Abstract
Background
The aim of our study was to determine through a systematic review and meta-analysis the incubation period of COVID-19. It was conducted based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA). Criteria for eligibility were all published population-based primary literature in PubMed interface and the Science Direct, dealing with incubation period of COVID-19, written in English, since December 2019 to December 2020. We estimated the mean of the incubation period using meta-analysis, taking into account between-study heterogeneity, and the analysis with moderator variables.
Results
This review included 42 studies done predominantly in China. The mean and median incubation period were of maximum 8 days and 12 days respectively. In various parametric models, the 95th percentiles were in the range 10.3–16 days. The highest 99th percentile would be as long as 20.4 days. Out of the 10 included studies in the meta-analysis, 8 were conducted in China, 1 in Singapore, and 1 in Argentina. The pooled mean incubation period was 6.2 (95% CI 5.4, 7.0) days. The heterogeneity (I2 77.1%; p < 0.001) was decreased when we included the study quality and the method of calculation used as moderator variables (I2 0%). The mean incubation period ranged from 5.2 (95% CI 4.4 to 5.9) to 6.65 days (95% CI 6.0 to 7.2).
Conclusions
This work provides additional evidence of incubation period for COVID-19 and showed that it is prudent not to dismiss the possibility of incubation periods up to 14 days at this stage of the epidemic.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-021-01648-y.
Keywords: COVID-19, Infectious disease incubation period, Contact tracing, Coronavirus
Background
Since December 2019, the world is facing the pandemic of COVID-19. As of December 8, 2020, a total of cumulative confirmed cases were estimated at more than 68 million and 1.5 million cumulative deaths with a case fatality rate of 2.28% [1, 2].
While awaiting a vaccine, massive public health interventions such as social awareness, social distancing, isolation, quarantine, contact tracing, targeted screening, and border controls have been implemented nationally and globally to limit transmissibility and contain the epidemic since late January [3–6]. The incubation period, one of the key epidemiological parameters, is essential to epidemiological case definition, to determine the appropriate duration of quarantine and to estimate the size of the epidemics. Therefore, it was rapidly being studied from incoming case reports as the epidemic continues. Several studies have confirmed that cases are infectious during the asymptomatic period (latency period) prior to onset and that disease transmission may be carried out [7–10]. Up to this point, the quarantine and isolation duration of exposed or suspected cases is set at 14 days, which is the longest incubation time expected based on initial observations of SARS-CoV-2 and similarity to severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) [11].
The distribution of the incubation period in most of the literature is either described through a parametric model or its empirical distribution based on the observed incubation period from the contact-tracing data (specific data indicating the time of exposure). However, the contact-tracing data are challenging and expensive to obtain, and their accuracy can be highly influenced by recall bias. Therefore, previous population dynamics studies tend to make assumptions about the distribution of the incubation period without using the observed data called modeling studies, and we are talking here about an estimate of the incubation period.
Furthermore, estimating and standardizing the incubation period of COVID-19 may vary depending on climate [12], on age or the genetic of the individuals, their pathologies, or their treatment like the long-term use of glucocorticoids which might cause atypical infections and a long incubation period [13].
Thus, a specific maximum duration of the incubation period is needed to answer if the 14-day quarantine is sufficient to protect against the spread of the pandemic. In this systematic review and meta-analysis, we tried to identify studies that recruited symptomatic patients, regardless of sex or age diagnosed with COVID-19, and calculated or estimated the incubation period between December 2019 and December 2020.
Methods
Criteria for considering studies for this review
Types of studies
The protocol for this review was registered with PROSPERO (international prospective register of systematic reviews) under the number CRD42020196347 (https://www.crd.york.ac.uk/prospero/).
This systematic review was conducted based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA) to study the length of incubation period during the COVID-19 pandemic.
Criteria for eligibility were all published population-based primary literature dealing with incubation period of COVID-19, since December 2019. We included full-text publications and excluded all articles not accepted or peer reviewed, not written in English, editorials, perspective, letter to the editor, review, article info, and comments. We only took articles that used RT-PCR for the diagnosis of COVID-19. Since randomized controlled trials (RCTs) do not apply to this topic, only observational studies with no limit on the number of participants were included. There were no limitations given the types of outcome measures: we accepted all documents that presented results even without a statistical parameter of variability.
Types of participants
We included individuals with a confirmed diagnosis of COVID-19 regardless of the severity of symptoms or associated comorbidities. There were no age, gender, or ethnicity restrictions. We excluded studies including populations with other coronavirus diseases (severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS)). We also excluded studies including populations with mixed viral diseases (e.g., COVID-19 plus influenza).
Types of outcome measures
The incubation period was defined as the amount of time between the exposure to SARS-CoV-2 and the onset of symptoms [14].
Estimated and calculated incubation period
Incubation periods were calculated from observed data based on specific dates indicating the time of exposure. Measures of central tendency were ranges, mean, and median with appropriate dispersion parameters (interquartile range (IQR) and standard deviation (SD)).
Incubation periods were estimated on incomplete data or imprecise exposure time using several models of distribution such as log-normal, Weibull, and Gamma distribution [15, 16]. They were presented by the mean and its 95% confidence interval (95% CI) with percentiles of the distribution in some studies.
Search methods for identification of studies
Electronic searches
The literature search was developed by WD and verified by a research librarian. It was carried out on Medline via its PubMed interface, through the following documentary query, as of 01/12/2020: (“Infectious Disease Incubation Period”[Mesh]) AND (“COVID-19”[All Fields] OR “COVID-2019”[All Fields] OR “severe acute respiratory syndrome coronavirus 2”[Supplementary Concept] OR “severe acute respiratory syndrome coronavirus 2”[All Fields] OR “2019-nCoV”[All Fields] OR “SARS-CoV-2”[All Fields] OR “2019nCoV”[All Fields] OR ((“Wuhan”[All Fields] AND (“coronavirus”[Mesh Terms] OR “coronavirus”[All Fields])) AND (2019/12[PDAT] OR 2020[PDAT]))). No filter was applied.
The research was also conducted on Science Direct through its advanced research (only the research article using COVID-19 and the incubation period in the title, abstract, or keywords specified by the author). The literature search was completed on 01/12/2020.
Data collection and analysis
Selection of studies and data extraction
The references were managed using the Zotero software. Firstly, and after exclusion of duplicates, all titles and abstracts of publications identified through the initial primary search were single reviewed for relevance.
