Abstract
Background
The optimal anticoagulant for end-stage renal disease patients for stroke prophylaxis is unknown. The efficacy and safety of warfarin in this population are debatable. In addition, real-world evidence of direct oral anticoagulants in patients with end-stage renal disease is limited. The aim of this study was to evaluate the clinical outcomes of rivaroxaban compared with warfarin in Taiwanese patients with end-stage renal disease with nonvalvular atrial fibrillation in a real-world setting.
Methods and results
This was a retrospective population-based cohort study conducted using Taiwan’s National Health Insurance Research Database. Patients with nonvalvular atrial fibrillation and end-stage renal disease who started on rivaroxaban or warfarin between February 2013 and September 2017 were eligible to participate in the study. The inverse probability of treatment weighting approach was used to balance baseline characteristics. Bleeding and thromboembolic outcomes were compared using competing risk analyses. The study population consisted of 3358 patients (173 and 3185 patients on rivaroxaban and warfarin, respectively). In the rivaroxaban group, 50.8%, 38.7%, and 10.4% of the patients received 10, 15, and 20 mg of the drug, respectively. The cumulative incidence of major bleeding was similar between the two groups; however, the gastrointestinal bleeding rate was lower in the rivaroxaban group (adjusted subdistribution hazard ratio [SHR]: 0.56, 95% confidence interval [CI]: 0.34–0.91) than in the warfarin group. Furthermore, the composite risk of ischemic stroke or systemic embolism was significantly lower in the rivaroxaban group (adjusted SHR: 0.36, 95% CI: 0.17–0.79). Similar findings were observed for patients who received 10 mg of rivaroxaban.
Conclusions
In Taiwanese patients with end-stage renal disease and nonvalvular atrial fibrillation, rivaroxaban may be associated with a similar risk of major bleeding but a lower risk of thromboembolism compared with warfarin. The potential benefit of 10 mg of rivaroxaban in this population requires further investigation.
Introduction
Nonvalvular atrial fibrillation (NVAF) is common in patients with chronic kidney disease, and the prevalence markedly increases as renal function declines [1, 2]. An estimated 13%–27% of patients with end-stage renal disease (ESRD) have NVAF [3, 4], a substantially higher prevalence than in the general population. In addition, chronic kidney disease increases the stroke risk independent of other risk factors in patients with NVAF [5]. Despite an increased thromboembolism risk in patients with ESRD and NVAF, anticoagulant use in this population has been controversial because it lacks sufficient benefits, and anticoagulant users have had more adverse effects than nonusers [6, 7]. Moreover, stroke prevention is complex because renal dysfunction is an independent risk factor for major bleeding [1, 8].
To date, the optimal anticoagulant for the ESRD population for stroke prophylaxis is unknown. The efficacy and safety of warfarin in patients with ESRD for stroke prophylaxis are debatable. Numerous observational studies and meta-analyses have suggested that warfarin has no clear benefit and indicated that it is associated with increased bleeding compared with no anticoagulant and direct oral anticoagulant use in patients with ESRD [6, 9–13]. Direct oral anticoagulants have been demonstrated to be beneficial over warfarin in patients with NVAF in phase 3 clinical trials [14–18]. However, patients with ESRD were excluded from these trials, considering that direct oral anticoagulants are primarily eliminated through the kidney and that this population has high mortality and morbidity risks. A recent randomized controlled trial compared the efficacy and safety of apixaban with warfarin for stroke prevention in patients with NVAF and ESRD [19]. However, the trial was stopped early, leaving the results inconclusive. Direct oral anticoagulant use in this population has been investigated using real-world data in the United States, but Caucasians were the large majority in these study populations, and conflicting results were obtained [20–23].
