Abstract
A self‐measured home blood pressure (BP)‐guided strategy is an effective practical approach to hypertension management. The Asia BP@Home study is the first designed to investigate current home BP control status in different Asian countries/regions using standardized home BP measurements taken with the same validated home BP monitoring device with data memory. We enrolled 1443 medicated hypertensive patients from 15 Asian specialist centers in 11 countries/regions between April 2017 and March 2018. BP was relatively well controlled in 68.2% of patients using a morning home systolic BP (SBP) cutoff of <135 mm Hg, and in 55.1% of patients using a clinic SBP cutoff of <140 mm Hg. When cutoff values were changed to the 2017 AHA/ACC threshold (SBP <130 mm Hg), 53.6% of patients were well controlled for morning home SBP. Using clinic 140 mm Hg and morning home 135 mm Hg SBP thresholds, the proportion of patients with well‐controlled hypertension (46%) was higher than for uncontrolled sustained (22%), white‐coat (23%), and masked uncontrolled (9%) hypertension, with significant country/regional differences. Home BP variability in Asian countries was high, and varied by country/region. In conclusion, the Asia BP@Home study demonstrated that home BP is relatively well controlled at hypertension specialist centers in Asia. However, almost half of patients remain uncontrolled for morning BP according to new guidelines, with significant country/regional differences. Strict home BP control should be beneficial in Asian populations. The findings of this study are important to facilitate development of health policies focused on reducing cardiovascular complications in Asia.
Keywords: Asia, Asia BP@Home study, blood pressure control, blood pressure variability, home blood pressure, Home study, validated blood pressure monitoring device
1. INTRODUCTION
Hypertension is a major risk factor for cardiovascular disease and other age‐related conditions, such as dementia and chronic kidney disease, worldwide. Strict blood pressure (BP) control is one of the most effective approaches to prevent cardiovascular events.1, 2, 3 Recently released 2017 American Heart Association (AHA)/American College of Cardiology (ACC) guidelines lowered thresholds for the diagnosis and management of hypertension from 140/90 to 130/80 mm Hg.4
This new threshold may be particularly relevant in Asia due to the different characteristics of hypertension and related cardiovascular disease in Asians.5, 6, 7 Stroke and non‐ischemic heart failure, both of which are closely related to hypertension, are more common in Asia than in Western countries.5, 7 The BP‐associated slope of cardiovascular events, especially stroke, is steeper in Asia than in Westerners.8 Thus, the benefits associated with strict BP control should be even greater in Asia.5, 8, 9, 10
Out‐of‐clinic measurement of BP using ambulatory BP monitoring (ABPM) or home BP monitoring (HBPM) is now recommended for the management of hypertension.11, 12, 13, 14 This is because ambulatory or home BP measurements are more closely associated with cardiovascular event risk and organ damage than clinic BP.4, 13, 15, 16 Again, this is likely to be of greater importance in Asia due to the presence of Asian‐specific characteristics in the 24‐hour BP profile.5, 17, 18, 19 In addition, the prevalence of masked uncontrolled hypertension (normotensive for clinic BP and hypertension based on out‐of‐office BP), excessive morning BP surge and morning hypertension, and nocturnal hypertension is higher in Asians than in Westerners.17, 20, 21, 22, 23, 24
A self‐measured home BP‐guided strategy has been stressed as the most effective practical approach of the management of hypertension in Asia.13, 25, 26, 27 There are several studies highlighting the importance of home BP for improving cardiovascular prognosis.28, 29, 30, 31, 32, 33 However, the findings of some of these studies are limited due to a lack of standardization in the BP measurement schedule, BP measurement device, and recording of home BP measurements, as well as a lack of measurement of nocturnal BP, which is an emerging important cardiovascular risk factor. In addition, all the home BP data in these studies were obtained at least 5 years ago and may not reflect the current status of home BP. Given that guideline‐driven antihypertensive medication with a lower home BP target (<135/85 mm Hg) has been widely introduced in Asian hypertension specialist centers over the last 10 years, home BP control status in Asia might now be quite different. However, there are no recent studies looking at home BP control status, and none have employed the same BP measurement schedule using the same validated HBPM device with data memory.
We have recently established the Hypertension, brain, cardiovascular and renal Outcome Prevention and Evidence in Asia (HOPE Asia) Network to improve management of hypertension and organ protection for “zero” cardiovascular events in Asia. The HOPE Asia Network has three key initiatives: (a) to understand the current evidence; (b) to achieve consensus; and (c) to conduct clinical studies on the current status.34 As part of the last initiative, the Asia BP@Home study was designed to investigate the current 2017‐2018 home BP control status in 11 Asian countries/regions using standardized home BP measurements taken with the same validated HBPM device with data memory.35
2. METHODS
2.1. Study design
The Asia BP@Home study design has been described in detail previously.35 In brief, the study was a prospective, multicenter, non‐interventional trial designed to collect home BP data from outpatients living in Asian countries and regions. All patients provided written informed consent before study enrollment, and the study protocol was approved by independent ethics committees or institutional review boards for every study center. All procedures were conducted in accordance with the ethical principles of the Declaration of Helsinki. The study was registered on the ClinicalTrials.gov website (NCT03096119).
2.2. Study participants
Patients aged 20 years and older with a diagnosis of hypertension who had been receiving stable doses of antihypertensive medications for ≥3 months were recruited from 15 Asian specialist hypertension centers in China, India, Indonesia, Japan, Korea, Malaysia, Pakistan, Philippines, Singapore, Taiwan, and Thailand. Patients were enrolled between April 2017 and March 2018. The predefined number of study patients was approximately 100 patients from each center.
