension who had OSA.14 We demonstrated in our earlier investigations10, 16 that BP surges monitored by TNP could be reduced by continuous positive airway pressure (CPAP). However, this BP‐lowering effect of CPAP is not perfect. The 2017 American Heart Association/American College of Cardiology guidelines24 note that the effectiveness of CPAP for reducing BP is not well established in adults with hypertension and OSA.25, 26, 27, 28, 29 The key factor of CPAP therapy and its effectiveness for cardiovascular protection may be the patient’s adherence to CPAP.30 There is a possibility that the use of TNP could improve adherence to CPAP therapy because patients can estimate their cardiovascular risk of BP surges induced by OSA and its suppression by using CPAP in day‐by‐day nocturnal BP monitoring.
4.2. Mean nocturnal SBP: the average of the nocturnal SBP values measured by the fixed‐interval function
In the present study, SpO2 < 90% was significantly correlated with mean nocturnal SBP in the univariable analysis. Some studies have reported that intermittent exposure to hypoxia induced sympathetic nerve activation by enhancing c‐fos in the brain stem, causing the nerve cells to memorize the hypoxic condition.31, 32 These results indicate that SpO2 < 90%, which reflects continuous hypoxia, is a more useful index than either apnea‐hypopnea episodes or the arousal index in terms of assessing increased mean nocturnal SBP, which is regarded as the strongest predictor of future cardiovascular events. This is clinically meaningful because SpO2 < 90% could be obtained by a simple screening test of OSA with pulse oximetry at home, rather than PSG, which requires hospitalization. However, no significant relationship between mean nocturnal SBP and any polysomnographic variables was revealed by the multivariable analysis. This might indicate that mean nocturnal SBP measured at 30‐minute fixed intervals is more vulnerable to other polysomnographic variables such as sodium intake together with salt sensitivity33 or the loss of sleep quality attributable to the disturbance of sleep by cuff inflation34, 35, 36 compared with hypoxia‐peak SBP measured by the oxygen‐triggered function.
4.3. Minimum nocturnal SBP: the lowest SBP value among all of the nocturnal SBP values measured by either the oxygen‐triggered or fixed‐interval functions
In the present study, minimum nocturnal SBP had a significant positive relationship with lowest SpO2 and a significant negative relationship with BMI. In addition, BMI had a close relationship with AHI in the univariable analysis. These results indicate that there were many cases in which the minimum nocturnal SBP was detected as a decreased BP overshoot at the phase after the peak of an SBP surge, because we defined minimum nocturnal SBP as the minimum SBP measured by both the fixed‐interval function and the hypoxia‐triggered function, which takes three consecutive BP measurements when the SpO2 falls below the threshold. In fact, when we defined minimum nocturnal SBP as the minimum SBP measured by only a fixed‐interval function, minimum nocturnal SBP had no significant independent determinant in the multivariate analysis. Because nocturnal BP frequently varies throughout the night, especially in patients with OSA, it might be difficult to determine an actual basal nocturnal SBP that reflects the stable basal condition of sympathetic nerve activity and is determined by structural changes of the small resistance arteries or circulating volume. To exclude the reactive hypotension just after OSA‐related BP surge, we are now trying to improve the algorithm triggered by the stable lowest heart rate (for a significant time period without a cluster of OSA episodes) in order to more accurately determine the basal nocturnal BP.
4.4. OSA‐related nocturnal SBP surge: the difference between the hypoxia‐peak SBP and the average of the SBP values measured by the fixed‐interval function within 30 minutes before and after the hypoxia‐peak SBP
The present study shows that nocturnal SBP surge had a stronger association with PSG‐derived sleep parameters (AHI, apnea index, ArI, lowest SpO2, and SpO2 < 90%) than the SD of nocturnal SBP. This may indicate that nocturnal SBP surge more precisely reflects OSA‐related BPV than the more generalized SD of nocturnal SBPs, because the former is derived from direct BP measurement in synchronization with OSA episodes. Frequent and large fluctuations of BP levels over time might have a role as a reliable risk factor of cardiovascular morbidity and mortality. Indeed, there is a great deal of evidence that BPV can also be a determinant of neurocognitive dysfunction37, 38, 39, 40 by a multitude of putative mechanisms including cerebral hemodynamic instability and blood flow imbalance with repeated episodes of cerebral tissue hypoxia,41, 42 microvascular damage, arterial remodeling and impairment of cerebrovascular reactivity,43 and inflammatory response and oxidative stress.44
In the present study, old age and high BMI were independent determinants of an increased SD of nocturnal SBP (ie, the SD of SBP measured by the fixed‐interval function). It is reasonable that age could be positively correlated with arterial stiffness, and the reduced elasticity of the vascular bed could mediate the higher BP fluctuation. Moreover, artery remodeling can make the cerebral blood flow highly dependent on the BP levels and magnify the harmful effects of the BP fluctuations. Dysautonomia and impairment of baroreceptor functions are also common in the elderly, and they could partly explain these findings. Moreover, the SD of nocturnal BP may include not only OSA‐related nocturnal BP surge, but also non–OSA‐related BP surges such as arousal‐induced or REM sleep–induced BP surge. In fact, the present study demonstrates that the arousal index is correlated with short‐term BP elevation induced by OSA. This suggests that arousal during sleep may be a target of medication from the viewpoint of preventing cardiovascular events.