In Taiwan, rivaroxaban is approved for stroke prophylaxis in NVAF patients with creatinine clearance of ≥15 mL/min. In addition to 15 mg of rivaroxaban, 10 mg of rivaroxaban is approved in Taiwan and Japan for patients with creatinine clearance between 15 and 50 mL/min. The approval was based on the findings of a phase 3 randomized controlled trial in Japan [24], and in that trial, a lower dosage was chosen for investigation based on previous pharmacokinetic data in Japanese patients. The elimination of rivaroxaban is less dependent on renal clearance compared with dabigatran and edoxaban [25–28], which makes it a potential option for patients with severe renal dysfunction. To our knowledge, no real-world data are available regarding the evaluation of the off-label use of rivaroxaban for stroke prophylaxis in Asian patients with ESRD. The study objective was to investigate the effectiveness and safety of rivaroxaban compared with warfarin in Asian patients with NVAF and ESRD in a real-world setting.
Methods
Study design and data sources
This was a retrospective population-based cohort study conducted using Taiwan’s National Health Insurance Research Database. This database contains insurance claims from 99% of Taiwan residents. The database captures enrollment records; International Classification of Diseases, Ninth and Tenth Revision (ICD-9 and ICD-10) diagnosis codes; procedure codes; and prescription records from both inpatient and outpatient services. This study was approved by the Joint Institutional Review Board of Taipei Medical University (TMU-JIRB No. N201911006). Because all data were de-identified, the Institutional Review Board waived the need for informed consent.
Study cohort
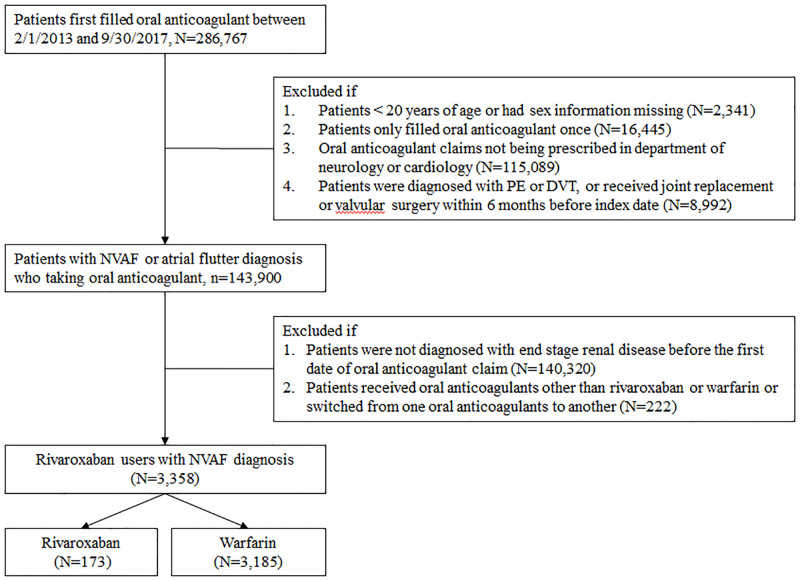
We used prescriptions records to select a study cohort to minimize the possibility of underreporting and incomplete diagnosis coding because the National Health Insurance Research Database only captured up to five diagnoses for each visit. We selected patients who received oral anticoagulant prescriptions between February 2013 and September 2017. We excluded patients from the cohort if (1) they were aged <20 years; (2) their anticoagulant prescription was filled only once during the study period; (3) anticoagulants were not prescribed by neurologists or cardiologists; (4) they received a diagnosis of pulmonary embolism or deep vein thrombosis within 6 months before the index date; and (5) they received joint replacement or valvular surgery within 6 months before the index date [29]. Among patients on oral anticoagulants with a diagnosis of NVAF or atrial flutter, we selected patients on rivaroxaban or warfarin with an ESRD diagnosis as our final study cohort. ESRD, which was defined based on a diagnosis of stage 5 chronic kidney disease or patients being on regular dialysis in this study, was identified through ICD-9 codes in the Registry of Catastrophic Illness and medical records indicating the use of erythropoiesis-stimulating agents. According to National Health Insurance policies, patients who have received renal replacement therapy for at least 3 months are eligible for catastrophic illness certification, and the use of erythropoiesis-stimulating agents is limited to patients with ESRD regardless of the dialysis status. For erythropoiesis-stimulating drug users, we specifically included those with an ESRD diagnosis identified through ICD-9 codes to eliminate patients using these agents for off-label indications. The patient selection process is shown in Fig 1. The study cohort was followed from the date of the first anticoagulant prescription to the date of the clinical event of interest or until December 31, 2017, whichever came first.
