Abstract
Renal denervation and spironolactone have both been proposed for the treatment of resistant hypertension, but their effects on preclinical target organ damage have not been compared. Twenty‐four patients with 24‐hour systolic blood pressure ≥140 mm Hg despite receiving three or more full‐dose antihypertensive drugs, one a diuretic, were randomized to receive spironolactone or renal denervation. Changes in 24‐hour blood pressure, urine albumin excretion, arterial stiffness, carotid intima‐media thickness, and left ventricular mass index were evaluated at 6 months. Mean baseline‐adjusted difference between the two groups (spironolactone vs renal denervation) at 6 months in 24‐hour systolic blood pressure was −17.9 mm Hg (95% confidence interval [CI], −30.9 to −4.9; P = .01). Mean baseline‐adjusted change in urine albumin excretion was −87.2 (95% CI, −164.5 to −9.9) and −23.8 (95% CI, −104.5 to 56.9), respectively (P = .028). Mean baseline‐adjusted variation of 24‐hour pulse pressure was −13.5 (95% CI, −18.8 to −8.2) and −2.1 (95% CI, −7.9 to 3.7), respectively (P = .006). The correlation of change in 24‐hour systolic blood pressure with change in log‐transformed urine albumin excretion was r = .713 (P < .001). At 6 months there was a reduction in albuminuria in patients with resistant hypertension treated with spironolactone as compared with renal denervation.
Keywords: albuminuria, arterial stiffness, carotid wall thickness, end organ damage, left ventricular hypertrophy, renal denervation, spironolactone
1. INTRODUCTION
Patients whose blood pressure (BP) remains above goal in spite of the use of at least three antihypertensive drugs of different classes given at optimal doses, ideally one being a diuretic, are considered to have resistant hypertension (RH).1 Around 11% to 13% of patients with hypertension are believed to have RH,2, 3, 4, 5 with a prevalence that lowers to near 5% when secondary causes, nonadherence to drug treatment, and white‐coat RH are reasonably discarded.6 RH is known to be associated with a higher cardiovascular risk than controlled BP, with an associated increased prevalence of major cardiovascular and renal outcomes and mortality.7 In the continuum of vascular disease, asymptomatic organ damage is considered as an intermediate stage and a determinant of overall cardiovascular risk. Four markers of organ damage (microalbuminuria, increased pulse wave velocity [PWV], left ventricular (LV) hypertrophy, and carotid plaques and/or increased wall thickness) have been shown to be reliable predictors of cardiovascular mortality independently of Systemic Coronary Evaluation (SCORE) stratification.8 Remarkably, preclinical target organ involvement is more prevalent in patients with uncontrolled hypertension than in those with controlled BP,9 therefore justifying their increased vascular risk. The past 2 decades have witnessed the development of new approaches to treat RH, with special focus on nonpharmacological methods. Sympathetic renal denervation (RDN) showed initial promising results, although the randomized controlled trial Symplicity HTN‐3 failed to demonstrate a significant BP decrease compared with the sham control group.10 On the other hand, spironolactone, a longstanding drug that acts as a mineralocorticoid receptor antagonist, has emerged to treat RH. Thus, in the ASPIRANT (Addition of Spironolactone in Patients With Resistant Arterial Hypertension) trial11 spironolactone as add‐on treatment showed significant decreases in BP. Recently, results from the PATHWAY‐2 (Optimum Treatment for Drug‐Resistant Hypertension) trial12 have shown that spironolactone is superior to other drugs as add‐on therapy in patients with RH. We designed a randomized clinical trial with a head‐to‐head comparison of these two strategies in patients with RH, reporting that spironolactone was superior to RDN in reducing 24‐hour ambulatory BP.13 As a prespecified secondary outcome, we have also compared changes on markers of preclinical target organ damage between both add‐on treatments, which are now reported.