2.3. BP measurements
Patients were provided with the same validated automatic, oscillometric HBPM device (Omron HEM‐7130‐AP or HEM‐7131‐E; Omron Healthcare, Kyoto, Japan)36 and instructed to measure their BP at home for at least 7 days during a 15‐day HBPM period. Patients were asked not to change their medication during the monitoring period. To avoid reporting bias, BP data were automatically stored in device memory, then entered into the study database by a physician or nurse.
Home BP measurements were performed according to the Expert Panel Consensus Recommendations for HBPM (the HOPE Asia Network),26, 27 which modified guidelines from the European Society of Hypertension (ESH),11, 12 Japanese Society of Hypertension (JSH),13 and the Korean Society of Hypertension,37 and the China consensus document on HBPM.38 Patients measured their BP at home using the device provided twice in the morning (morning home BP) and twice at bedtime (evening home BP) as follows. Patients were instructed to take morning BP measurements, within 1 hour after waking, following urination, before taking any medications, before eating breakfast, and after 2 minutes of rest while in a sitting position (leaning against a seat back and resting both feet on the floor), with no moving or talking. Bedtime measurements were to be taken immediately before going to bed and after 2 minutes of rest while in a sitting position (as for morning BP measurement). For both morning and evening home BP, the interval between the two measurements should be at least 1 minute, and BP measurements should be taken from the upper area of the nondominant arm. However, if BP readings taken in the right and left arms differed significantly, patients were instructed to use the arm with the highest BP (as determined at the first study visit) for all subsequent home BP measurements. Patients were asked to record BP values on the sheet provided and to return this at the second visit.
Clinic BP was measured twice at the first and second (if applicable) study visit.
2.4. Definition of hypertension control subgroups
Definition was based on the morning home SBP measured by HBPM and clinic SBP. Using the clinic 140 mm Hg and morning home 135 mm Hg SBP thresholds, the proportion of patients with well‐controlled, white‐coat, masked uncontrolled, and sustained morning hypertension was determined. We also defined this classification using the new 2017 ACC/AHA guideline thresholds of 130 mm Hg for morning and clinic BP.
2.5. Day‐to‐day home BP variability
We calculated coefficient of variation (CV) for morning home SBP based on the patients' home BP readings during the study period. Average real variability (ARV) is the average absolute difference between successive BP measurements and, in contrast to CV, takes the order of the BP measurements into account. Both CV and ARV are partially dependent on the overall mean BP levels over time, and this issue may not be resolved even if mean BP level over time is used as an adjustment factor. Therefore, we used BP variability independent of the mean (VIM), another BP variability measure that has no correlation with mean BP levels. These variability measures have been used in previously reported BP variability studies.39, 40, 41
2.6. Comparison with historical home BP study
Home BP control status and home BP variability profiles from the Asia BP@Home study were compared with those of the Japanese nationwide general practitioner‐based home BP study (Japan Morning Surge‐Home Blood Pressure [J‐HOP] study) which used the same home BP measurement schedule and the same validated, automatic, oscillometric HBPM device,42 and collected baseline data over the period 2005‐2012.30, 39 Characteristics for patients enrolled in the J‐HOP study (mean age 64.8 years, 47.0% male, body mass index 24.3 kg/m2, 23.5% with diabetes) are described elsewhere in detail.43
2.7. Statistical analyses
All statistical analyses were performed using SAS version 9.4 software (SAS Institute Inc) at Super Circulation Monitoring With High Technology R&D Center, Jichi Medical University COE Cardiovascular Research and Development Center (JCARD) (Tochigi, Japan). The mean morning home SBP (average of all morning BP measurements during the monitoring period) vs the mean clinic SBP (average of two measurements at the first visit) values for each patient were plotted to obtain the distribution, overall and by hypertension subtype.
3. RESULTS
3.1. Patient demographics and characteristics
A total of 1467 patients with medicated hypertension from 15 hypertension specialist centers in 11 countries/regions were recruited. Of these, 1443 were included in the analysis (Figure S1). Overall, 47.4% of patients were men (mean age 62.3 ± 12.1 years); the prevalence of diabetes and chronic kidney disease was 25.1% and 5.6%, respectively (Table 1).
Table 1.