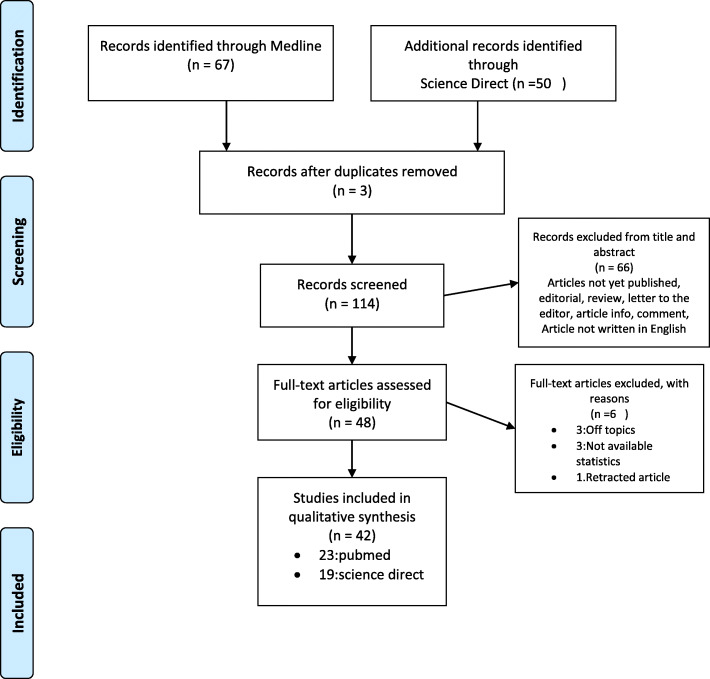
Secondly, the final selection of the articles was based on the full texts of papers by retrieving and checking for relevance by two authors (WD, AI) independently of each other, with referral to MJ in the case of discordant opinions. Studies were excluded if they were off topic or if they gave no number or statistics. One article was retracted from Medline during the process of final selection. We documented the study selection process in a flow chart and showed the total numbers of retrieved references and the numbers of included and excluded studies (Fig. 1).
Fig. 1.
PRISMA study flow diagram for search up to 1 December 2020
One review author WD performed all data extractions. Two other review authors (AI and MJ) verified the accuracy and the plausibility of extractions.
All authors participated in quality assessment, level of evidence, and grades of recommendations. DW and AI independently reviewed all studies, with disagreements resolved by referral to MJ.
The following bibliometric, epidemiological data were extracted: authors, study design, country or geographical region, period of study, sample size, data and source collection, general characteristics of the studied population (age, sex ratio), exposure history, and duration of incubation period.
The meta-analysis was based on the mean of the distributions either in observed or estimated log-normal distribution data. The meta-analysis included all studies that reported the mean with its SD of the observed incubation period or the mean and corresponding CI of the normal log distribution. Excluded studies where those representing outcome reporting bias. The selection of studies to include in the meta-analysis was conducted by the primary author WD.
Assessment of risk of bias
Quality assessment was done according to recommendation of “Quality Assessment Tool for Quantitative Studies” developed in Canada by the Effective Public Health Practice Project (EPHPP) [17]. Once the assessment is fulfilled using a number pre-determined criteria, each examined practice receives a mark ranging between “strong,” “moderate,” and “weak” in three categories (study design, data collection practices, and selection bias). After discussing the ratings and resolving any discrepancy, the global rating for each paper was according to the sum of “weak” ratings given to the three categories (1: strong=0 “weak”; 2: moderate=1 “weak”; and 3: weak=≥2 “weak”) (Additional file 1).
Risk of bias was done according to Chapter 25 of Cochrane Handbook: assessing risk of bias in a non-randomized study [: /handbook/current/chapter-25]; assessing risk of bias was presented using Revman 5 tools (Review Manager (RevMan) [Computer program] version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.).
Level of evidence and grades of recommendations were assessed according to the Scottish Intercollegiate Guidelines Network (SIGN) [18].
Levels of evidence were graded into 8 levels from 1++ (high quality meta-analyses, systematic reviews of randomized controlled trials (RCTs), or RCTs with a very low risk of bias) to 4 (expert opinion).
There were 4 grades of recommendations (A, B, C, and D) based on the results of the level of evidence. D is given if the evidence level was 3 or 4 or extrapolated from studies rated as 2+ [18].
Data synthesis
A random effects meta-analysis was conducted in the Open Meta Analyst software [19], of the calculated and estimated mean of the log-normal distribution. Forest plots were produced using the same package. Heterogeneity between the studies was assessed using both the I2 statistic with a cutoff of 50% and the χ2 test with P-value <0.10 and investigated by conducting subgroup analyses of the data set following these moderator variables: population of studies (Chinese or not), severity (hospitalized or not), sex ratio (> or < 1), study quality, and method of calculation (estimated or calculated).
Results
Results of the search
We identified 117 records through Medline and Science Direct database searches. After removing 3 duplicates, we screened 114 records based on their titles and abstracts, leaving 48 full manuscripts to be assessed for eligibility (Fig. 1). As a result of this assessment, 42 studies met the inclusion criteria.
Study characteristics
Over the 42 observational studies, the quality assessment gave 9 strong, 19 moderate, and 14 weak studies (Additional file 1). Most of the studies had a retrospective data collection; 10 had a prospective one [7–9, 20–26]. The sampling methods and sample size recorded varied substantially across studies. In some cases, entire provinces or villages were selected [8, 9, 20, 24–37] whereas in others the focus was on serial cases or family clusters [7, 8, 24, 38–42], hospitals, and laboratory [43–50]. Almost studies were done in China (30 studies), including a study of around 8579 people in 30 provinces [23]. Four studies was conducted in Korea [9, 28, 34, 51], three in Singapore [21, 48, 52], one in France [41], one in Brunei [32], one in Argentina [33], one in Saudi Arabia [53], and two in Germany [37, 42]. The period of all studies was between January and May 2020 (Table 1).
Table 1.