2. METHODS
2.1. Study design and patients
The DENERVHTA (Denervación en Hipertensión Arterial) study is a prospective, multicenter, open‐label, randomized, controlled trial (clinicaltrials.gov identifier: NCT02039492) aimed to compare changes in 24‐hour systolic BP (SBP) between patients with RH randomized to receive either RDN or spironolactone as add‐on therapies. Details about the DENERVHTA trial have been published elsewhere.13 In brief, all participants were aged between 18 and 80 years at study entry and had office SBP ≥150 mm Hg and 24‐hour SBP ≥ 140 mm Hg while on treatment with three or more full‐dose antihypertensive medications, one of them a diuretic, but without mineralocorticoid receptor antagonists. Patients were randomized (in a 1:1 ratio) to receive RDN (one single operator, median 10 shots) or spironolactone (50 mg once daily), in addition to current antihypertensive treatment. Estimated glomerular filtration rate <45 mL/min per 1.73 m2 or the occurrence of a major vascular event (myocardial infarction, unstable angina, or stroke) within 6 months before study enrollment were exclusion criteria for entry into the DENERVHTA trial. The primary end point was the between‐group comparison of mean changes in ambulatory 24‐hour SBP from baseline to 6 months. Here, we report additional analysis focused on the effect of both treatments on preclinical target organ damage, according to prespecified secondary end points.
2.2. Procedures
All of the following measurements were obtained at prerandomization and at 6 months.
2.3. Office and 24‐hour ambulatory BP measurements
Office BP was assessed in patients after 5 minutes of rest in the sitting position using a validated oscillometric semiautomatic device (Omron 705IT) with appropriately sized cuffs, between 8 and 10 am and before taking any antihypertensive drug. Three measurements spaced by 1 to 2 minutes were averaged to determine the final office BP values.
A 24‐hour ambulatory BP monitoring registry was obtained by validated Spacelabs 90207 devices and suitably sized cuffs. The monitoring started at around 8 to 10 am of a working day, with ambulatory BP readings obtained at 20‐minute intervals throughout both awake and asleep periods. A good technical quality recording (minimum 80% of valid readings) was required for a 24‐hour ambulatory BP monitoring registry to be evaluable.
2.4. Urine albumin excretion
Urine albumin excretion (UAE) was recorded by standard methods (using a turbidimetric method) and determined as the average of the ratio of concentration of albumin to creatinine in two spot first‐morning void urine samples collected on separate days. Urine creatinine was measured by an enzymatic modified Jaffe reaction (CREA, Roche Diagnostics) using the Hitachi Modular System Analyzer (Roche Diagnostics). Microalbuminuria was defined following the European Society of Hypertension Guidelines,14 as an albumin‐creatinine ratio of 30 to 300 mg/g.
2.5. Arterial stiffness
Carotid‐femoral PWV (cfPWV) was evaluated by noninvasive applanation tonometry on carotid and femoral arteries (Sphygmocor, AtCor Medical).15 The values of two valid consecutive measurements (all performed in a single center by the same trained nurse) were averaged at each visit. cfPWV was computed as the distance traveled by the pulse wave divided by pulse wave transit time. Travel distance was measured to the nearest centimeter with an external tape measure over the body surface. The transit time was determined as the time difference between the feet of carotid and femoral arterial waveforms gated to electrocardiography. Arterial stiffness was defined by cfPWV >10 m/s.14 In addition, two other measurements were used to assess arterial stiffness. From derived central waveforms, data were obtained for augmentation pressure, and central augmentation index (AIx) was defined as the ratio of augmentation pressure to pulse pressure and normalized to a heart rate of 75 beats per minute (AIx75) to minimize the influence of heart rate.
2.6. Carotid ultrasound
An ultrasound examination of both carotid arteries (left and right) to measure intima‐media thickness (IMT) and/or the presence of plaques was performed in all patients. IMT was measured at three different sites, ie, the common carotid artery (1 cm distal to bulb), bulb, and internal carotid (1 cm proximal to bulb) by an Esaote ultrasound device and specific measurement software. The final value for IMT was the average of these six measures. Carotid ultrasound was abnormal if wall thickening (IMT >0.9 mm) or plaques were found.14
2.7. Echocardiographic measurements
All echocardiography examinations were performed and read by one single experienced physician blinded to randomization and all other information. Cardiac dimensions and wall thickness were measured according to standard recommendations.14, 16 LV internal dimension and wall thickness were measured at end diastole and LV mass was calculated and indexed to body surface area to calculate LV mass index (LVMI). The diagnosis of LV hypertrophy was considered if LVMI was >115 g/m2 for men and >95 g/m2 for women. Alterations in diastolic dysfunction17 were assessed by pulsed‐wave tissue Doppler recordings of peak early (E‐wave) and late (A‐wave) diastolic flow velocities and the E/A ratio, as well as by recordings of the lateral portion of the mitral annulus to obtain the early diastolic e′‐wave velocity. The mitral inflow E velocity to tissue Doppler e′ (E/e′) ratio was used as an index of LV filling. Left atrial volume and area indexes were also measured.