Baseline characteristics
| N = 1443 | |
|---|---|
| Age, years | 62.3 ± 12.1 |
| Male, % | 47.4 |
| BMI, kg/m2 | 26.0 ± 4.5 |
| Habitual drinking, % | 10.6 |
| Current smoking, % | 8.4 |
| Shift worker, % | 3.1 |
| Current disease, % | |
| Hyperlipidemia | 52.0 |
| Diabetes | 25.1 |
| Carotid artery disease | 12.1 |
| Thoracic aortic aneurysm | 0.6 |
| Chronic kidney disease | 5.6 |
| Atrial fibrillation | 3.7 |
| Antihypertensive medication, % | |
| ARB | 49.4 |
| ACE | 11.6 |
| CCB | 66.0 |
| Alpha‐blocker | 3.7 |
| Beta‐blocker | 29.7 |
| Diuretics | 17.6 |
| Other | 1.0 |
| Bedtime dosing | 8.0 |
| Number of antihypertensive medications, % | |
| 1 | 45.6 |
| 2 | 34.2 |
| 3 | 13.9 |
| 4 | 5.1 |
| 5 | 0.9 |
| 6 | 0.3 |
| Medical history, % | |
| Angina pectoris | 10.2 |
| Myocardial infarction | 3.4 |
| Aortic dissection | 0.1 |
| Heart failure | 3.9 |
| Peripheral artery disease | 0.7 |
| Stroke | 6.4 |
| Clinic blood pressure | |
| Systolic, mm Hg | 138.8 ± 18.4 |
| Diastolic, mm Hg | 82.2 ± 11.1 |
| Pulse rate, bpm | 74.3 ± 11.8 |
| Self‐measured blood pressure at home | |
| Morning measurement | |
| Systolic, mm Hg | 130.4 ± 14.6 |
| Diastolic, mm Hg | 80.6 ± 9.5 |
| Pulse rate, bpm | 70.3 ± 9.6 |
| Evening measurement | |
| Systolic, mm Hg | 129.1 ± 15.4 |
| Diastolic, mm Hg | 78.1 ± 9.7 |
| Pulse rate, bpm | 73.1 ± 10.5 |
Data are shown as the mean ± SD, % or median (interquartile range).
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker.
Morning and evening home SBP was 130.4 ± 14.6 and 129.1 ± 15.4 mm Hg, respectively (Table 1). The mean number of the days of the BP measurements was 8.2 ± 2.7 days, and the total measurement of morning and evening BPs per person was 16.2 ± 5.6 and 16.0 ± 5.6, respectively. The mean values of morning and evening home SBP are significantly lower than those for patients in the J‐HOP study (138.4 ± 15.8 and 130.1 ± 14.9 mm Hg, respectively). Differences between morning and evening BP readings were 1.3 mm Hg for SBP and 2.5 mm Hg for diastolic BP (Table 1), much lower than 8.3 and 6.5 mm Hg, respectively, at baseline in the J‐HOP study. The most popular antihypertensive agents were calcium channel blockers (CCBs, 66.0% of patients), followed by angiotensin receptor blockers (ARBs; 49.4%), beta‐blockers (29.7%), diuretics (17.6%), and angiotensin converting enzyme (ACE) inhibitors (11.6%); other antihypertensive drug classes were used by less than 10% of patients (Table 1). Amlodipine was the most commonly used CCB (48.8% of the total population, 73.9% of all CCBs).
Using the clinic 140 mm Hg and morning home 135 mm Hg SBP thresholds, the proportion of patients with well‐controlled hypertension (46%) was higher than that for uncontrolled sustained (22%), white‐coat (23%), and masked uncontrolled (9%) hypertension. Corresponding values calculated using the 2017 ACC/AHA guideline thresholds of 130 mm Hg for morning and clinic BP were 26% of patients with well‐controlled hypertension and 40%, 28%, and 6%, respectively, with uncontrolled sustained, white‐coat or masked uncontrolled hypertension.
3.2. Home BP control status
Well‐controlled morning home SBP (SBP <135 mm Hg; Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [JNC 7] threshold44) was documented in 68.2% of patients, and 55.1% had well‐controlled clinic SBP (Figure 1A, black). Even using the new 2017 AHA/ACC threshold of 130 mm Hg,4 53.6% of patients were well controlled for morning home SBP and 32.3% were well controlled for clinic SBP <130 mm Hg (Figure 1A, red). The prevalence of well‐controlled hypertension based on both clinic and home morning systolic BP (SBP) was highest (45.6%) when control was classified using the clinic SBP 140 mm Hg and morning home SBP 135 mm Hg thresholds. Adopting the 2017 AHA/ACC classification thresholds (clinic SBP 130 mm Hg and morning home SBP 130 mm Hg), the prevalence of well‐controlled hypertension decreased from 45.6% to 26.0%, and that of masked uncontrolled hypertension decreased from 9.5% to 6.3%.
Figure 1.

Distributions of blood pressure (BP) control status based on different clinic and morning home blood pressure thresholds in (A) the Asia BP@Home study (N = 1443) and (B) the J‐HOP study (N = 4310). Black lines show cutoff values of 140 mm Hg for clinic systolic BP (SBP) and 135 mm Hg for home SBP, and black numbers show the proportion of patients with different types of hypertension based on those cutoff values. Red lines show cutoff values of 130 mm Hg for both clinic SBP and home SBP, and red numbers show the proportion of patients with different types of hypertension based on those cutoff values
3.3. Country/region difference
There were significant country/region differences in the demographics of study participants (Table S1) and home BP control status. However, the prevalence of controlled hypertension was higher than that of white‐coat, masked uncontrolled or uncontrolled hypertension in 11 out of 15 centers when the clinic SBP 140 mm Hg and morning home SBP 135 mm Hg thresholds were used (Figure 2A). With the new, lower thresholds (clinic SBP 130 mm Hg and morning home SBP 130 mm Hg), the prevalence of well‐controlled hypertension exceeded that of white‐coat, masked uncontrolled, and uncontrolled hypertension in 5/15 centers (Figure 2B).
Figure 2.