Fig 1. Patient selection process.
A total of 3358 patients with NVAF and ESRD receiving either rivaroxaban or warfarin were enrolled in this study, consisting of 173 and 3185 rivaroxaban and warfarin users, respectively. NVAF = nonvalvular atrial fibrillation; DVT = deep vein thrombosis; PE = pulmonary embolism.
Comorbidities and medications
Thromboembolic and bleeding risks at the baseline were assessed using established scoring systems, namely the CHA2DS2-VASc and ORBIT scores. The CHA2DS2-VASc score outperformed the CHADS2 score in predicting thromboembolic risk in the Taiwanese population with NVAF [30]. The ORBIT score had better accuracy than other bleeding risk scoring systems in predicting major bleeding in patients with NVAF and was validated in a large cohort of patients receiving rivaroxaban or warfarin [31]. Specific diagnosis and medication codes for comorbidities and medications are listed in S1 Table.
Study outcomes
The outcomes of interest are safety and efficacy [29]. Safety outcomes include hospitalization for major bleeding, defined as fatal bleeding, symptomatic bleeding in a critical area or organ, or bleeding leading to transfusions, and non-major clinically relevant bleeding. The definition of major bleeding was based on the recommendations of the International Society on Thrombosis and Haemostasis [32]. Non-major clinically relevant bleeding was defined as any hemorrhage that did not satisfy the criteria for major bleeding but led to hospitalization or medical visits. Efficacy outcomes included the composite endpoint of ischemic stroke or systemic embolism and individual components of the composite endpoint. Study outcomes were identified based on disease diagnosis codes and procedure codes, which are provided in S1 Table. Because 10 mg of rivaroxaban accounted for a large proportion of usage and is only approved in Taiwan and Japan, we performed analyses comparing clinical outcomes between users of 10 mg of rivaroxaban and warfarin.
Statistical analysis
To reduce potential selection bias, we used inverse probability of treatment weighting (IPTW) based on the propensity score to balance the baseline characteristics of patients receiving warfarin and rivaroxaban, resulting in similar baseline characteristics between the two groups. Instead of matching two treatment groups based on the selected confounders, IPTW involves using the entire cohort and can address numerous confounding variables. IPTW allows for the estimation of marginal hazard ratios with minimal bias while retaining data from all participants [33, 34]. Each patient was assigned a weight based on the likelihood of exposure to the treatment effect, which was estimated through logistic regression. We considered all baseline characteristics when estimating the weight. Standardized mean difference was used to compare baseline characteristics between the two groups, and a value of <0.1 indicated a negligible difference between the variables of the treatment groups. Because the outcomes of interest were thromboembolic and bleeding events, we considered death as a competing risk. Therefore, the cumulative incidence of competing risk was used to estimate the incidence of the selected outcomes. The adjusted subdistribution hazard ratio (SHR) was calculated using the competing risk model adjusted for sex, age, comorbidities, and prescribed medications. The warfarin group served as the reference cohort. During the follow-up period, patients who switched from rivaroxaban to warfarin and vice versa were excluded. Analyses were performed using SAS/STAT 9.4 software (SAS Institute Inc., Cary, NC, USA) and STATA 14 software (Stata Corp LP, College Station, TX, USA). A p-value of <0.05 was considered statistically significant.
Results
A total of 286,767 patients were selected from February 2013 to September 2017. Of these, 3358 patients met the eligibility criteria and were included in the analysis (173 and 3185 patients receiving rivaroxaban or warfarin, respectively). In the rivaroxaban group, 88 (50.8%), 67 (38.7%), and 18 (10.4%) patients received 10, 15, and 20 mg of the drug, respectively. The mean follow-up durations for the rivaroxaban and warfarin groups were 19.1 and 27.4 months, respectively. Before IPTW, patients in the rivaroxaban group were older and had more comorbidities than those in the warfarin group. The mean CHA2DS2-VASc and ORBIT scores of those in the rivaroxaban group were higher than those in the warfarin group (CHA2DS2-VASc and ORBIT scores were 4.0 vs. 3.7 and 2.9 vs. 2.7, respectively). After IPTW, the baseline characteristics were balanced between the two groups. Detailed baseline characteristics are listed in Table 1.