2.8. Statistical analyses
Variables following normal distribution are summarized as mean ± standard deviation or median (interquartile range) if asymmetrically distributed, and categorical data are presented as frequencies and percentages. Comparisons of baseline characteristics of patients in one treatment strategy arm or another were performed by unpaired t tests in continuous normally distributed data, by nonparametric either Wilcoxon or Mann–Whitney tests in asymmetrically distributed data, or by chi‐square test in categorical data.
Between‐group comparisons of changes in BP or in preclinical target organ damage markers were performed using generalized linear models adjusted by respective baseline values. When the independent variable was the change in different markers of target organ damage, the variation of 24‐hour SBP was also included in the model. For this analysis, both UAE and AIx75 were log‐transformed because of skewed distribution. Spearman's rho was calculated for correlations. A two‐sided P value ≤.05 was considered statistically significant. Ordinary statistical methods were performed with statistical package SPSS for Windows version 22.0 (SPSS Inc).
3. RESULTS
In total, 24 randomized patients had complete data on ambulatory BP monitoring and target organ damage at baseline and the final visit (6 months) and were analyzed. Thirteen patients were allocated in the spironolactone group and 11 patients underwent RDN. The mean age was 63.5 ± 7.5 years and 63% were men. Mean office SBP and diastolic BP were 170.1 ± 20.4 mm Hg and 91.8 ± 12.0 mm Hg, respectively. Baseline clinical characteristics and BP values in both groups are shown in Table 1. As observed, there were no statistically significant differences between groups (P = not significant for all comparisons). All included patients with diabetes mellitus had type 2. In patients with diabetes mellitus, baseline glycated hemoglobin was 7.1% ± 0.9 and 7.8% ± 0.8 (P = .2) for the RDN and the spironolactone groups, respectively. Baseline antihypertensive treatment was also comparable between groups (Table S1). All patients in the RDN group and 92% of patients in the spironolactone group received renin‐angiotensin system blockers. According to the prespecified protocol, but attending to ethical and safety reasons, there were few changes in the baseline antihypertensive regimen, and no statistically significant differences between groups were observed. Many of these changes consisted of a dosage adjustment, and in no case did spironolactone be added to any of the RDN group patients. Table 2 shows baseline data on markers of preclinical target organ damage, both as quantitative and qualitative variables, compared between groups. Baseline UAE and 24‐hour pulse pressure tended to be higher in the spironolactone group. No other between‐group differences were observed in preclinical organ damage.
Table 1.
Patients’ demographics and baseline laboratory and BP characteristics
| Variable | RDN (n = 11) | Spironolactone (n = 13) | P value |
|---|---|---|---|
| Clinical characteristics | |||
| Age, y | 61.9 ± 6.6 | 64.9 ± 8.2 | .35 |
| Men, No. (%) | 6 (55) | 9 (69) | .68 |
| Body mass index, kg/m2 | 33.7 ± 7.4 | 30.6 ± 3.6 | .23 |
| Diabetes mellitus | 4 (36) | 8 (62) | .41 |
| Dyslipidemia | 11 (100) | 11 (85) | .48 |
| Previous CVD | 2 (18) | 3 (23) | .64 |
| Duration of hypertension, y | 13.6 ± 6.9 | 14.2 ± 7.7 | .82 |
| Antihypertensive drugs | 4.3 ± 0.8 | 3.9 ± 0.6 | .13 |
| Office and ambulatory BP | |||
| Office SBP, mm Hg | 168.0 ± 13.8 | 171.2 ± 16.8 | .74 |
| Office DBP, mm Hg | 89.6 ± 12.8 | 90.2 ± 16.1 | .79 |
| 24‐h SBP, mm Hg | 149.2 ± 6.9 | 155.4 ± 9.9 | .09 |
| 24‐h DBP, mm Hg | 81.3 ± 8.8 | 80.9 ± 9.7 | .93 |
| 24‐h PP, mm Hg | 68.0 ± 6.9 | 74.5 ± 10.6 | .09 |
| Daytime SBP, mm Hg | 152.6 ± 7.9 | 158.9 ± 9.4 | .10 |
| Daytime DBP, mm Hg | 83.8 ± 10.5 | 83.4 ± 9.3 | .92 |
| Daytime PP, mm Hg | 68.5 ± 6.8 | 75.5 ± 9.7 | .06 |
| Nighttime SBP, mm Hg | 141.9 ± 11.4 | 147.7 ± 15.5 | .32 |
| Nighttime DBP, mm Hg | 75.7 ± 8.8 | 75.9 ± 11.7 | .98 |
| Nighttime PP, mm Hg | 66.2 ± 9.2 | 71.9 ± 14.2 | .26 |
Abbreviations: BP, blood pressure; CVD, cardiovascular disease; DBP, diastolic blood pressure; PP, pulse pressure; RDN, renal denervation; SBP, systolic blood pressure.