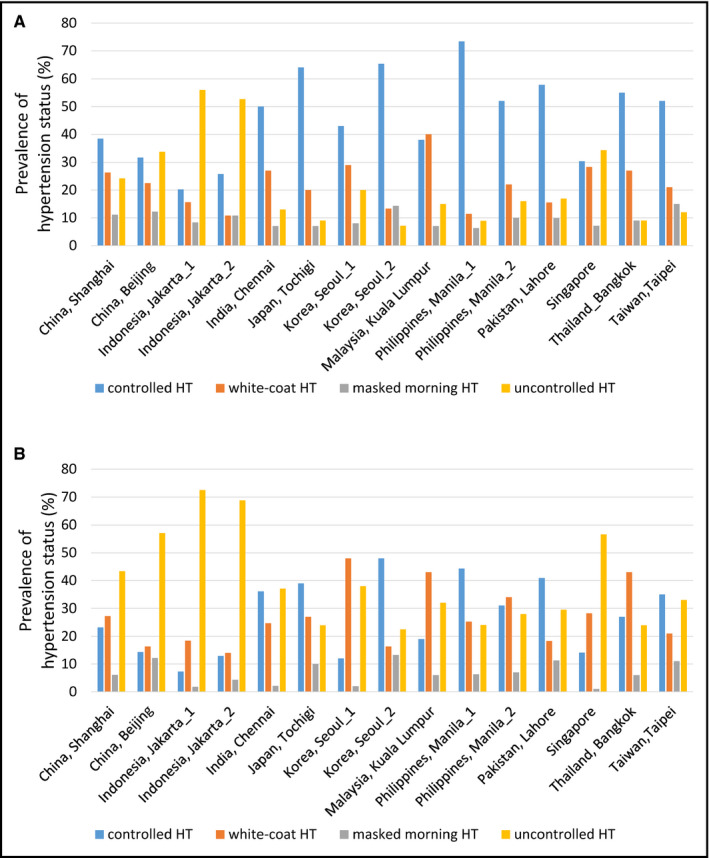
Country/regional differences in blood pressure (BP) control status based on different clinic and morning home blood pressure thresholds. A, Cutoff values of 140 mm Hg for clinic systolic BP (SBP) and 135 mm Hg for home SBP. B, Cutoff values of 130 mm Hg for both clinic SBP and home SBP. HT, hypertension, masked morning HT, masked uncontrolled morning hypertension
The prevalence of well‐controlled morning home SBP (well‐controlled hypertension plus white‐coat hypertension) was higher than that of uncontrolled morning home SBP (masked uncontrolled hypertension plus uncontrolled hypertension) in 13/15 centers using the higher thresholds (Figure 2A) and in 11/15 centers using the new, lower thresholds (Figure 2B).
3.4. Home BP variability
Despite relatively good BP control rates based on mean morning home BP (Figure 2), the prevalence of exaggerated BP variability based on CV, ARV, and VIM was quite high in Asian countries, with clear country/regional differences (Figure 3A‐C). The highest quartile (Q4) of day‐by‐day morning home SBP variability measures (CV ≥ 6.1, ARV ≥ 8.5 and VIM ≥ 8.2) of the J‐HOP study39 was seen more often in this study (Figure 3D). In addition, more than 25% of patients were in the highest quartile for BP variability at 13/15 centers (the exceptions were India and Japan) (Figure 3D).
Figure 3.
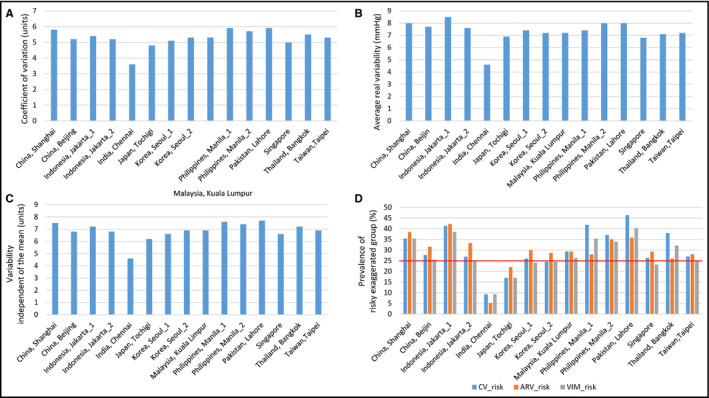
Country/regional difference in measures of day‐by‐day morning home systolic blood pressure variability (A. coefficient of variation; B. average real variability; C. variability independent of the mean) and the prevalence of risky exaggerated home blood pressure variability groups (D) in different countries/regions
4. DISCUSSION
The Asia BP@Home study is the first Asian simultaneous cross‐sectional study on home BP control status in major hypertension specialist centers across Asia using the same measurement schedule and the same validated device with memory. The results from 15 centers in 11 countries/regions showed that home BP control status in 2017‐2018 for patients managed at hypertension specialist centers in Asia was relatively good. However, almost one in three patients (31.8%) still did not reach their morning home SBP goal according to the conservative guidelines, and almost half (46.4%) were uncontrolled based on the new 2017 AHA/ACC threshold of 130 mm Hg.4 In addition, there were significant country/regional differences in home BP control status and the degree of home BP variability.
In the 2017 ACC/AHA guidelines compared with other or previous documents, the office SBP target has decreased by 10 mm Hg lower (SBP <140 mm Hg to <130 mm Hg) and the home SBP target is 5 mm Hg lower (SBP <135 mm Hg to <130 mm Hg). Needless to say, this will make uncontrolled hypertension more common while fewer patients will have controlled hypertension. Similarly, white‐coat effect will be found more often because some patients previously classified as having controlled hypertension will now be defined as having white‐coat effect, while masked uncontrolled hypertension should be found less often because many of these patients will now fit the criteria for uncontrolled hypertension (although some controlled hypertension will become masked uncontrolled hypertension).