Characteristics of studies calculating incubation period SARS-COV-2
| Authors | Country (province) | Period | Data and source collection | General characteristics of the population study | Exposure history | ||
|---|---|---|---|---|---|---|---|
| N | Age┼┼ (years) | Sex ratio (M/F) | |||||
| Guan et al. [27] | China | 11/12/2019 to 29/01/2020 | 552 hospitals’ medical records | 1099 |
Median =47 IQR [35-58] |
1.43 |
Contact with wildlife Resident or travelers to Wuhan |
| Ki and Task Force for 2019-nCoV [28] | Korea | 20/01/2020 to 10/02/2020 |
CDC Additional data announced by the press |
28 |
Mean= 42 Range (20–73) |
1.15 | Close contact with confirmed cases |
| Chen et al. [43] | China (Chongqing) | 28/01/2020 to 11/02/2020 | 3 hospitals’ medical records | 12 | Mean=14.5 range (7M–17Y) | 1 |
Resident or travelers to Wuhan Close contact with confirmed cases |
| Gao et al. [7] | China (Wuxi) | January–March 2020 | Data of scientific investigation from “Public Health Emergency Reporting Management Information System” | 15 |
Median= 51 Range (9–74) |
1.5 | Close contact with confirmed cases |
| Huang et al. [8] | China (Anhui) | January–February 2020 | Information from patients and contacts. | 17 |
Median=22 Range (16–23) |
0.75 | Close Contact with confirmed cases |
| Pung et al. [38] | Singapore | February 2020 | The ministry of health (first three clusters) | 36 |
- - |
- |
Close contact with a tourist group from China Public place (company conference/church) |
| Song et al. [39] | China (Beijing) | 16/01/2020 to 29/01/2020 | 1 hospital’s medical records (4 families) | 24 | Range (9M–86 Y) | 0.37 | Close contact with confirmed cases |
| Tian et al. [44] | China (Beijing) | 20/01/2020 to 10/02/2020 | 57 hospitals’ medical records | 262 |
Median =47.5 Range (6M–94 Y ) |
0.94 |
Wuhan travel Close contact with confirmed cases Family cluster cases |
| Wang et al. [25] | China (Jiangsu ) | 22/01/2020 to 18/02/2020 | Websites of bureau of health and the people’s government. | 631 |
- - |
>1 |
Resident or travelers to Hubei province Close contact with confirmed cases Others |
| Xia et al. [40] | China (Chongqing) | 23/01/2020 to 18/02/2020 | Hospital electronic medical record system of patient with severe acute respiratory syndrome | 10 | Mean =56.5±11.16 | 1.5 | Close contact with confirmed cases |
| Xu et al. [54] | China (Changzhou) | 23/01/2020 to 18/02/2020 | Laboratory-confirmed cases | 51 |
Mean =35.0 Range (29–51) |
0.96 |
Resident or travelers to Wuhan Close contact with confirmed or suspected cases |
| Bernard et al. [41] | France | 24/01/2020 to 12/02/2020 | Unspecified | 3 | - | - | - |
| Yu et al. [30] | China (Shanghai) | As of February 19th, 2020. | CDC | 333 | Median =50 | 1.06 |
Cases with a travel history in Wuhan Close contact with confirmed cases |
| Li et al. [20] | China (Wuhan) | December 2019 to 22 January 2020 | Laboratory-confirmed cases of infected pneumonia(NCIP) | 425 |
Median= 59 Range(15 to 89) |
1.29 |
Contact with wildlife Close contact with suspected cases |
| Zhang et al. [23] | China outside Hubei | 19/01/2020 to 17/02/ 2020 | Laboratory-confirmed cases | 8579 |
Median= 44 Range (33–56) |
- |
Contact with wildlife Close contact with confirmed or suspected cases Resident or travelers to Wuhan |
| Linton et al. [29] | China | December 2019 to January 2020 | Official reports from governmental institutes | 158 | Most (30–59) | 0.58 | Resident or travelers to Wuhan |
| Backer et al. [55] | China | 20 to 28 January 2020 | CDC | 88 | Range (2–72) | 1.84 | Travelers to Wuhan |
| Lauer et al. [56] |
China 24 countries outside China |
04/01/2020 to 24/02/2020 | Public health reports and news | 181 |
Median= 44.5 IQR [34.0–55.5] |
1.56 |
Resident or travelers to Wuhan Close contact with confirmed cases or travelers from Hubei |
| Wang et al. [57] | China (Henan) | 21/01/2020 to 14/02/2020 | CDC | 1212 | Most (21–60) | 1.22 |
Travelers to Wuhan Close contact with confirmed cases |
| Bi et al. [24] | China (Shenzhen) | 14/01/2020 to 12/02/2020 | CDC | 3911 | Mean=45 | 0.91 |
Travelers to Wuhan Close contact with confirmed cases |
| Zheng et al. [45] | China (Hubei) | 16/01/2020 to 04/02/2020 | Taihe Hospital medical records | 73 | Mean=43 | 1.73 |
Huanan China Seafood Market 12 cases had no exposure history Family cluster cases |
| Zhao et al. [46] | China (Jiangsu ) | 16/01/2020 to 17/02/2020 | Jiangsu Hospital medical records | 136 |
Median=49 IQR [33-63] |
1 |
Resident or travelers to Wuhan Close contact with confirmed cases Family cluster cases 14 cases had no exposure history |
| Zhang et al. [59] | China (Hubei) | 22/01/2020 to 28/02/2020 |
Huanggang Hospital Shandong First Medical University |
194 | Median=48.3 | 1.25 |
Contact with wildlife Resident or travelers to Wuhan |
| Yang et al. [26] | China (Hubei) | 20/01/2020 to 29/02/2020 | CDC | 672 | Range (35–64) | 1.08 |
Wuhan-imported Close contact of imported cases Locally infected |
| Xiao et al. [31] | China (Hubei, Qinghai, Tibet) | As of February 21th, 2020. | CDC | 4741 | Median =50 | 1.06 |
Resident or travelers to Wuhan Local community transmission |
| Wong et al. [32] | Brunei | 09/03/2020 to 05/04/2020 | CDC | 135 |
Median= 36 Range(0.5–72) |
1.54 |
Travel history outside Brunei Local community transmission |
| Wang et al. [47] | China (Wuhan) | 05/01/2020 to 12/02/2020 | Wuhan Union Hospital | 35 |
Median= 37 Range(25–88) |
0.59 |
27 health care worker 10 relatives to health care worker |
| Viego et al. [33] | Argentina (Bahia Blanca) | 20/03/2020 to 08/05/2020 | Local health authorities | 36 | - | - |
Travel history outside city Local community transmission |
| Tindale et al. [52] | Singapore | 23/01/2020 to 26/02/2020 | Publicly available data | 93 | - | - | |
| China (Tianjin) | 21/01/2020 to 22/02/2020 | 135 | - | ||||
| Tan et al. [48] | Singapore | 23/01/2020 to 02/04/2020 | Hospital records | 164 | Mean = 44.2 ±15.8 | 0.88 |