Data are expressed as mean ± standard deviation or number (percentage).
Table 2.
Baseline data of markers of preclinical target organ damage
| Variable | RDN (n = 11) | Spironolactone (n = 13) | P value |
|---|---|---|---|
| UAE, mg/ga | 9.0 (6.8–104.1) | 28.8 (19.1–222.1) | .07 |
| Microalbuminuria or macroalbuminuria | 4 (36) | 7 (54) | .44 |
| eGFR, mL/min per 1.73 m2 | 75.9 ± 20.0 | 81.3 ± 16.1 | .47 |
| Renal dysfunctiona | 3 (27) | 1 (8) | .30 |
| AIx75 | 20.0 (15.0–28.5) | 24.0 (21.0–26.5) | .46 |
| 24‐h PP, mm Hg | 68.0 ± 6.9 | 74.5 ± 10.6 | .09 |
| cfPWV, m/s | 12.4 ± 2.9 | 13.4 ± 2.9 | .42 |
| Arterial stiffnessb | 8 (73) | 11 (92) | .32 |
| Carotid IMT, mm | 0.67 ± 0.05 | 0.66 ± 0.09 | .82 |
| IMT >0.9 mm and/or plaques | 7 (64) | 10 (83) | .37 |
| LVMI, g/m2 | 121.6 ± 36.6 | 117.6 ± 21.8 | .74 |
| LV hypertrophy | 8 (73) | 9 (75) | 1 |
| E/e’ | 10.1 ± 2.3 | 10.0 ± 4.3 | .97 |
| E/e’ ≥13 | 1 (9) | 1 (8) | 1 |
Abbreviations: AIx75, augmentation index normalized to a heart rate of 75 beats per minute; E/e’, E‐wave/annular e’ velocities ratio; IMT, intima‐media thickness; LV, left ventricular; LVMI, left ventricular mass index; PP, pulse pressure; RDN, renal denervation; UAE, urinary albumin excretion.
Data are expressed as median (p25–p75), mean ± standard deviation, or number (percentage).
Estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2.
Carotid‐femoral pulse wave velocity (cfPWV) >10 m/s.
As previously reported,13 spironolactone was superior to RDN in reducing both 24‐hour SBP (−17.9 mm Hg; 95% confidence interval, −30.9 to −4.9 mm Hg [P = .01]) and 24‐hour diastolic BP (−6.6 mm Hg; 95% confidence interval, −12.9 to −0.3 [P = .04]). Changes in markers of preclinical target organ damage at 6 months between both treatment groups are shown in Table 3. For the whole cohort, UAE (P = .013) and arterial stiffness, as assessed by cfPWV (P = .001) and 24‐hour pulse pressure (P = .001) showed a statistically significant decrease at 6 months compared with baseline values. These changes generally occurred in the spironolactone group. Individual changes in albuminuria within each treatment group are shown in Figure 1. However, these differences lost statistical significance after adjusting for both respective baseline values and the variation of 24‐hour SBP (data not shown). As shown in Figure 2, changes in UAE correlated with changes in 24‐hour SBP in the whole cohort (r = .713; r 2 = .508 [P < .001]).
Table 3.