Table 1. Baseline characteristics of eligible patients received rivaroxaban and warfarin.
| Before IPTW | After IPTW | |||||
|---|---|---|---|---|---|---|
| Rivaroxaban (n = 173) | Warfarin (n = 3185) | Rivaroxaban (n = 173) | Warfarin (n = 3185) | |||
| % | % | SMD | % | % | SMD | |
| Male | 55 | 51 | 0.08 | 43 | 49 | 0.13 |
| Age, mean ± SD (y) | 75 ± 9 | 69 ± 12 | 0.54 | 69 ± 11 | 69 ± 12 | 0.02 |
| 20–64 | 15 | 35 | 0.49 | 39 | 34 | 0.09 |
| 65–74 | 32 | 31 | 0.04 | 29 | 31 | 0.03 |
| 75+ | 53 | 34 | 0.39 | 32 | 35 | 0.06 |
| Charlson–Deyo index, mean ± SD | 6 ± 3 | 5 ± 2 | 0.35 | 5 ± 2 | 5 ± 2 | 0.13 |
| 0–2 | 9 | 13 | 0.13 | 7 | 13 | 0.18 |
| 3 | 13 | 18 | 0.16 | 17 | 18 | 0.02 |
| 4+ | 78 | 69 | 0.22 | 76 | 69 | 0.14 |
| CHA2DS2-VASc score, mean ± SD | 4.0 ± 1.5 | 3.7 ± 1.6 | 0.18 | 3.8 ± 1.5 | 3.7 ± 1.6 | 0.05 |
| 0–2 | 20 | 25 | 0.12 | 20 | 25 | 0.11 |
| 3 | 17 | 25 | 0.13 | 24 | 22 | 0.03 |
| 4+ | 63 | 52 | 0.21 | 56 | 53 | 0.06 |
| ORBIT score | 2.9 ± 1.4 | 2.7 ± 1.4 | 0.13 | 2.8 ± 1.5 | 2.7 ± 1.4 | 0.05 |
| 0–2 | 49 | 55 | 0.14 | 54 | 55 | 0.02 |
| 3 | 22 | 20 | 0.07 | 20 | 20 | 0.004 |
| 4+ | 29 | 25 | 0.09 | 26 | 25 | 0.02 |
| Comorbidities | ||||||
| Ischemic stroke | 19 | 13 | 0.15 | 16 | 13 | 0.08 |
| GI bleeding | 13 | 12 | 0.03 | 15 | 12 | 0.09 |
| Myocardial infarction | 10 | 7 | 0.09 | 11 | 8 | 0.12 |
| Congestive heart failure | 33 | 37 | 0.08 | 43 | 37 | 0.13 |
| Peptic ulcer disease | 29 | 22 | 0.16 | 24 | 22 | 0.04 |
| Hypertension | 82 | 78 | 0.09 | 78 | 78 | 0.02 |
| Diabetes | 41 | 51 | 0.19 | 51 | 50 | 0.02 |
| Chronic liver disease | 9 | 7 | 0.08 | 6 | 7 | 0.07 |
| Hyperlipidemia | 28 | 23 | 0.11 | 18 | 23 | 0.13 |
| COPD | 16 | 13 | 0.08 | 10 | 13 | 0.10 |
| Valvular heart disease | 11 | 11 | 0.002 | 12 | 11 | 0.04 |
| Malignancy | 47 | 14 | 0.77 | 13 | 16 | 0.08 |
| Medication history | ||||||
| NSAID | 35 | 27 | 0.17 | 24 | 28 | 0.08 |
| Glucocorticoids | 23 | 15 | 0.20 | 14 | 16 | 0.05 |
| Antiplatelet agents | 57 | 52 | 0.09 | 50 | 53 | 0.06 |
| PPI | 19 | 14 | 0.14 | 16 | 14 | 0.06 |
| HMG-CoA reductase inhibitors | 25 | 21 | 0.10 | 17 | 21 | 0.10 |
| ACE inhibitors | 9 | 6 | 0.12 | 8 | 6 | 0.08 |
| Angiotensin II antagonists | 43 | 34 | 0.19 | 35 | 35 | 0.003 |
ACE = angiotensin-converting enzyme; CHA2DS2-VASc score was based on the presence of congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/transient ischemic attack, vascular disease, age 65–74 years, sex category (female); Charlson–Deyo index was based on the presence of myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, diabetes, diabetes with chronic complications, hemiplegia or paraplegia, renal disease, moderate or severe liver disease, acquired immune deficiency syndrome; COPD = chronic obstruction pulmonary disease; GI = gastrointestinal; HMG-CoA = 3-hydroxy-3-methylglutaryl coenzyme A; IPTW = inverse probability of treatment weighting; NSAID = nonsteroidal anti-inflammatory drugs; ORBIT score was based on the presence of age ≥ 74 years, anemia, bleeding history, chronic kidney disease, treatment with antiplatelet; PPI = proton pump inhibitor; SD = standard deviation; SMD = standardized mean difference.