4.5. Effect of antihypertensive drugs
The present study showed that the association between lowest SpO2 and hypoxia‐peak SBP was stronger in nonmedicated patients with hypertension than in all patients. Antihypertensive medication lowered the slope of the regression line (absolute value) between hypoxia‐peak SBP and lowest SpO2. These trends were also found in the association between lowest SpO2 and OSA‐related nocturnal SBP surge. These facts might indicate that hypertensive medication could reduce hypoxia‐peak SBP, especially in the patients with low lowest SpO2. Specifically, in the present study, calcium channel blockers significantly reduced the regression line (absolute value) between hypoxia‐peak SBP and lowest SpO2, and α‐adrenergic or β‐adrenergic blockers significantly reduced that between nocturnal SBP surge and lowest SpO2. These results for the different classes of antihypertensive drug were the same as those in our previous crossover study,14 which evaluated the effects of single‐dose nighttime administration of vasodilating (nifedipine 40 mg) vs sympatholytic (carvedilol 20 mg) antihypertensive agents on nocturnal BP parameters measured by TNP in patients with hypertension who had OSA. In that study, sympatholytic antihypertensive agents significantly reduced OSA‐related short‐term BP surge.
4.6. Limitations and perspectives
The present study has some limitations. The BP values obtained by the oxygen‐triggered function of the TNP system were measured by a cuff inflation–based BP monitor. This device may have underestimated BP surges induced by OSA compared with the actual peak of the BP surges induced by an OSA episode. In addition, the present study was a cross‐sectional study. Therefore, a future drug intervention study using TNP is needed to demonstrate the direct effects on OSA‐triggered nocturnal BP.
5. CONCLUSIONS
The hypoxia‐peak SBP and the nocturnal SBP surge measured by the oxygen‐triggered function were much more closely associated with PSG‐derived sleep parameters than the maximum nocturnal SBP and mean nocturnal SBP obtained by a fixed‐interval function. Lowest SpO2 was the strongest independent determinant of hypoxia‐peak SBP and also nocturnal SBP surge. Our findings might indicate that the severity of the drops of SpO2 and the frequency of these drops would be important indicators for the identification of high‐risk patients who are likely to develop cardiovascular events specifically during sleep.
CONFLICT OF INTEREST
The authors have no disclosures to declare.
Supporting information
ACKNOWLEDGMENTS
We gratefully acknowledge Mrs Ayako Okura for her editorial support.
Kuwabara M, Tomitani N, Shiga T, Kario K. Polysomnography‐derived sleep parameters as a determinant of nocturnal blood pressure profile in patients with obstructive sleep apnea. J Clin Hypertens. 2018;20:1039–1048. 10.1111/jch.13308
Funding information
This research was supported by a Grant‐in‐Aid for Scientific Research (B) (21390247) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, to K.K., 2009–2013, and by the MEXT‐Supported Program for the Strategic Research Foundation at Private Universities, 2011–2015.