Changes in markers of preclinical target organ damage at 6 months
| Variable | All patients (N = 24) Δ at 6 mo | P value | RDN (n = 11) Δ at 6 mo | Spironolactone (n = 13) Δ at 6 mo | P valueΔRDN vs Δspironolactone |
|---|---|---|---|---|---|
| Renal damage | |||||
| UAE, mg/g | −82.7 (−158.5 to −7.0) | .013 | −23.8 (−104.5 to 56.9) | −87.2 (−164.5 to −9.9) | .028 |
| Carotid artery | |||||
| Carotid IMT, mm | 0.01 (−0.04 to 0.05) | .719 | −0.29 (−0.05 to 1.29) | −0.01 (−0.13 to 0.05) | .396 |
| Arterial stiffness | |||||
| cfPWV, m/s | −1.03 (−1.61 to −0.44) | .001 | −0.7 (−1.5 to 0.2) | −1.3 (−2.2 to −0.5) | .259 |
| AIx75, % | −1.11 (−5.87 to 3.64) | .417 | −0.4 (−7.6 to 6.9) | −1.8 (−8.4 to 4.9) | .768 |
| 24‐h PP, mm Hg | −8.3 (−12.8 to −3.8) | .001 | −2.1 (−7.9 to 3.7) | −13.5 (−18.8 to −8.2) | .006 |
| Echocardiographic parameters | |||||
| LVMI, g/m2 | −2.0 (−14.4 to 10.6) | .750 | 1.83 (−16.6 to 20.2) | −5.41 (−23.0 to 12.2) | .561 |
| E/e’ | −0.47 (−1.59 to 0.65) | .393 | −0.32 (−2.0 to 2.58) | −0.61 (−2.6 to 2.0) | .795 |
Abbreviations: Δ, change; AIx75, augmentation index normalized to a heart rate of 75 beats per minute; cfPWV, carotid‐femoral pulse wave velocity; E/e’, E‐wave/annular e’ velocities ratio; IMT, intima‐media thickness; LVMI, left ventricular mass index; PP, pulse pressure; RDN, renal denervation; UAE, urinary albumin excretion.
Values are expressed as mean (95% confidence interval).
Figure 1.
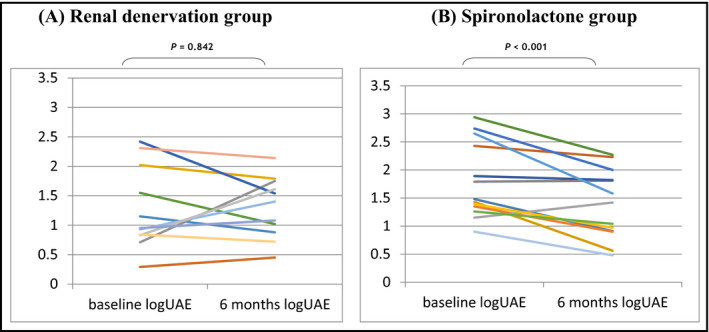
Individual changes in albuminuria from baseline to 6 months in the (A) renal denervation group and the (B) spironolactone group. Log‐UAE indicates log‐transformed urine albumin excretion
Figure 2.
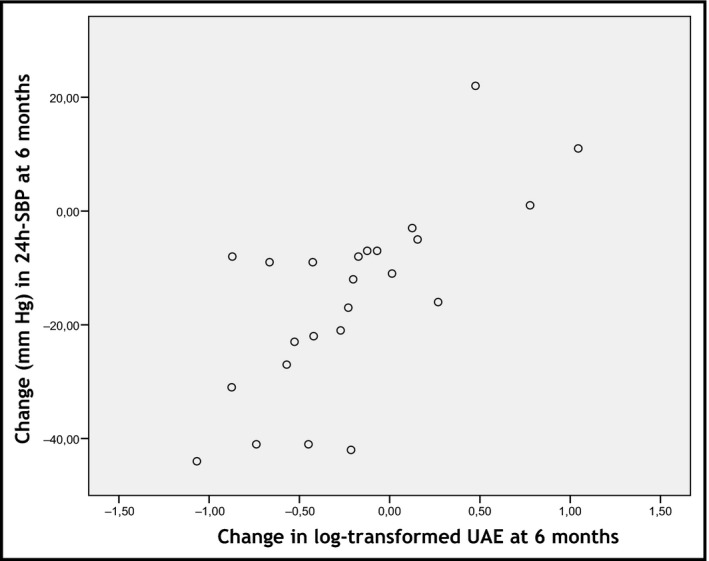
Correlation between changes in 24‐hour systolic blood pressure (SBP) and urine albumin excretion (UAE) at 6 months
Changes in additional echocardiographic parameters in systolic and diastolic function and left ventricular and atrial parameters were also analyzed. As shown in Table S2, no statistically significant between‐group differences were found.