Travel history Local community transmission |
| Ryu et al. [34] | South Korea | 20/01/2020 to 21/04/2020 | Local public health authorities | 2023 |
Median= 42 Range(1–102) |
0.24 |
Clusters Imported from Daegu-Gyeongsangbuk or abroad Local transmission |
| Qin et al. [35] |
China (outside Hubei) Other countries |
as of 15 February 2020 |
Publicly available data in China The ministries of health in other countries |
1084 |
Mean = 41.31 Median= 40 |
1.31 | - |
| Nie et al. [60] | China (outside Hubei) | 19/01/2020 to 08/02/2020 |
The website of the National Health Commission of the People’s Republic of China The health commission website of each province or city. |
7015 |
Mean = 44.24 Range (2 m–97 y) |
1.11 |
Resident or travelers to Wuhan Close contact with confirmed cases |
| Lou et al. [50] | China | 19/01/2020 to 09/02/2020 | Hospital records | 80 |
Median=55 IQR [45-64] |
1.58 | - |
| Liu et al. [62] | China (Shenzhen) | 04/01/2020 to 05/02/2020 | 365 |
Median=46 Range (1–86) |
0.99 |
Contact with confirmed case-patients Wuhan Cities other than Wuhan in Hubei Province No definite exposure |
|
| Li et al. [49] | China (Shenzhen) | 21/01/2020 to 09/02/2020 | Hospital records | 74 | Mean = 44.26 | 0.89 |
Travelers to Wuhan Family clusters Sporadic cases |
| Lee et al. [51] | South Korea | 20/02/2020 to 03/03/2020 | Publicly available data | 47 |
Median=30 Range (17–83) |
- | - |
| Chun et al. [9] | South Korea | 23/01/2020 to 31/03/2020 | Public reports of confirmed COVID-19 patients by the government and each municipal website in South Korea | 72 |
Median=40 IQR [24-54] |
0.89 | Contact with confirmed cases |
| Alsofayan et al. [53] | Saudi Arabia | 01/03/2020 to 31/03/2020 | Health Electronic Surveillance Network (HESN) Database | 1519 | Median=36 | 1.18 |
History of recent travel outside KSA Local community transmission |
| Bohmer et al. [42] | Germany (Bavaria) | 27/01/2020 to 16/02/2020 | Bavarian Health and Food Safety Authority and national level (Robert Koch Institute) public health authorities and four public health laboratories. | 16 |
Median=35 IQR [27-42] Range (2–58) |
3.0 | Cluster |
| You et al. [36] | China (outside Hubei province) | 01/01/2020 to 31/03/2020 | The National Health Commission (NHC) of China | 169 | - | - | Resident or travelers to Hubei |
| Wieland [37] | Germany | 15/02/2020 to 31/03/2020 | Official German case data | 107 | - | - | - |
IQR interquartile range, 95% CI 95% confidence interval, ± standard deviation
┼Proportion of cases on which was calculated the incubation period among all participants in the study
┼┼Age expressed by median [IQR] or range (x–x) or mean ±SD (years)
Risk of bias within studies
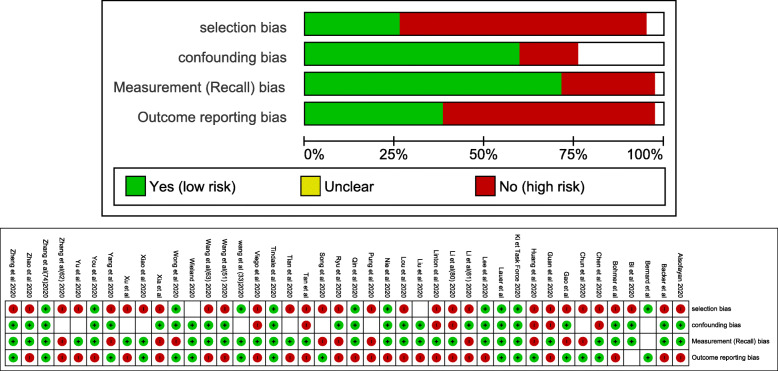
All the 42 observational studies had the third level of evidence (non-analytic studies) with a grading D of recommendation.
Most of study had the risk of recall bias (Fig. 2).
Fig. 2.
Overall and detailed risk of bias assessment among the 42 studies
Results of individual studies
Based on studies calculating incubation period for SARS-CoV-2
The median incubation period was calculated in 17 studies ranging from 2 to 12 days with an IQR lower bound of 2 days and higher bound of 14 days. In 9 studies, the mean was ranging from 3.9 to 8.98 days.
The total incubation period ranged in 9 studies from 0 to 26 days. One study was restricted to pediatric patients infected with SARS-CoV-2 from 7 months to 17 years old. The average incubation period was 8 days ranged from 1 day to 13 days [43] (Table 2).
Table 2.
Overview of studies calculating incubation period for SARS-CoV-2
| Authors | (n/N)┼ | Incubation period (days) | ||
|---|---|---|---|---|
| Median [IQR] | Mean±SD | Range | ||
| Guan et al. [27] | 291/1099 | 4 [2.0–7.0] | - | - |
| Ki and Task Force for 2019-nCoV [28] | 10/28 | 3 | 3.9 | 0–15 |
| Chen et al. [43] | 12/ 12 | - | 8 | 1–13 |
| Gao et al. [7] | 6/15 | 10 | - | 3–12 |
| Huang et al. [8] | 6/8 | 2.0 | - | 1–4 |
| Pung et al. [38] | 19/36 | 4 [3.0–6.0] | - | - |
| Song et al. [39] | 22/24 | - | - | 2–13 |
| Tian et al. [44] | 262/262 | - | 6.7 ±5.2 | - |
| Wang et al. [25] | 631/631 | - | Max 19 | |
| Xia et al. [40] | 9/10 | - | 7 ± 2.59 | 2–14 |
|
Xu et al. [54] Imported Secondary Tertiary |
15/51 17/51 19/51 |
8 [4.0–10.0] 8 [4.0–11.0] 12 [9.0–14.0] |
- | - |
| Bernard et al. [41] | 3/3 | - | - | 3–7 |
| Yu et al. [30] |
132/333 G1 (n=64) G2 (n=57) G3 (n=11) |
7.8 [5.0–8.2] 7.5 [5.0–7.9] 9 [5.0–8.0] |
- - - |
0.5–20 0.5–23 1–14 |
| Zheng et al. [45] | 61/73 | - | - | Max 26 |
| Zhao et al. [46] | 6/136 | 6 [4.0–11.0] | - | 1–21 |
| Zhang et al. [59] | 194 | - | 7.44 | 0.08–18 |
| Xiao et al. [31] | 2555/ 4741 | - | 8.98 | - |
| Wong et al. [32] | 135/135 | 5.0 | - | 1–11 |
| Tan et al. [48] | 164 | 5.0 | 5.7±3.5 | 1–17 |
| Nie et al. [60] | 2907/7015 | 5.0 [2.0–8.0] | - | Max 24 |
| Lou et al. [50] | 45/80 | 5.0 [2.0–10.0] | - | 0–23 |
| Liu et al. [62] | 58/365 | 5.0 [3.0–8.0] | 6.0 | 1–16 |
| Li et al. [49] | 74/74 | 5.0 [4.0–7.0] | - | - |
| Alsofayan et al. [53] | 309/1519 | 6.0 [7.5] | - | - |
| Bohmer et al. [42] | 16 | 4.0 [2.3–4.3] | - | - |
| You et al. [36] | 169/198 | 7.0 [4.5–10] | 8.0±4.75 | 0–23.5 |
┼Proportion of cases on which was calculated the incubation period among all participants in the study
SD standard deviation, IQR interquartile range
Based on studies estimating incubation periods for SARS-CoV-2
The log-normal distribution was the best fitting to the data in 7 studies with an estimated mean ranging from 5.0 to 7.4 (95% CI, 2 to 20 days) (Table 3). The median was estimated in 9 studies and had a maximum value of 7.2 (95% CI, 6.4 to 7.9 days) [58]. The estimated 95th percentile of the distribution had a maximum value of 16.32 days. The maximum 97.5th and 99th percentile of the distribution was 11.5 days and 20.4 days respectively (Table 3).
Table 3.