The control rates of 68.2% for morning home SBP <135 mm Hg and 53.6% using the new 2017 AHA/ACC threshold of 130 mm Hg4 are higher than those reported in previous general practitioner‐based home BP studies conducted in Asian and Western countries 29, 31, 32 Home BP control rates achieved in this study from 2017 to 2018 were higher than those reported in the Japanese nationwide general practitioner‐based J‐HOP study from 2005 to 2012.30 Given that Asian patients with hypertension have different characteristics from those in Western populations, including higher salt intake and higher salt sensitivity,5, 17 it could theoretically be more difficult to achieve BP control during antihypertensive treatment in these patients. The better home BP control status of Asia BP@Home study than previous studies may partly be increased use of more potent and long‐acting antihypertensive agents. The most commonly used antihypertensive agent in the Asia BP@Home study was the long‐acting CCB, amlodipine (used by 48.8% of patients). The BP‐lowering effect of amlodipine persists for 24 hours and is not affected by salt sensitivity and salt intake, although it is dependent on baseline BP.45, 46 Thus, amlodipine is an appropriate choice for Asian hypertensive patients with high salt intake and high salt sensitivity. The use of long‐acting, effective antihypertensives could also have contributed to the smaller morning‐evening difference in home SBP and DBP seen in the current study compared with J‐HOP.30 Another difference between the Asia BP@Home and J‐HOP studies was the setting in which patients were treated. Our study was conducted in hypertension specialist centers (organized by members of HOPE Asia Network), whereas J‐HOP patients were managed by general practitioners.
For Asian patients, we recommend measurement of morning BP before taking medication as the first target of a hypertension management strategy.47, 48, 49, 50 Lifestyle factors in Asia mean that evening BP is difficult to measure before dinner at home, and evening home BP measured at bedtime after dinner is significantly affected by alcohol consumption and bathing,51 making this approach much less reliable, as has been shown previously.52 In addition, as highlighted above, there is a good body of data from Asia showing that morning home BP is an independent predictor of cardiovascular events.28, 30, 31, 33 The results of the prospective J‐HOP study clearly demonstrated that patients with well‐controlled morning home SBP (<135 mm Hg at baseline) were at lower risk of having a stroke than those with a higher morning home SBP.30 In addition, the large real‐world observational prospective HONEST study of more than 20 000 hypertensive patients receiving olmesartan‐based antihypertensive medication showed that on‐treatment morning home SBP <125 mm Hg was associated with a significantly lower rate of both stroke and coronary artery disease events compared with a morning home SBP of ≥145 mm Hg, especially in high‐risk patients.53 Furthermore, the home BP‐guided interventional HOMED‐BP study demonstrated that achievement of lower home SBP was significantly associated with better cardiovascular prognosis.28 Considering these results, the well‐controlled home BP achieved with specialist management in the Asia BP@Home study should result in a significant reduction of cardiovascular events in Asian countries. The challenge will be to ensure that these practices and control rates are translated to other clinical settings, such as for the majority of patients with hypertension who are managed in primary care.
The prevalence of masked uncontrolled morning hypertension (clinic SBP <140 mm Hg, and morning home SBP ≥135 mm Hg) in the Asia BP@Home study was 9.5% of the total sample, almost half the 19% rate found in the J‐HOP study.30 Masked uncontrolled hypertension is an important clinical issue. In the international ABPM registry of hypertensive patients, ARTEMIS, the prevalence of masked uncontrolled hypertension was higher in Asians than in the Westerners.24 In an analysis of data from the J‐HOP study of Japanese patients, the increased stroke risk associated with the presence of masked uncontrolled hypertension was comparable to that of sustained hypertension (clinic SBP ≥140 mm Hg and home SBP ≥135 mm Hg).54 A recent largest Spanish ABPM registry demonstrated that the risk associated with masked uncontrolled hypertension was highest compared with other hypertension subgroups (controlled, uncontrolled, and white‐coat).55 This may be a result of out‐of‐clinic hypertension that had been unrecognized by doctors for a long time. Again, this difference may be due to improvements in hypertension therapies over time, or care in the specialist vs primary care setting. Whatever, the underlying reason, the lower rates of masked uncontrolled hypertension seen in the Asia BP@home study suggest effective management of hypertension and should translate to reduced cardiovascular risk.
Home BP variability was greater in the Asia BP@Home study with a significant country/regional difference, even though home BP control status was better in the Asia BP@Home vs J‐HOP studies. Differences in environmental factors and lifestyles between counties may contribute to increased home BP variability. In the current study, we used three measures of day‐by‐day home BP variability (CV, ARB, and VIM) because values in the highest quartiles of these three measures (CV ≥ 6.1, ARV ≥ 8.5, and VIM ≥ 8.2) were significantly associated with stroke prognosis independent of mean home BP in the J‐HOP study.39 Similar findings were reported in other population‐based studies, where day‐by‐day home BP variability was a significant predictor of cardiovascular prognosis independent of the average home BP.40, 56, 57 The pathological thresholds of day‐by‐day home BP variability in treated patients with well‐controlled average home BP need to be determined in the future prospective studies.