4. DISCUSSION
In patients with RH, we found a reduction in preclinical target organ damage, specifically in albuminuria, in those who received spironolactone as add‐on therapy. As these results show, spironolactone is not only superior to RDN in lowering BP but also in organ damage regression. At 6 months, there were no changes in other markers of preclinical target organ damage in the DENERVHTA study, except a trend towards a reduction in arterial stiffness when assessed by pulse pressure but not with PWV or AIx.
The main goal in treating RH is to minimize target organ damage and, therefore, to reduce cardiovascular morbidity and mortality. Recently, two of the approaches to treat RH highlighted in the clinical research are sympathetic RDN, a minimally invasive nonpharmacological procedure with controversial results for efficacy in lowering BP,10, 18 and spironolactone, a longstanding antihypertensive drug with good results shown in several studies of RH.11, 12, 13 Beyond efficiency to reduce BP, the effect of these treatments on markers of target organ damage has separately been reported, although most of the studies are nonrandomized and results are generally inconclusive or lack sufficient consistency. A few studies have assessed their effect on UAE. A significant reduction in UAE was shown in 59 patients with RH with either microalbuminuria or macroalbuminuria who underwent RDN in a noncontrolled study, which correlated with changes in office SBP but not with changes in ambulatory SBP.19 Moreover, Verloop and colleagues20 reported the effects of RDN on target organ damage in 54 patients with RH, showing no change in UAE after 12 months. The addition of spironolactone in both patients without RH21 and those with RH22 produced a significant decrease in albuminuria compared with placebo, although the relationship with changes in BP is not shown in these reports. There are some studies that analyze the variation in arterial stiffness after treatment with RDN or spironolactone in patients with RH. Contrary to expectations, in the noncontrolled study by Verloop and colleagues20 assessing the effects of RDN, an increase in PWV at 12 months and no change in AIx75 were found. On the other hand, several trials have explored the effects of mineralocorticoid receptor blockers on arterial stiffness. In patients with early chronic kidney disease treated with a renin‐angiotensin system blocker, the addition of spironolactone led to a reduction in both PWV and AIx75 even after adjusting for BP variation.21 In another study,23 the addition of eplerenone in patients with uncontrolled hypertension reduced arterial stiffness as evaluated according to the cardio‐ankle vascular index, and this reduction was not associated with changes in BP. Even less information is available on changes in carotid wall thickness with both treatments, and none is available in patients with RH. In fact, we found only a single study on the effect of RDN in carotid IMT in 12 patients,24 showing no change, and only two small studies showing a reduction of their progression in hemodialysis patients25 and a regression in patients with primary aldosteronism, but not in those with essential hypertension,26 when treated with spironolactone. Finally, several studies have analyzed the effect of both treatments on cardiac ultrasound parameters. In patients with RH, some studies have shown an improvement in echocardiographic parameters after RDN, mostly related to LVMI or diastolic function parameters, but none were randomized controlled trials.27, 28 On the other hand, in a well‐conducted trial by Azizi and colleagues29 when spironolactone was given as add‐on therapy in patients with RH in comparison to dual renin‐angiotensin system blockade, a decrease in LVMI after adjustment for ambulatory BP was found. One possible explanation for not finding a decrease in LVMI in our study may be that, although in both groups there was approximately 75% of patients with LV hypertrophy, the absolute values of LVMI were relatively low in the cohort. This could explain why the decrease in LVMI was not statistically significant.
Overall, information on the effect of RDN or spironolactone on preclinical target organ damage in patients with RH is scarce. Although both treatments seem to have a positive effect in improving organ damage, irrespective of the variation on BP, there are no trials comparing both treatment strategies. Here, we report for the first time the effect of spironolactone and RDN as add‐on therapy in patients with RH on several markers of preclinical target organ damage in a randomized controlled trial. We found a higher decrease in albuminuria in the spironolactone group, compared with patients who underwent RDN. Even though we found a significant correlation between changes in albuminuria and variation in BP, our results (r 2 = .508) indicate that half of the variation in log‐transformed UAE is caused by something other than changes in 24‐hour SBP. Thus, a specific role for spironolactone beyond its effect on lowering BP cannot be discarded. Whatever the reason, the important finding is that spironolactone as add‐on treatment in patients with RH lowers both BP and preclinical organ damage more than RDN. Based on this, it seems reasonable to hypothesize that these patients have better cardiovascular prognosis, although longer‐term studies are needed to confirm this findings.