Overview of studies estimating incubation periods for SARS-CoV-2
| Study | n | Distribution | Mean (days) | 95th percentile(days) | ||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95%CI | |||
| Li et al. [20] | 10 | Log normal | 5.2 | 4.1–7.0 | 12.5 | 9.2–18 |
| Zhang et al. [23] | 49 | Log normal | 5·2 | 1.8–12.4 | 10.5 | |
| Linton et al. [29] | 52€ | Log normal* |
5.0 5.6 ┼ |
4.2–6.0 4.4–7.4 |
10.6 12.3 |
8.5–14.1 9.1–19.8 |
| Weibull | 5.4 | 4.3–6.6 | 12.0 | 9.8–15.6 | ||
| Gamma | 5.3 | 4.3–6.6 | 11.3 | 9.2–14.5 | ||
| 158€ € | Log normal* | 5.6 | 5.0–6.3 | 10.8 | 9.3–12.9 | |
| Weibull | 5.8 | 5.2–6.5 | 11.0 | 9.6–12.9 | ||
| Gamma | 6.0 | 5.3–6.7 | 11.7 | 10.3–13.4 | ||
| Backer et al. [55] | 88 | Weibull* | 6.4 | 5.6–7.7 | 10.3 | 8.6–14.1 |
| Gamma | 6.5 | 5.6–7.9 | 11.3 | 9.1–15.7 | ||
| Lauer et al. [56] | 181 | Log normal* |
5.5 5.1 a |
- 4.5–5.8 |
11.5b | 8.2–15.6 |
| Wang et al. [57] | 483 | Log normal* | 7.4 | 2–20 | - | - |
| Bi et al. [24] | 183 | Log normal | 4.8a | 4.2–5.4 | 14.0 | 12.2–15.9 |
| Yu et al. [30] | 132 | Gamma | 7.2a | 6.4 -7.9 |
16.0 20.4 c |
- - |
| Yang et al. [26] | 178 | Weibull* | 6 a | - | 13.7 | 12.5–14.9 |
| Wang et al. [47] | 14 | Log normal | 4.5 | 3.0–6.4 | 11.4 | 4.0–12.0 |
| Viego et al. [33] | 12 | Log normal | 7.50 | 4.11–10.89 | - | - |
| Tindale et al. [52] | 93 | Gamma | 5.99 | 4.97–7.14 | - | - |
| 135 | Gamma | 8.68 | 7.72–9.7 | - | - | |
| Ryu et al. [34] | 181 | Log normal* | 4.7 a | 0.1–15.6 | - | - |
| Qin et al(69) | 1084 | Weibull | 8.29 | 7.67–8.90 | 16.32 | 15.62-17.04 |
| Lee et al. [51] | 47 | Log normal* | 3.0 a | 0.6–8.2 | - | - |
| Chun et al. [9] | 74 | Weibull | 3.10 a | 2.54–3.71 | - | - |
| Gamma | 2.99 a | 2.44–3.60 | - | - | ||
| Log normal* | 2.87 a | 2.33–3.50 | - | - | ||
| Wieland [37] | 107 | - | - | - | 5.6 b | - |
€Excluding Wuhan resident
€€Including Wuhan resident
*Best fit distribution to the data
┼Estimation with right truncation
aMedian
b97.5th percentile
c99th percentile
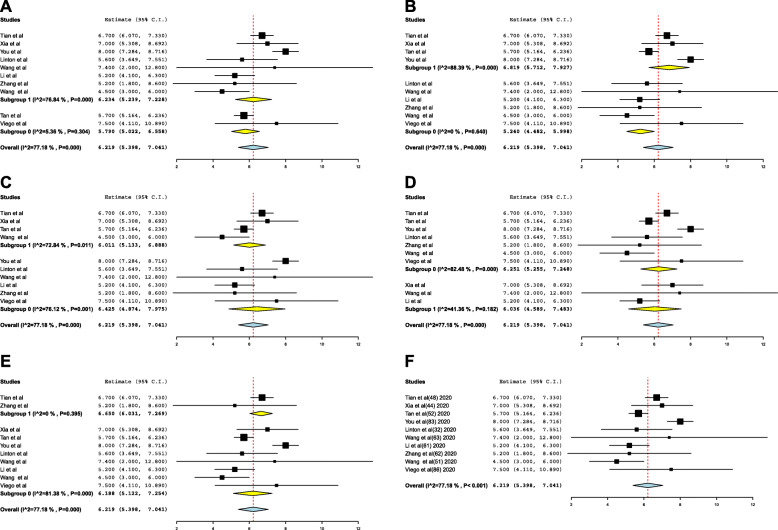
Mean incubation period and meta-analysis
The estimated mean incubation period obtained from the included studies and the pooled mean are presented in Fig. 3. Out of the 10 included studies in the meta-analysis, 8 were conducted in China, 1 in Singapore, and 1 in Argentina. The pooled mean incubation period was 6.2 (95% CI 5.4, 7.0) days. Heterogeneity testing (I2 = 77.1%; p < 0.001) revealed notable differences among the included studies in the meta-analysis.
Moderator variables were analyzed to identify and eliminate the observed heterogeneity: population of studies, severity, sex-ratio, study quality, and method of calculation (Table 4).
Table 4.
Estimation of days of incubation with moderator variables
| Estimate | SE | 95% CI | p-value | |
|---|---|---|---|---|
| Intercept | 6.219 | 0.419 | (5.398; 7.041) | < 0.001 |
| Population | ||||
| Chinese | 6.234 | 0.507 | (5.239; 7.228) | < 0.001 |
| Not Chinese | 5.790 | 0.392 | (5.022; 6.558) | < 0.001 |
| Severity | ||||
| Hospitalized | 6.011 | 0.448 | (5.133; 6.888) | < 0.001 |
| Not hospitalized | 6.425 | 0.791 | (4.874; 7.975) | < 0.001 |
| Sex ratio | ||||
| >1 | 6.036 | 0.738 | (4.589; 7.483) | < 0.001 |
| <1 | 5.805 | 0.435 | (4.952; 6.659) | < 0.001 |
| Quality of study | ||||
| Strong | 6.650 | 0.316 | (6.031; 7.269) | < 0.001 |
| Moderate to weak | 6.188 | 0.419 | (5.122; 7.254) | < 0.001 |
CI confidence interval, SE standard error
The heterogeneity was decreased when we included the study quality and the method of calculation used as moderator variables (I2 0%). The mean incubation period ranged from 5.2 (95% CI 4.4 to 5.9) to 6.65 days (95% CI 6.0 to 7.2) (Fig. 3).
Fig. 3.
Forest-plot for mean incubation period in days
Discussion
This review includes 42 studies done predominantly in China showing a mean and median incubation period of maximum 8 days and 12 days respectively. The pooled mean incubation period for COVID-19 is 6.2 (95% CI 5.4, 7.0) and may vary depending on population of studies, severity, sex-ratio, study quality, and method of calculation. In various parametric models, the 95th percentiles were in the range 10.3–16 days, which was not consistent with the first WHO reports [61]. While it was difficult to estimate the right hand tail of the incubation period distribution based on small sample sizes, the highest 99th percentile would be as long as 20.4 days, and this indicates that long incubation periods are possible. Lauer et al. [56] estimated that 101 out of 10,000 cases (99th percentile=482) would develop symptoms after 14 days of active monitoring or quarantine. Wang et al. [57] reported that about 7.45% patients were overestimated with longer than 14 days of incubation periods. Although many studies did not match with the inclusion criteria in our review, they are worthy to be mentioned. In a research letter studying serial cases of 6 patients infected with SARS-CoV-2 in China, Bai et al. reported that the incubation period of patient 1 was 19 days [63]. Based on 175 case details reported by 64 web pages between January20, 2020, and February 12, 2020, Leung estimated a mean of 7.2 (95% CI 6.1 to 8.4) with a 95th percentile of the Weibull distribution of 14.6 days (95% CI, 12.1 to 17.1) [64]. On the other hand, in the beginning of the pandemic of COVID-19, some studies found that there is no observable difference between the incubation time for SARS-CoV-2, severe acute respiratory syndrome coronavirus (SARS-CoV), and Middle East respiratory syndrome coronavirus (MERS-CoV) [11].