There were significant country/regional differences in home BP control status, and in home BP variability. Rates of uncontrolled hypertension were much higher in some regions, while controlled hypertension was more common in others. These trends were accentuated when the 2017 ACC/AHA thresholds were applied. In addition, there are significant country/regional difference in cardiovascular death rates in Asia.7 Each county leader of the HOPE Asia Network plans to explore these inter‐country differences and their underlying reasons; results will be reported in due course.
This study also has some limitations. Firstly, the sample size in each country/region was small. Secondly, this study may not be directly applicable to routine clinical care in all participating countries. It is also possible that study patients enrolled from specialist hypertension centers, as was the case in this study, may be more motivated than patients treated in general practice or hospital clinics. An important next step is to create a real‐world database of outpatients recruited from general practitioners in each country. We did not collect urine or blood samples to confirm adherence to antihypertensive therapy, although we asked patients not to change their medication during the monitoring period. Finally, there is not yet any data on the clinical relevance of the four hypertension phenotype classifications using the lower new thresholds in the 2017 ACC/AHA guidelines. Therefore, the prognosis for hypertension subgroups based on this classification needs to be determined.
5. CONCLUSIONS
The Asia BP@Home study showed that hypertension specialist centers in Asia can achieve relatively good home BP control status for their patients. However, morning SBP remained above 130 mm Hg in the entire sample, and there were significant country/regional differences. Considering the characteristics of hypertension in Asians, strict home BP control would be expected to be beneficial. The findings of this study are important to facilitate development of health policies targeting the reduction of cardiovascular disease complications in Asia. A key component of this is transferring expertise in hypertension management from specialist centers to primary care, where the majority of patients with hypertension are managed.
DISCLOSURES
CH Chen has received honoraria for serving as a speaker or member of a speaker bureau for AstraZeneca, Bayer AG, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Merck & Co, Novartis, Pfizer, Sanofi, Servier and Takeda. YC Chia has received honoraria for serving as a speaker or advisor for Abbott, Bayer, Boehringer Ingelheim, Merck, MSD, Novartis, Pfizer, Reckitt Benckiser, Sanofi, Servier and Takeda; sponsorship to scientific conferences from Pfizer and Takeda; and research grants from Pfizer. K Kario has received research grants from A&D Co., Bayer Yakuhin, Boehringer Ingelheim, Daiichi Sankyo, EA Pharma, Fukuda Denshi, Medtronic, Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Omron Healthcare, Otsuka, Pfizer, Takeda and Teijin Pharma; and honoraria from Daiichi Sankyo, Omron Healthcare and Takeda. S Park has received honoraria from Astellas and Pfizer; and consultation fees from Takeda. S Siddique has received honoraria from Bayer, Novartis, Pfizer, Sanofi Aventis and Servier; and travel, accommodation and conference registration support from Atco Pharmaceutical, Bayer, Highnoon Laboratories, Novartis, Pfizer, Sanofi Aventis and Servier. GP Sogunuru has received a research grant related to hypertension monitoring and treatment from Pfizer. All other authors report no potential conflict of interest in relation to this article.
Supporting information
ACKNOWLEDGMENTS
This study was supported by an Investigator Initiated Research grant from Pfizer, and Omron Healthcare who provided the use of computer servers to store study‐related data. The protocol for the study was developed by Jichi Medical University School of Medicine. Pfizer was not involved in the development of the protocol nor this manuscript. The authors acknowledge editorial support from Ayako Okura, editorial coordinator of Jichi Medical University School of Medicine, Japan. English language editing assistance was provided by Nicola Ryan, independent medical writer.
Kario K, Tomitani N, Buranakitjaroen P, et al. Home blood pressure control status in 2017‐2018 for hypertension specialist centers in Asia: Results of the Asia BP@Home study. J Clin Hypertens. 2018;20:1686–1695. 10.1111/jch.13415
REFERENCES
- 1. Campbell NR, Khalsa T, Lackland DT et al. High blood pressure 2016: why prevention and control are urgent and important. The World Hypertension League, International Society of Hypertension, World Stroke Organization, International Diabetes Foundation, International Council of Cardiovascular Prevention and Rehabilitation, International Society of Nephrology. J Clin Hypertens. 2016;18:714‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weber MA, Lackland DT. Hypertension: cardiovascular benefits of lowering blood pressure. Nat Rev Nephrol. 2016;12:202–204. [DOI] [PubMed] [Google Scholar]
- 3. Weber MA, Lackland DT. Contributions to hypertension public policy and clinical practice: a review of recent reports. J Clin Hypertens. 2016;18:1063‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13‐e115. [DOI] [PubMed] [Google Scholar]
- 5. Kario K, Chen CH, Park S, et al. Consensus document on improving hypertension management in Asian patients. Taking into account Asian characteristics. Hypertension. 2018;71:375‐382. [DOI] [PubMed] [Google Scholar]