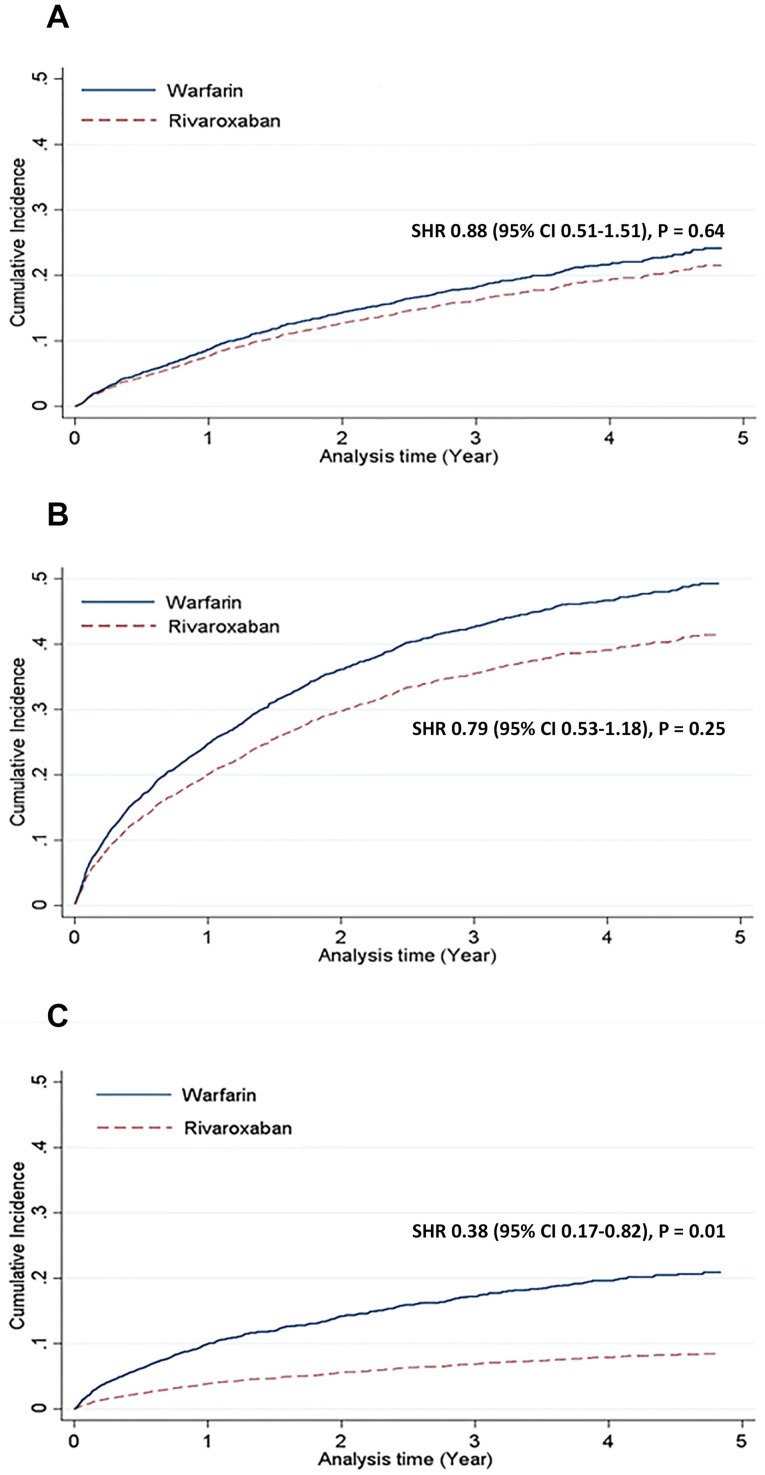
The cumulative incidence and competing risk of safety outcomes are shown in Fig 2 and Table 2. The major bleeding risk was similar between rivaroxaban and warfarin users (adjusted SHR: 0.86, 95% confidence interval [CI]: 0.50–1.47, p = 0.59). No significant difference was observed in the risk of non-major clinically relevant bleeding (adjusted SHR: 0.74, 95% CI: 0.48–1.13, p = 0.16). We further classified these bleeding events based on bleeding origin. The gastrointestinal bleeding risk was significantly lower in the rivaroxaban group than in the warfarin group (adjusted SHR: 0.56, 95% CI: 0.34–0.91, p = 0.02), whereas the intracranial bleeding risk was similar between the groups (adjusted SHR: 0.62, 95% CI: 0.24–1.61, p = 0.33).
Fig 2. Bleeding and thromboembolic outcomes: Rivaroxaban versus warfarin.
Compared with warfarin users, rivaroxaban users were associated with similar risks of major bleeding (A) and non-major clinically relevant bleeding (B), but a lower risk of ischemic stroke or systemic embolism (C). CI = confidence interval; SHR = subdistribution hazard ratio.
Table 2. Cumulative incidence and SHR of bleeding and thromboembolic outcomes: Rivaroxaban versus warfarin.
| Outcomes | Rivaroxaban (n = 173) | Warfarin (n = 3185) | Crude SHR (95% CI) | P-value | Adjusted SHR* (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Major bleeding | ||||||