REFERENCES
- 1. Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA 1999;282:539‐46. [DOI] [PubMed] [Google Scholar]
- 2. Kikuya M, Ohkubo T, Asayama K, et al. Ambulatory blood pressure and 10‐year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension. 2005;45:240‐5. [DOI] [PubMed] [Google Scholar]
- 3. Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219‐29. [DOI] [PubMed] [Google Scholar]
- 4. The ABC‐H Investigators , Roush GC, Fagard RH, et al. Prognostic impact from clinic, daytime, and night‐time systolic blood pressure in nine cohorts of 13,844 patients with hypertension. J Hypertens. 2014;32:2332‐40. [DOI] [PubMed] [Google Scholar]
- 5. Palatini P, Reboldi G, Beilin LJ, et al. Added predictive value of night‐time blood pressure variability for cardiovascular events and mortality the ambulatory blood pressure–international study. Hypertension. 2014;64:487‐93. [DOI] [PubMed] [Google Scholar]
- 6. Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811‐7. [DOI] [PubMed] [Google Scholar]
- 7. Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol. 1989;67:2101‐6. [DOI] [PubMed] [Google Scholar]
- 8. Somers VK, Mark AL, Zavala DC, Abboud FM. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J Appl Physiol. 1989;67:2095‐100. [DOI] [PubMed] [Google Scholar]
- 9. Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic‐nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303‐7. [DOI] [PubMed] [Google Scholar]
- 10. Kario K. Obstructive sleep apnea syndrome and hypertension: ambulatory blood pressure. Hypertens Res. 2009;32:428‐32. [DOI] [PubMed] [Google Scholar]
- 11. Kario K. Obstructive sleep apnea syndrome and hypertension: mechanism of the linkage and 24‐h blood pressure control. Hypertens Res. 2009;32:537‐41. [DOI] [PubMed] [Google Scholar]
- 12. Shirasaki O, Yamashita S, Kawara S, et al. A new technique for detecting sleep apnea–related “midnight” surge of blood pressure. Hypertens Res. 2006;29:695‐702. [DOI] [PubMed] [Google Scholar]
- 13. Shirasaki O, Kuwabara M, Saito M, Tagami K, Washiya S, Kario K. Development and clinical application of a new technique for detecting ‘nocturnal blood pressure surges’ in sleep apnea patients based on a variable desaturation threshold. Hypertens Res. 2011;34:922‐8. [DOI] [PubMed] [Google Scholar]
- 14. Kario K, Kuwabara M, Hoshide S, Nagai M, Shimpo M. Effects of nighttime single‐dose administration of vasodilating vs sympatholytic antihypertensive agents on nocturnal blood pressure in hypertensive patients with sleep apnea syndrome. J Clin Hypertens (Greenwich). 2014;16:459‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kario K, Hamasaki H. Nocturnal blood pressure surge behind morning surge in obstructive sleep apnea syndrome: another phenotype of systemic semodynamic atherothrombotic syndrome. J Clin Hypertens (Greenwich). 2015;17:682‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuwabara M, Kario K. Development of a triggered nocturnal blood pressure monitoring which detects nighttime blood pressure surges in sleep apnea syndrome. Curr Hypertens Rev. 2016;12:27‐31. [DOI] [PubMed] [Google Scholar]
- 17. Yoshida T, Kuwabara M, Hoshide S, Kario K. Recurrence of stroke caused by nocturnal hypoxia‐induced blood pressure surge in a young adult male with severe obstructive sleep apnea syndrome. J Am Soc Hypertens. 2016;10:201‐4. [DOI] [PubMed] [Google Scholar]
- 18. Yoshida T, Kuwabara M, Hoshide S, Kario K. The effect of the bedtime‐dosing doxazosin on nocturnal hypoxia‐triggered blood pressure surge in a young adult man with severe obstructive sleep apnea syndrome and a history of three recurrent sleep‐onset strokes. Blood Press Monit. 2017;22:173‐4. [DOI] [PubMed] [Google Scholar]
- 19. Kuwabara M, Hamasaki H, Tomitani N, Shiga T, Kario K. Novel triggered nocturnal blood pressure monitoring for sleep apnea syndrome: distribution and reproducibility of hypoxia‐triggered nocturnal blood pressure measurements. J. Clin Hypertens (Greenwich). 2017;19:30‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viera AJ, Hinderliter AL. Validation of the HEM‐780REL with easy wrap cuff for self‐measurement of blood pressure according to the European society of hypertension international protocol. Blood Press Monit. 2007;12:335‐8. [DOI] [PubMed] [Google Scholar]
- 21. Kario K, Bhatt DL, Brar S, Cohen SA, Fahy M, Bakris GL. Effect of catheter‐based renal denervation on morning and nocturnal blood pressure. Insights from SYMPLICITY HTN‐3 and SYMPLICITY HTN‐Japan. Hypertension. 2015;66:1130‐7. [DOI] [PubMed] [Google Scholar]