- 6. Perkovic V, Huxley R, Wu Y, Prabhakaran D, MacMahon S. The burden of blood pressure‐related disease: a neglected priority for global health. Hypertension. 2007;50:991‐997. [DOI] [PubMed] [Google Scholar]
- 7. Ueshima H, Sekikawa A, Miura K et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702‐2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kario K. Global impact of 2017 American Heart Association/American College of Cardiology Hypertension Guidelines: a perspective from Japan. Circulation. 2018;137:543‐545. [DOI] [PubMed] [Google Scholar]
- 9. Kario K, Wang JG. Could 130/80 mm Hg be adopted as the diagnostic threshold and management goal of hypertension in consideration of the characteristics of Asian populations? Hypertension. 2018;71:979‐984. [DOI] [PubMed] [Google Scholar]
- 10. Wang JG, Liu L. Global impact of 2017 American College of Cardiology/American Heart Association Hypertension Guidelines: a perspective from China. Circulation. 2018;137:546‐548. [DOI] [PubMed] [Google Scholar]
- 11. Mancia G, Fagard R, Narkiewicz K et al. ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2013;2014(23):3‐16. [DOI] [PubMed] [Google Scholar]
- 12. O'Brien E, Parati G, Stergiou G et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731‐1768. [DOI] [PubMed] [Google Scholar]
- 13. Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37:253‐390. [DOI] [PubMed] [Google Scholar]
- 14. Stergiou GS, Kario K, Kollias A, et al. Home blood pressure monitoring in the 21st century. J Clin Hypertens. 2018;20:1116‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams B, Giuseppe M, Spiering W, et al.European Society of Cardiology (ESC) and European Society of Hypertension (ESH) Joint Guidelines for the Management of Arterial Hypertension ‐ first look. Presented at the European Society of Hypertension conference. Barcelona, Spain. 9 July 2018. 2018. [Google Scholar]
- 16. Ntineri A, Kalogeropoulos PG, Kyriakoulis KG, et al. Prognostic value of average home blood pressure and variability: 19‐year follow‐up of the Didima study. J Hypertens. 2018;36:69‐76. [DOI] [PubMed] [Google Scholar]
- 17. Kario K. Essential Manual of 24‐hour Blood Pressure Management from Morning to Nocturnal Hypertension; Up‐to‐date for Anticipation Medicine. London, UK: Wiley‐Blackwell; 2018:1‐309. [Google Scholar]
- 18. Kario K. Evidence and perspectives on the 24‐hour management of hypertension: hemodynamic biomarker‐initiated 'anticipation medicine' for zero cardiovascular event. Prog Cardiovasc Dis. 2016;59:262‐281. [DOI] [PubMed] [Google Scholar]
- 19. Kario K, Tomitani N, Kanegae H, et al. Development of a new ICT‐based multisensor blood pressure monitoring system for use in hemodynamic biomarker‐initiated anticipation medicine for cardiovascular disease: The National IMPACT Program Project. Prog Cardiovasc Dis. 2017;60:435‐449. [DOI] [PubMed] [Google Scholar]
- 20. Hoshide S, Kario K, de la Sierra A, et al. Ethnic differences in the degree of morning blood pressure surge and in its determinants between Japanese and European hypertensive subjects: data from the ARTEMIS study. Hypertension. 2015;66:750‐756. [DOI] [PubMed] [Google Scholar]
- 21. Kario K, Bhatt DL, Brar S, Bakris GL. Differences in dynamic blood pressure variability between Japanese and American treatment‐resistant hypertensive populations. Circulation J. 2017;81:948‐957. [DOI] [PubMed] [Google Scholar]
- 22. Li Y, Staessen JA, Lu L, Li LH, Wang GL, Wang JG. Is isolated nocturnal hypertension a novel clinical entity? Findings from a Chinese population study. Hypertension. 2007;50:333‐339. [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Wang JG. Isolated nocturnal hypertension: a disease masked in the dark. Hypertension. 2013;61:278‐283. [DOI] [PubMed] [Google Scholar]
- 24. Omboni S, Aristizabal D, De la Sierra A, et al. Hypertension types defined by clinic and ambulatory blood pressure in 14 143 patients referred to hypertension clinics worldwide. DATA from the ARTEMIS study. J Hypertens. 2016;34:2187‐2198. [DOI] [PubMed] [Google Scholar]
- 25. Chia YC, Buranakitjaroen P, Chen CH, et al. Current status of home blood pressure monitoring in Asia: Statement from the HOPE Asia Network. J Clin Hypertens. 2017;19:1192‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kario K, Park S, Buranakitjaroen P, et al. Guidance on home blood pressure monitoring: a statement of the HOPE Asia Network. J Clin Hypertens. 2018;20:456‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park S, Buranakitjaroen P, Chen CH, et al. Expert panel consensus recommendations for home blood pressure monitoring in Asia: the Hope Asia Network. J Hum Hypertens. 2018;32:249‐258. [DOI] [PubMed] [Google Scholar]
- 28. Asayama K, Ohkubo T, Metoki H, et al. Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self‐measured home blood pressure. Hypertens Res. 2012;35:1102‐1110. [DOI] [PubMed] [Google Scholar]
- 29. Bobrie G, Chatellier G, Genes N, et al. Cardiovascular prognosis of "masked hypertension" detected by blood pressure self‐measurement in elderly treated hypertensive patients. JAMA. 2004;291:1342‐1349. [DOI] [PubMed] [Google Scholar]
- 30. Hoshide S, Yano Y, Haimoto H, et al. Morning and evening home blood pressure and risks of incident stroke and coronary artery disease in the Japanese General Practice Population: the Japan morning surge‐home blood pressure study. Hypertension. 2016;68:54‐61. [DOI] [PubMed] [Google Scholar]