STUDY LIMITATIONS AND STRENGTHS
We found no significant changes regarding other secondary end points, ie, PWV, echocardiographic parameters, or carotid IMT. One possible reason is the time interval that was evaluated. Six months is probably not long enough to observe changes in these markers of organ damage. In fact, the expected time for an intervention to produce changes is more than 6 months for albuminuria and arterial stiffness and more than 12 months for carotid IMT and echocardiographic measurements.14 Moreover, the high variability of some of these markers could account for the lack of other between‐group differences. Other limitations of our study include not using cardiac magnetic resonance imaging to determine LV mass and other cardiac measurements, which is considered more reliable than echocardiography. Moreover, it is well known that no study has assessed the effectiveness of the RDN procedure, and the extent and duration of RDN effects are unknown. Finally, the small number of patients included in this trial limits our findings.
Strengths of our study include the fact that investigators who performed the echocardiographic, carotid, and cfPWV measurements were the same for all patients and were blinded to the allocation treatment group. Moreover, most of the patients in both groups remained on the same baseline pharmacological treatment during follow‐up, as reported.13 In the present study, the BP‐lowering effect was determined using 24‐hour ambulatory BP measurements, reinforcing the reliability of the findings. Finally, we cannot rule out different results in other clinical settings such as patients with mild hypertension or using other RDN catheters, other devices to measure arterial stiffness, or other mineralocorticoid receptor blockers. Nor can we rule out possible side effects on long‐term spironolactone therapy, not observed in this 6‐month follow‐up period.
CONCLUSIONS
After 6 months of add‐on treatment an improvement in preclinical target organ damage was seen in patients with RH treated with spironolactone but not in those undergoing RDN.
INVESTIGATORS’ LIST
Pedro Armario, MD; Francina Barbosa, MD; Angela Barrera, MD; Albert Clarà, MD; Alejandro de la Sierra, MD; Anna Faura, RN; Lidia González, MD; Tai Mooi Ho, RN; Omar Ibrik, MD; Carme Llort, MD; Carmen Martin, RN; Meritxell Mellado, MD; Luis Molina, MD; Anna Oliveras, MD; Júlia Pareja, MD; Julio Pascual, MD; Carmen Roman, RN; Laia Sans, MD; Marta Serra, MD; Susana Vázquez, MD; Maria Vera, RN; Ernest Vinyoles, MD.
CONFLICT OF INTEREST
The corresponding author (AO) contributes to scientific advisory with Medtronic Ibérica, SA. The remaining authors have no conflicts of interest to disclose.
Supporting information
Oliveras A, Armario P, Sans L, et al. Organ damage changes in patients with resistant hypertension randomized to renal denervation or spironolactone: The DENERVHTA (Denervación en Hipertensión Arterial) study. J Clin Hypertens. 2018;20:69–75. 10.1111/jch.13156
Funding information
This work was supported by the Spanish Health Authority, grant reference: EC11‐426.
Alejandro de la Sierra and Julio Pascual contributed equally to this work and are the co‐last authors.
Clinical Trial Registration—URL: http://www.clinicaltrials.gov. Unique identifier: NCT02039492.