In our results, studies with contact tracing or exposure history of close contact showed a range of incubation period not exceeding 14 days [8, 38, 40, 41]. In fact, potential direct transmission could be related to a higher infecting dose and higher virulence of the strain that could lead to a shorter incubation period [65]. Indeed, Yu et al. showed that the incubation period was significantly shorter among patients who had multiple exposures to confirmed cases in the same province (Shanghai) (median 7.5 days; interquartile range (IQR) 5–7.9 days) compared with patients who had travel history in Wuhan (median 7.8days; (IQR) 5–8.2days) [30]. These results strengthen the hypothesis that a higher infecting dose could have been transmitted by the index case leading to a shorter incubation period compared with cases associated with “indirect” transmission.
In our study, there was considerable heterogeneity investigated with subgroup analysis. Several articles have shown that the incubation period differs between individuals according to their age or sex. Tan et al. showed that age-specific mean incubation periods were statistically significantly different across different age categories. The longest was observed among those aged 70+ (7.56 days, 95% CI 5.31–9.80) while the shortest was among those aged 60–69 years (4.69 days, 95% CI 3.86–5.52) and <30 years (4.95 days, 95% CI 4.31–5.58) [48]. However, Qin et al. concluded that there is no evidence that the incubation time depends on age [35].
Systematic reviews and meta-analyses conducted from 1 December 2019 to 11 March showed that the pooled incubation period mean was 5.68 (99% CI: 4.78, 6.59) days with heterogeneity testing (I2 = 98.4%) [66]. As in our findings, this heterogeneity test revealed notable differences among the included studies.
On the other hand, and based on the log-normal distribution, McAloon et al. [67] found in a meta-analysis conducted from December 1, 2019, to April 8, 2020, a mean of 5.8 days (95% CI 5.0–6.7) for the corresponding incubation period. However, our results with an estimated incubation mean of 5.2 (95% CI, 4.4–5.9) were more reliable since the heterogeneity test was zero.
Our study has some notable limitations. First, in most studies, the data were collected retrospectively, resulting in a recall bias (uncertain exact dates of exposure) and some missing data that would inevitably influence our assessment. Second, due to urgent timeline for data extraction and analysis, many studies have estimated the incubation period in a limited case number in a short period of time, which necessitates the cautious interpretation of the generalizability of our findings. The numbers were too small to detect systematic differences in incubation time with age or sex. Third, not all studies (except Tian et al. [44]) paid attention to asymptomatic patients, so our review may represent an erroneous incubation period. Although there is interest on asymptomatic transmission, we were unable to address this point in our review, and further studies should be done to better understand disease transmissibility of asymptomatic cases.
Conclusions
This work provides additional evidence of incubation period for COVID-19 and showed that it is prudent not to dismiss the possibility of incubation periods up to 14 days at this stage of the epidemic. As the epidemic continues, it remains important to collect more information on incubation periods through longitudinal studies with more patients so that we can conduct subgroup analysis and better understand the transmissibility of.
Supplementary Information
Additional file 1:. Quality assessment of the included studies.
Acknowledgements
Not applicable.
Abbreviations
- EPHPP
Effective Public Health Practice Project
- MERS-CoV
Middle East respiratory syndrome coronavirus
- IQR
Interquartile range
- RCTs
Randomized controlled trials
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- SD
Standard deviation
- SIGN
Scottish Intercollegiate Guidelines Network
- 95% CI
95% confidence interval
Authors’ contributions
WD: conceptualization, research literature, and writing—original draft.
IA and JM: research literature and quality assessment. NZ, RG, SBF: contributor in writing the manuscript. HG: revision of all manuscript. All authors read and approved the final manuscript.
Funding
No funding
Availability of data and materials
Not applicable
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coronavirus COVID-19 (2019-nCoV) [Internet]. [cited 2020 Dec 8]. Available from: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6.
- 2.Weekly operational update on COVID-19 - 7 December 2020 [Internet]. [cited 2020 Dec 8]. Available from: https://www.who.int/publications/m/item/weeklyoperational-update-on-covid-19%2D%2D-7-december-2020.
- 3.Quilty BJ, Clifford S, Flasche S, Eggo RM, CMMID nCoV working group. Effectiveness of airport screening at detecting travellers infected with novel coronavirus (2019-nCoV). Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25(5). [DOI] [PMC free article] [PubMed]
- 4.Gostic K, Gomez AC, Mummah RO, Kucharski AJ, Lloyd-Smith JO. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. eLife. 9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7060038/. [cited 2020 Dec 8]. [DOI] [PMC free article] [PubMed]
- 5.Tang B, Wang X, Li Q, Bragazzi NL, Tang S, Xiao Y, et al. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. J Clin Med. 2020;9(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7074281/. [cited 2020 Dec 8]. [DOI] [PMC free article] [PubMed]
- 6.Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Y, Shi C, Chen Y, Shi P, Liu J, Xiao Y, Shen Y, Chen E. A cluster of the corona virus disease 2019 caused by incubation period transmission in Wuxi, China. J Infect. 2020;80(6):666–670. doi: 10.1016/j.jinf.2020.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L, Zhang X, Zhang X, Wei Z, Zhang L, Xu J, Liang P, Xu Y, Zhang C, Xu A. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: a prospective contact-tracing study. J Infect. 2020;80(6):e1–13. doi: 10.1016/j.jinf.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun JY, Baek G, Kim Y. Transmission onset distribution of COVID-19. Int J Infect Dis. 2020;99:403–407. doi: 10.1016/j.ijid.2020.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jing Q-L, Liu M-J, Zhang Z-B, Fang L-Q, Yuan J, Zhang A-R, Dean NE, Luo L, Ma MM, Longini I, Kenah E, Lu Y, Ma Y, Jalali N, Yang ZC, Yang Y. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(10):1141–1150. doi: 10.1016/S1473-3099(20)30471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang X, Rayner S, Luo M-H. Does SARS-CoV-2 has a longer incubation period than SARS and MERS? J Med Virol. 2020;92(5):476–478. doi: 10.1002/jmv.25708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Şahin M. Impact of weather on COVID-19 pandemic in Turkey. Sci Total Environ. 2020;728:138810. doi: 10.1016/j.scitotenv.2020.138810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y, Jiang M, Xia D, He L, Lv X, Liao X, Meng J. COVID-19 in a patient with long-term use of glucocorticoids: a study of a familial cluster. Clin Immunol Orlando Fla. 2020;214:108413. doi: 10.1016/j.clim.2020.108413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armenian HK, Lilienfeld AM. Incubation period of disease. Epidemiol Rev. 1983;5(1):1–15. doi: 10.1093/oxfordjournals.epirev.a036254. [DOI] [PubMed] [Google Scholar]
- 15.Virlogeux V, Li M, Tsang TK, Feng L, Fang VJ, Jiang H, Wu P, Zheng J, Lau EHY, Cao Y, Qin Y, Liao Q, Yu H, Cowling BJ. Estimating the distribution of the incubation periods of human avian influenza A(H7N9) virus infections. Am J Epidemiol. 2015;182(8):723–729. doi: 10.1093/aje/kwv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishiura H. Early efforts in modeling the incubation period of infectious diseases with an acute course of illness. Emerg Themes Epidemiol. 2007;4(1):2. doi: 10.1186/1742-7622-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quality assessment tool for quantitative studies [Internet]. Effective Public Healthcare Panacea Project. [cited 2020 Dec 8]. Available from: https://www.ephpp.ca/quality-assessment-tool-for-quantitative-studies/
- 18.Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323(7308):334–336. doi: 10.1136/bmj.323.7308.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.OpenMetaAnalyst: Wallace, Byron C, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, et al. Closing the gap between methodologists and end-users: R as a computational back-End. J Statistical Software. 49(2012):5.