| No. of events | 23 | 560 | 0.88 (0.51–1.51) | 0.64 | 0.86 (0.50–1.47) | 0.59 |
| CICR (%) | 21.5 | 24.1 | ||||
| Non-major clinically relevant bleeding | ||||||
| No. of events | 53 | 1267 | 0.79 (0.53–1.18) | 0.25 | 0.74 (0.48–1.13) | 0.16 |
| CICR (%) | 41.4 | 49.3 | ||||
| Gastrointestinal bleeding | ||||||
| No. of events | 32 | 1010 | 0.62 (0.39–0.99) | 0.04 | 0.56 (0.34–0.91) | 0.02 |
| CICR (%) | 27.1 | 40.1 | ||||
| Intracranial bleeding | ||||||
| No. of events | 7 | 236 | 0.62 (0.24–1.61) | 0.32 | 0.62 (0.24–1.61) | 0.33 |
| CICR (%) | 6.2 | 9.8 | ||||
| Composite endpoints of ischemic stroke or systemic embolism | ||||||
| No. of events | 10 | 520 | 0.38 (0.17–0.82) | 0.01 | 0.36 (0.17–0.79) | 0.01 |
| CICR (%) | 8.4 | 20.9 | ||||
| Ischemic stroke | ||||||
| No. of events | 7 | 236 | 0.62 (0.24–1.61) | 0.32 | 0.62 (0.24–1.61) | 0.33 |
| CICR (%) | 6.2 | 9.8 | ||||
| Systemic embolism | ||||||
| No. of events | 6 | 311 | 0.38 (0.12–1.24) | 0.10 | 0.36 (0.11–1.12) | 0.08 |
| CICR (%) | 4.9 | 12.2 | ||||
*Adjusted for sex, age, Charlson–Deyo index, CHA2DS2-VASc score, ORBIT score, comorbidities, and medications listed in Table 1. CI = confidence interval; CICR = cumulative incidence for competing risk; SHR = subdistribution hazard ratio.