REFERENCES
- 1. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403‐1419. [DOI] [PubMed] [Google Scholar]
- 2. Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076‐1080. [DOI] [PubMed] [Google Scholar]
- 3. de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898‐902. [DOI] [PubMed] [Google Scholar]
- 4. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calhoun DA, Booth JN III, Oparil S, et al. Refractory hypertension: determination of prevalence, risk factors, and comorbidities in a large, population‐based cohort. Hypertension. 2014;63:451‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Judd E, Calhoun DA. Management of resistant hypertension: do not give up on medication. Nephrol Self Assess Program. 2014;13:57‐63. [PMC free article] [PubMed] [Google Scholar]
- 7. Sim JJ, Bhandari SK, Shi J, et al. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int. 2015;88:622‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sehestedt T, Jeppesen J, Hansen TW, et al. Thresholds for pulse wave velocity, urine albumin creatinine ratio and left ventricular mass index using SCORE, Framingham and ESH/ESC risk charts. J Hypertens. 2012;30:1928‐1936. [DOI] [PubMed] [Google Scholar]
- 9. de la Sierra A, Banegas JR, Oliveras A, et al. Clinical differences between resistant hypertensives and patients treated and controlled with three or less drugs. J Hypertens. 2012;30:1211‐1216. [DOI] [PubMed] [Google Scholar]
- 10. Bhatt DL, Kandzari DE, O'Neill WW, et al. SYMPLICITY HTN‐3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393‐1401. [DOI] [PubMed] [Google Scholar]
- 11. Václavík J, Sedlák R, Plachy M, et al. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomised, double‐blind, placebo‐controlled trial. Hypertension. 2011;57:1069‐1075. [DOI] [PubMed] [Google Scholar]
- 12. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug‐resistant hypertension (PATHWAY‐2): a randomised, double‐blind, crossover trial. Lancet. 2015;386:2059‐2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oliveras A, Armario P, Clarà A, et al. Spironolactone versus sympathetic renal denervation to treat true resistant hypertension: results from the DENERVHTA study––a randomized controlled trial. J Hypertens. 2016;34:1863‐1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281‐1357. [DOI] [PubMed] [Google Scholar]
- 15. van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30:445‐448. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233‐270. [DOI] [PubMed] [Google Scholar]
- 17. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321‐1360. [DOI] [PubMed] [Google Scholar]
- 18. Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardized antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open‐label, randomized controlled trial. Lancet. 2015;385:1957‐1965. [DOI] [PubMed] [Google Scholar]
- 19. Ott C, Mahfoud F, Schmid A, et al. Improvement of albuminuria after renal denervation. Int J Cardiol. 2014;173:311‐315. [DOI] [PubMed] [Google Scholar]
- 20. Verloop WL, Vink EE, Spiering W, et al. Effects of renal denervation on end organ damage in hypertensive patients. Eur J Prev Cardiol. 2015;22:558‐567. [DOI] [PubMed] [Google Scholar]
- 21. Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early‐stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54:505‐512. [DOI] [PubMed] [Google Scholar]
- 22. Vácklavic J, Sedlák R, Jarkovsky J, Kociánová E, Táborsky M. Effect of spironolactone in resistant arterial hypertension. A randomized, double‐blind, placebo‐controlled trial (ASPIRANT‐EXT). Medicine. 2014;93:e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shibata T, Tsutsumi J, Hasegawa J, et al. Effects of add‐on therapy consisting of a selective mineralocorticoid receptor blocker on arterial stiffness in patients with uncontrolled hypertension. Intern Med. 2015;54:1583‐1589. [DOI] [PubMed] [Google Scholar]
- 24. Baroni M, Nava S, Giupponi K, et al. Effects of renal sympathetic denervation on arterial stiffness and blood pressure control in resistant hypertensive patients: a single centre prospective study. High Blood Press Cardiovasc Prev. 2015;22:411‐416. [DOI] [PubMed] [Google Scholar]
- 25. Vukusich A, Kunstmann S, Varela C, et al. A randomized, double‐blind, placebo‐controlled trial of spironolactone on carotid intima‐media thickness in nondiabetic hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1380‐1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holaj R, Rosa J, Zelinka T, et al. Long‐term effect of specific treatment of primary aldosteronism on carotid intima‐media thickness. J Hypertens. 2015;33:874‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mahfoud F, Urban D, Teller D, et al. Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multicentre cardiovascular magnetic resonance imaging trial. Eur Heart J. 2014;35:2224‐2231. [DOI] [PubMed] [Google Scholar]
- 28. Palionis D, Berukstis A, Misonis N, et al. Could careful patient selection for renal denervation warrant a positive effect on arterial stiffness and left ventricular mass reduction? Acta Cardiol. 2016;71:173‐183. [DOI] [PubMed] [Google Scholar]
- 29. Azizi M, Perdrix K, Bobrie G, et al. Greater efficacy of aldosterone blockade and diuretic reinforcement vs. Dual renin‐angiotensin blockade for left ventricular mass regression in patients with resistant hypertension. J Hypertens. 2014;32:2038‐2044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