- 20.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pung R, Chiew CJ, Young BE, Chin S, Chen MI-C, Clapham HE, Cook AR, Maurer-Stroh S, Toh MPHS, Poh C, Low M, Lum J, Koh VTJ, Mak TM, Cui L, Lin RVTP, Heng D, Leo YS, Lye DC, Lee VJM, Kam KQ, Kalimuddin S, Tan SY, Loh J, Thoon KC, Vasoo S, Khong WX, Suhaimi NA, Chan SJH, Zhang E, Oh O, Ty A, Tow C, Chua YX, Chaw WL, Ng Y, Abdul-Rahman F, Sahib S, Zhao Z, Tang C, Low C, Goh EH, Lim G, Hou Y', Roshan I, Tan J, Foo K, Nandar K, Kurupatham L, Chan PP, Raj P, Lin Y, Said Z, Lee A, See C, Markose J, Tan J, Chan G, See W, Peh X, Cai V, Chen WK, Li Z, Soo R, Chow ALP, Wei W, Farwin A, Ang LW. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. The Lancet. 2020;395(10229):1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song Y-S, Hao Y-B, Liu W-W, Zhang S-S, Wang P, Fan T-L. Clinical features of 17 patients with 2019-nCoV. Eur Rev Med Pharmacol Sci. 2020;24(20):10896–10901. doi: 10.26355/eurrev_202010_23454. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Litvinova M, Wang W, Wang Y, Deng X, Chen X, Li M, Zheng W, Yi L, Chen X, Wu Q, Liang Y, Wang X, Yang J, Sun K, Longini IM, Jr, Halloran ME, Wu P, Cowling BJ, Merler S, Viboud C, Vespignani A, Ajelli M, Yu H. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect Dis. 2020;20(7):793–802. doi: 10.1016/S1473-3099(20)30230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, Liu X, Wei L, Truelove SA, Zhang T, Gao W, Cheng C, Tang X, Wu X, Wu Y, Sun B, Huang S, Sun Y, Zhang J, Ma T, Lessler J, Feng T. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K, Gao J, Wang H, Wu X, Yuan Q, Guo F, et al. Epidemiology of 2019 novel coronavirus in Jiangsu Province, China after wartime control measures: a population-level retrospective study. Travel Med Infect Dis. 2020;35:101654. doi: 10.1016/j.tmaid.2020.101654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Dai J, Zhao J, Wang Y, Deng P, Wang J. Estimation of incubation period and serial interval of COVID-19: analysis of 178 cases and 131 transmission chains in Hubei province, China. Epidemiol Infect. 2020;148:e117. doi: 10.1017/S0950268820001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ki M. Task Force for 2019-nCoV. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiol. Health. 2020;42:e2020007. doi: 10.4178/epih.e2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linton NM, Kobayashi T, Yang Y, Hayashi K, Akhmetzhanov AR, Jung S, et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9(2) Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7074197/. [cited 2020 Dec 9]. [DOI] [PMC free article] [PubMed]
- 30.Yu X, Sun X, Cui P, Pan H, Lin S, Han R, Jiang C, Fang Q, Kong D, Zhu Y, Zheng Y, Gong X, Xiao W, Mao S, Jin B, Wu H, Fu C. Epidemiological and clinical characteristics of 333 confirmed cases with coronavirus disease 2019 in Shanghai. China. Transbound Emerg Dis. 2020;67(4):1697–1707. doi: 10.1111/tbed.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao Z, Xie X, Guo W, Luo Z, Liao J, Wen F, et al. Examining the incubation period distributions of COVID-19 on Chinese patients with different travel histories. J Infect Dev Ctries. 2020;14(4):323–327. doi: 10.3855/jidc.12718. [DOI] [PubMed] [Google Scholar]
- 32.Wong J, Chaw L, Koh WC, Alikhan MF, Jamaludin SA, Poh WWP, Naing L. Epidemiological investigation of the first 135 COVID-19 cases in Brunei: implications for surveillance, control, and travel restrictions. Am J Trop Med Hyg. 2020;103(4):1608–1613. doi: 10.4269/ajtmh.20-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viego V, Geri M, Castiglia J, Jouglard E. Incubation period and serial interval of Covid-19 in a chain of infections in Bahia Blanca (Argentina) Cienc Saude Coletiva. 2020;25(9):3503–3510. doi: 10.1590/1413-81232020259.20852020. [DOI] [PubMed] [Google Scholar]
- 34.Ryu S, Ali ST, Jang C, Kim B, Cowling BJ. Effect of nonpharmaceutical interventions on transmission of severe acute respiratory syndrome coronavirus 2, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2406–2410. doi: 10.3201/eid2610.201886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin J, You C, Lin Q, Hu T, Yu S, Zhou X-H. Estimation of incubation period distribution of COVID-19 using disease onset forward time: a novel cross-sectional and forward follow-up study. Sci Adv. 2020;6(33):eabc1202. doi: 10.1126/sciadv.abc1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.You C, Deng Y, Hu W, Sun J, Lin Q, Zhou F, Pang CH, Zhang Y, Chen Z, Zhou XH. Estimation of the time-varying reproduction number of COVID-19 outbreak in China. Int J Hyg Environ Health. 2020;228:113555. doi: 10.1016/j.ijheh.2020.113555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wieland T. A phenomenological approach to assessing the effectiveness of COVID-19 related nonpharmaceutical interventions in Germany. Saf Sci. 2020;131:104924. doi: 10.1016/j.ssci.2020.104924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pung R, Chiew CJ, Young BE, Chin S, Chen MI-C, Clapham HE, et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet Lond Engl. 2020;395(10229):1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song R, Han B, Song M, Wang L, Conlon CP, Dong T, Tian D, Zhang W, Chen Z, Zhang F, Shi M, Li X. Clinical and epidemiological features of COVID-19 family clusters in Beijing. China. J Infect. 2020;81(2):e26–e30. doi: 10.1016/j.jinf.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia X-Y, Wu J, Liu H-L, Xia H, Jia B, Huang W-X. Epidemiological and initial clinical characteristics of patients with family aggregation of COVID-19. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2020;127:104360. doi: 10.1016/j.jcv.2020.104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernard Stoecklin S, Rolland P, Silue Y, Mailles A, Campese C, Simondon A, et al. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Eurosurveillance. 2020;25(6) Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7029452/. [cited 2020 Dec 9]. [DOI] [PMC free article] [PubMed]