- 22. Kario K, Bhatt DL, Kandzari DE, et al. Impact of renal denervation on patients with obstructive sleep apnea and resistant hypertension: insights from the SYMPLICITY HTN‐3 trial. Circ J. 2016;80:1404‐12. [DOI] [PubMed] [Google Scholar]
- 23. Kario K, Ikemoto T, Kuwabara M, Ishiyama H, Saito K, Hoshide S. Catheter‐based renal denervation reduces hypoxia‐ trigger nocturnal blood pressure peak in obstructive sleep apnea syndrome. J Clin Hypertens. 2016;18:707‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guidelines for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13‐e115. [DOI] [PubMed] [Google Scholar]
- 25. Barbe F, Duran‐Cantolla J, Capote F, et al. Long‐term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718‐26. [DOI] [PubMed] [Google Scholar]
- 26. Lozano L, Tovar JL, Sampol G, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161‐8. [DOI] [PubMed] [Google Scholar]
- 27. Martinez‐Garcia MA, Capote F, Campos‐Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407‐15. [DOI] [PubMed] [Google Scholar]
- 28. Pedrosa RP, Drager LF, de Paula LKG, et al. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144:1487‐94. [DOI] [PubMed] [Google Scholar]
- 29. Muxfeldt ES, Margallo V, Costa LMS, et al. Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trial. Hypertension. 2015;65:736‐42. [DOI] [PubMed] [Google Scholar]
- 30. Kario K. Essential Manual on Perfect 24‐Hour Blood Pressure Management from Morning to Nocturnal Hypertension: Up‐to‐Date for Anticipation Medicine. London, England: Wiley‐Blackwell; 2018:16‐20. ISBN: 978‐1‐119‐08724‐3. [Google Scholar]
- 31. Greenberg HE, Sica AL, Scharf SM, Ruggiero DA. Expression of c‐fos in the rat brainstem after chronic intermittent hypoxia. Brain Res. 1999;816:638‐45. [DOI] [PubMed] [Google Scholar]
- 32. Sica AL, Greenberg HE, Scharf SM, Ruggiero DA. Immediate‐early gene expression in cerebral cortex following exposure to chronic‐intermittent hypoxia. Brain Res. 2000;870:204‐10. [DOI] [PubMed] [Google Scholar]
- 33. Castiglioni P, Parati G, Brambilla L, et al. Detecting sodium‐sensitivity in hypertensive patients: information from 24‐hour ambulatory blood pressure monitoring. Hypertension. 2011;57:180‐5. [DOI] [PubMed] [Google Scholar]
- 34. Dimsdale JE, Coy TV, Ancoli‐Israel S, Clausen J, Berry CC. The effect of blood pressure cuff inflation on sleep. A polysomnographic examination. Am J Hypertens. 1993;6:888‐91. [DOI] [PubMed] [Google Scholar]
- 35. Davies RJ, Jenkins NE, Stradling JR. Effect of measuring ambulatory blood pressure on sleep and on blood pressure during sleep. BMJ. 1994;308:820‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heude E, Bourgin P, Feigel P, Escourrou P. Ambulatory monitoring of blood pressure disturbs sleep and raises systolic pressure at night in patients suspected of suffering from sleep‐disordered breathing. Clin Sci (Lond). 1996;91:45‐50. [DOI] [PubMed] [Google Scholar]
- 37. Lattanzi S, Viticchi G, Falsetti L, et al. Visit‐to‐visit blood pressure variability in Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28:347‐51. [DOI] [PubMed] [Google Scholar]
- 38. Lattanzi S, Luzzi S, Provinciali L, Silvestrini M. Blood pressure variability predicts cognitive decline in Alzheimer’s disease patients. Neurobiol Aging. 2014;35:2282‐7. [DOI] [PubMed] [Google Scholar]
- 39. Lattanzi S, Luzzi S, Provinciali L, Silvestrini M. Blood pressure variability in Alzheimer’s disease and frontotemporal dementia: the effect on the rate of cognitive decline. J Alzheimers Dis. 2015;45:387‐94. [DOI] [PubMed] [Google Scholar]
- 40. Cho N, Hoshide S, Nishizawa M, Fujiwara T, Kario K. Relationship between blood pressure variability and cognitive function in elderly patients with good blood pressure control. Am J Hypertens. 2018;31:293‐8. [DOI] [PubMed] [Google Scholar]
- 41. Buratti L, Cagnetti C, Balucani C, et al. Blood pressure variability and stroke outcome in patients with internal carotid artery occlusion. J Neurol Sci. 2014;339:164‐8. [DOI] [PubMed] [Google Scholar]
- 42. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Blood pressure variability and clinical outcome in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2015;24:1493‐9. [DOI] [PubMed] [Google Scholar]
- 43. Lattanzi S, Carbonari L, Pagliariccio G, et al. Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy. Neurology. 2018;90:e307‐15. [DOI] [PubMed] [Google Scholar]
- 44. Kim KI, Lee JH, Chang HJ, et al. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circ J. 2008;72:293‐8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