The cumulative incidence and competing risk of efficacy outcomes are shown in Fig 2 and Table 2. The composite risk of ischemic stroke or systemic embolism was significantly lower in the rivaroxaban group than in the warfarin group (adjusted SHR: 0.36, 95% CI: 0.17–0.79, p = 0.01), whereas the individual components of the composite endpoint were similar between the two groups.
Because 10 mg rivaroxaban 10 is approved in Taiwan for stroke prevention in NVAF patients with mild to moderate renal insufficiency, we further performed a subgroup analysis of patients receiving 10 mg rivaroxaban to evaluate its effectiveness and safety in patients with ESRD compared with warfarin (Table 3). Although no significant difference was observed in overall bleeding events (major bleeding adjusted SHR: 0.58, 95% CI: 0.24–1.37, p = 0.21; non-major clinically relevant bleeding adjusted SHR: 0.75, 95% CI: 0.40–1.39, p = 0.36), gastrointestinal bleeding risk was lower in the 10 mg rivaroxaban group (adjusted SHR: 0.43, 95% CI: 0.22–0.83, p = 0.01) than in the warfarin group. Furthermore, the composite risk of ischemic stroke or systemic embolism was significantly lower in the 10 mg rivaroxaban group than in the warfarin group (adjusted SHR: 0.32, 95% CI: 0.12–0.85, p = 0.02). The reduction in the thromboembolism risk was primarily driven by ischemic stroke (adjusted SHR: 0.31, 95% CI: 0.11–0.86, p = 0.03).
Table 3. Cumulative incidence and SHR of bleeding and thromboembolic outcomes: Rivaroxaban 10 mg versus warfarin.
| Outcomes | Rivaroxaban 10 mg (n = 88) | Warfarin (n = 3185) | Crude SHR (95% CI) | P-value | Adjusted SHR* (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Major bleeding | ||||||
| No. of events | 8 | 559 | 0.60 (0.25–1.44) | 0.25 | 0.58 (0.24–1.37) | 0.21 |
| CICR (%) | 15.2 | 24.0 | ||||
| Non-major clinically relevant bleeding | ||||||
| No. of events | 28 | 1265 | 0.82 (0.46–1.46) | 0.49 | 0.75 (0.40–1.39) | 0.36 |
| CICR (%) | 42.4 | 49.1 | ||||
| Gastrointestinal bleeding | ||||||
| No. of events | 13 | 1009 | 0.49 (0.25–0.95) | 0.04 | 0.43 (0.22–0.83) | 0.01 |
| CICR (%) | 22.1 | 40.1 | ||||
| Intracranial bleeding | ||||||
| No. of events | 2 | 170 | 0.53 (0.11–2.61) | 0.44 | 0.59 (0.12–2.89) | 0.52 |
| CICR (%) | 4.2 | 7.7 | ||||
| Composite endpoints of ischemic stroke or systemic embolism | ||||||
| No. of events | 4 | 521 | 0.33 (0.13–0.89) | 0.03 | 0.32 (0.12–0.85) | 0.02 |
| CICR (%) | 7.5 | 20.9 | ||||
| Ischemic stroke | ||||||
| No. of events | 2 | 235 | 0.31 (0.11–0.86) | 0.02 | 0.31 (0.11–0.86) | 0.03 |
| CICR (%) | 3.1 | 9.8 | ||||
| Systemic embolism | ||||||
| No. of events | 3 | 313 | 0.34 (0.08–1.43) | 0.14 | 0.32 (0.08–1.33) | 0.12 |
| CICR (%) | 4.3 | 12.2 | ||||
*Adjusted for sex, age, Charlson–Deyo index, CHA2DS2-VASc score, ORBIT score, comorbidities, and medications listed in Table 1. CI = confidence interval; CICR = cumulative incidence for competing risk; SHR = subdistribution hazard ratio.