- 31. Kario K, Saito I, Kushiro T, et al. Home blood pressure and cardiovascular outcomes in patients during antihypertensive therapy: primary results of HONEST, a large‐scale prospective, real‐world observational study. Hypertension. 2014;64:989‐996. [DOI] [PubMed] [Google Scholar]
- 32. Niiranen TJ, Hanninen MR, Johansson J, Reunanen A, Jula AM. Home‐measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn‐Home study. Hypertension. 2010;55:1346‐1351. [DOI] [PubMed] [Google Scholar]
- 33. Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population‐based observation in Ohasama. Japan. J Hypertens. 1998;16:971‐975. [DOI] [PubMed] [Google Scholar]
- 34. Kario K. The HOPE Asia Network for "zero" cardiovascular events in Asia. J Clin Hypertens. 2018;20:212‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kario K, Tomitani N, Buranakitjaroen P, et al. Rationale and design for the Asia BP@Home study on home blood pressure control status in 12 Asian countries and regions. J Clin Hypertens. 2018;20:33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takahashi H, Yoshika M, Yokoi T. Validation of three automatic devices for the self‐measurement of blood pressure according to the European Society of Hypertension International Protocol revision 2010: the Omron HEM‐7130, HEM‐7320F, and HEM‐7500F. Blood Press Monit. 2015;20:92‐97. [DOI] [PubMed] [Google Scholar]
- 37. Shin J, Park JB, Kim KI, et al. 2013 Korean Society of Hypertension guidelines for the management of hypertension: part I‐epidemiology and diagnosis of hypertension. Clin Hypertens. 2015;21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang JG, for the China HBPM consensus expert panel . Home blood pressure monitoring: a China consensus document. Chin . J Hypertens. 2012;20:525‐529. [Google Scholar]
- 39. Hoshide S, Yano Y, Mizuno H, Kanegae H, Kario K. Day‐by‐day variability of home blood pressure and incident cardiovascular disease in clinical practice: The J‐HOP Study (Japan Morning Surge‐Home Blood Pressure). Hypertension. 2018;71:177‐184. [DOI] [PubMed] [Google Scholar]
- 40. Juhanoja EP, Niiranen TJ, Johansson JK, et al. Outcome‐driven thresholds for increased home blood pressure variability. Hypertension. 2017;69:599‐607. [DOI] [PubMed] [Google Scholar]
- 41. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895‐905. [DOI] [PubMed] [Google Scholar]
- 42. Coleman A, Freeman P, Steel S, Shennan A. Validation of the Omron 705IT (HEM‐759‐E) oscillometric blood pressure monitoring device according to the British Hypertension Society protocol. Blood Press Monit. 2006;11:27‐32. [DOI] [PubMed] [Google Scholar]
- 43. Hoshide S, Kario K, Yano Y, et al. Association of morning and evening blood pressure at home with asymptomatic organ damage in the J‐HOP Study. Am J Hypertens. 2014;27:939‐947. [DOI] [PubMed] [Google Scholar]
- 44. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 45. Eguchi K, Kario K, Hoshide Y, et al. Comparison of valsartan and amlodipine on ambulatory and morning blood pressure in hypertensive patients. Am J Hypertens. 2004;17:112‐117. [DOI] [PubMed] [Google Scholar]
- 46. Wang JG, Kario K, Lau T, et al. Use of dihydropyridine calcium channel blockers in the management of hypertension in Eastern Asians: a scientific statement from the Asian Pacific Heart Association. Hypertens Res. 2011;34:423‐430. [DOI] [PubMed] [Google Scholar]
- 47. Kario K. Time for focus on morning hypertension: pitfall of current antihypertensive medication. Am J Hypertens. 2005;18:149‐151. [DOI] [PubMed] [Google Scholar]
- 48. Kario K, Ishikawa J, Pickering TG, et al. Morning hypertension: the strongest independent risk factor for stroke in elderly hypertensive patients. Hypertens Res. 2006;29:581‐587. [DOI] [PubMed] [Google Scholar]
- 49. Wang JG, Kario K, Chen CH, et al. Management of morning hypertension: a consensus statement of an Asian expert panel. J Clin Hypertens. 2018;20:39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang JG, Kario K, Park JB, Chen CH. Morning blood pressure monitoring in the management of hypertension. J Hypertens. 2017;35:1554‐1563. [DOI] [PubMed] [Google Scholar]
- 51. Fujiwara T, Hoshide S, Nishizawa M, Matsuo T, Kario K. Difference in evening home blood pressure between before dinner and at bedtime in Japanese elderly hypertensive patients. J Clin Hypertens. 2017;19:731‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fujiwara T, Hoshide S, Kanegae H, Nishizawa M, Kario K. Reliability of morning, before‐dinner, and at‐bedtime home blood pressure measurements in patients with hypertension. J Clin Hypertens. 2018;20:315‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kario K, Saito I, Kushiro T, et al. Morning home blood pressure is a strong predictor of coronary artery disease: the HONEST Study. J Am Coll Cardiol. 2016;67:1519‐1527. [DOI] [PubMed] [Google Scholar]
- 54. Fujiwara T, Yano Y, Hoshide S, Kanegae H, Kario K. Association of cardiovascular outcomes with masked hypertension defined by home blood pressure monitoring in a Japanese general practice population. JAMA Cardiol. 2018;1:583‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between Clinic and Ambulatory Blood‐Pressure Measurements and Mortality. N Engl J Med. 2018;378:1509‐1520. [DOI] [PubMed] [Google Scholar]
- 56. Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home‐measured blood pressure and heart rate: the Finn‐Home Study. Hypertension. 2012;59:212‐218. [DOI] [PubMed] [Google Scholar]
- 57. Kikuya M, Ohkubo T, Metoki H, et al. Day‐by‐day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52:1045‐1050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