- 42.Böhmer MM, Buchholz U, Corman VM, Hoch M, Katz K, Marosevic DV, Böhm S, Woudenberg T, Ackermann N, Konrad R, Eberle U, Treis B, Dangel A, Bengs K, Fingerle V, Berger A, Hörmansdorfer S, Ippisch S, Wicklein B, Grahl A, Pörtner K, Muller N, Zeitlmann N, Boender TS, Cai W, Reich A, an der Heiden M, Rexroth U, Hamouda O, Schneider J, Veith T, Mühlemann B, Wölfel R, Antwerpen M, Walter M, Protzer U, Liebl B, Haas W, Sing A, Drosten C, Zapf A. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis. 2020;20(8):920–928. doi: 10.1016/S1473-3099(20)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Zhang Z-Z, Chen Y-K, Long Q-X, Tian W-G, Deng H-J, Hu JL, Zhang XX, Pu-Liao, Xiang JL, Wang DX, Hu P, Zhou FC, Li ZJ, Xu HM, Cai XF, Wang DQ, Hu Y, Tang N, Liu BZ, Wu GC, Huang AL. The clinical and immunological features of pediatric COVID-19 patients in China. Genes Dis. 2020;7(4):535–541. doi: 10.1016/j.gendis.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, Chen H, Wang D, Liu N, Liu D, Chen G, Zhang Y, Li D, Li J, Lian H, Niu S, Zhang L, Zhang J. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng Y, Xiong C, Liu Y, Qian X, Tang Y, Liu L, Leung ELH, Wang M. Epidemiological and clinical characteristics analysis of COVID-19 in the surrounding areas of Wuhan, Hubei Province in 2020. Pharmacol Res. 2020;157:104821. doi: 10.1016/j.phrs.2020.104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao C, Xu Y, Zhang X, Zhong Y, Long L, Zhan W, Xu T, Zhan C, Chen Y, Zhu J, Xiao W, He M. Public health initiatives from hospitalized patients with COVID-19. China. J Infect Public Health. 2020;13(9):1229–1236. doi: 10.1016/j.jiph.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Zhou Q, He Y, Liu L, Ma X, Wei X, et al. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J. 2020;55(6). [DOI] [PMC free article] [PubMed]
- 48.Tan WYT, Wong LY, Leo YS, Toh MPHS. Does incubation period of COVID-19 vary with age? A study of epidemiologically linked cases in Singapore. Epidemiol Infect. 2020;148:e197. doi: 10.1017/S0950268820001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Ding J, Chen L, Hong L, Yu X, Ye E, et al. Epidemiological and clinical characteristics of three family clusters of COVID-19 transmitted by latent patients in China. Epidemiol Infect. 2020;148:e137. doi: 10.1017/S0950268820001491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lou B, Li T-D, Zheng S-F, Su Y-Y, Li Z-Y, Liu W, et al. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur Respir J. 2020;56(2). [DOI] [PMC free article] [PubMed]
- 51.Lee H, Kim K, Choi K, Hong S, Son H, Ryu S. Incubation period of the coronavirus disease 2019 (COVID-19) in Busan, South Korea. J Infect Chemother Off J Jpn Soc Chemother. 2020;26(9):1011–1013. doi: 10.1016/j.jiac.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tindale LC, Stockdale JE, Coombe M, Garlock ES, Lau WYV, Saraswat M, et al. Evidence for transmission of COVID-19 prior to symptom onset. eLife. 2020;22:9. doi: 10.7554/eLife.57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alsofayan YM, Althunayyan SM, Khan AA, Hakawi AM, Assiri AM. Clinical characteristics of COVID-19 in Saudi Arabia: a national retrospective study. J Infect Public Health. 2020;13(7):920–925. doi: 10.1016/j.jiph.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu T, Chen C, Zhu Z, Cui M, Chen C, Dai H, et al. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020;94:68–71. doi: 10.1016/j.ijid.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25(5). [DOI] [PMC free article] [PubMed]
- 56.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;10 [cited 2020 Dec 9]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7081172/. [DOI] [PMC free article] [PubMed]
- 57.Wang P, Lu J, Jin Y, Zhu M, Wang L, Chen S. Statistical and network analysis of 1212 COVID-19 patients in Henan, China. Int J Infect Dis. 2020;95:391–398. doi: 10.1016/j.ijid.2020.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu P, Zhu J, Zhang Z, Han Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis. 2020;221(11):1757–1761. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, Shang W, Liu Q, Zhang X, Zheng M, Yue M. Clinical characteristics of 194 cases of COVID-19 in Huanggang and Taian. China. Infection. 2020;48(5):687–694. doi: 10.1007/s15010-020-01440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nie X, Fan L, Mu G, Tan Q, Wang M, Xie Y, et al. Epidemiological characteristics and incubation period of 7015 confirmed cases with coronavirus disease 2019 outside Hubei Province in China. J Infect Dis. 2020;222(1):26–33. doi: 10.1093/infdis/jiaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coronavirus disease (COVID-19) situation reports [Internet]. [cited 2020 Dec 9]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 62.Liu J, Liao X, Qian S, Yuan J, Wang F, Liu Y, Wang Z, Wang FS, Liu L, Zhang Z. Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerg Infect Dis. 2020;26(6):1320–1323. doi: 10.3201/eid2606.200239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bai Y, Yao L, Wei T, Tian F, Jin D-Y, Chen L, Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leung C. The difference in the incubation period of 2019 novel coronavirus (SARS-CoV-2) infection between travelers to Hubei and nontravelers: the need for a longer quarantine period. Infect Control Hosp Epidemiol. 2020;41(5):594–596. doi: 10.1017/ice.2020.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Virlogeux V, Fang VJ, Wu JT, Ho L-M, Malik Peiris JS, Leung GM, et al. Incubation period duration and severity of clinical disease following severe acute respiratory syndrome coronavirus infection. Epidemiol Camb Mass. 2015;26(5):666–669. doi: 10.1097/EDE.0000000000000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khalili M, Karamouzian M, Nasiri N, Javadi S, Mirzazadeh A, Sharifi H. Epidemiological characteristics of COVID-19: a systematic review and meta-analysis. Epidemiol Infect. 2020;148:e130. doi: 10.1017/S0950268820001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McAloon C, Collins Á, Hunt K, Barber A, Byrne AW, Butler F, et al. Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research. BMJ Open. 2020;10(8):e039652. doi: 10.1136/bmjopen-2020-039652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1:. Quality assessment of the included studies.
Data Availability Statement
Not applicable