Discussion
To our knowledge, this was the first study to evaluate the use of rivaroxaban compared with warfarin in Asian ESRD patients with NVAF using real-world data. A phase 3 randomized controlled trial comparing the clinical outcomes of rivaroxaban and warfarin included patients with moderate renal insufficiency, defined as creatinine clearance of 30–49 mL/min, and similar rates of stroke and major bleeding were observed in these patients [35]. A retrospective population-based US study using an ESRD database investigated the prescribing patterns and bleeding rates associated with rivaroxaban, dabigatran, and warfarin in chronic hemodialysis patients with NVAF [20]. Patients on rivaroxaban had a higher rate of major bleeding compared with those on warfarin, especially patients on 20 mg of rivaroxaban. Another retrospective cohort study in the United States compared the effectiveness and safety of rivaroxaban and warfarin in NVAF patients with stage 4 or 5 chronic kidney disease or those on hemodialysis [21]. Most patients in this study were using 20 mg of rivaroxaban. Although the risk of stroke or systemic embolism was similar between the two groups, rivaroxaban was associated with a lower rate of major bleeding compared with warfarin. These conflicting findings reflect the heterogeneity of anticoagulation responses in patients with NVAF and chronic kidney disease. In our study, we did not observe significant differences in major bleeding and non-major clinically relevant bleeding events, except lower gastrointestinal bleeding. Notably, we observed the risk of ischemic stroke or systemic embolism was lower in patients who used rivaroxaban than in those who used warfarin.
Our study revealed a high cumulative incidence of thromboembolism and bleeding in patients with ESRD on warfarin. Although warfarin has been the mainstay therapy for stroke prevention in patients with ESRD, the level of evidence is weak. According to several observational studies and meta-analyses, warfarin is associated with an increased bleeding risk, including intracranial hemorrhage, in patients with ESRD, without providing a protective effect against stroke [6, 9–12, 36]. In addition, the risk of warfarin-related major bleeding, particularly intracranial bleeding, is significantly higher in Asian populations [37]. Our study did not include patients with no anticoagulation because such a study design imposes a significant confounding effect that cannot be eliminated through statistical adjustments. Furthermore, direct oral anticoagulants have fewer drug and food interactions and monitoring requirements. In addition, some studies have indicated that factor Xa inhibition may ameliorate nephropathy, and rivaroxaban has been associated with a slower decline in renal function compared with warfarin [38, 39]. A pharmacokinetic study of rivaroxaban in chronic hemodialysis patients indicated that 10 mg of the drug administered to such patients had similar outcomes to 20 mg given to healthy volunteers [40], and drug accumulation was absent after multiple doses. Our study showed that 10 mg of rivaroxaban was associated with a lower rate of thromboembolism and gastrointestinal bleeding compared with warfarin, but the use of this dose in patients with NVAF and ESRD requires further investigation, considering the retrospective nature of our study. Other therapies such as antiplatelet drugs have been investigated in retrospective observational studies, and they did not confer stroke prevention benefits in patients with ESRD [41, 42]. The current guidelines do not recommend antiplatelet agent use for stroke prevention [43]. Therefore, our study did not compare clinical outcomes between rivaroxaban and antiplatelet agents.
The present study has several limitations. First, the data source was insurance claims, and thus, information on international normalized ratio levels was unavailable; for this reason, we could not evaluate the time in the therapeutic range of the warfarin group. Information of actual adherence rates were unavailable in our data source. Second, the sample size of the rivaroxaban group was small because rivaroxaban was used off-label. Further studies with a larger Asian ESRD population are required to evaluate the effectiveness and safety of rivaroxaban. Finally, the retrospective study design and differences between rivaroxaban and warfarin users at baseline indicated the potential for confounding effects. Therefore, we used IPTW to minimize the effect of confounding variables, and our analyses were adjusted for baseline characteristics.
Conclusions
In real-world clinical settings, warfarin is associated with a high incidence of thromboembolic and bleeding events in Taiwanese patients with NVAF and ESRD. Rivaroxaban use in this population resulted in fewer thrombotic events but similar major bleeding events when compared with warfarin. A prospective clinical study is required to confirm the findings of the present study.
Supporting information
(DOCX)
Acknowledgments
We appreciate the assistance provided by Health and Clinical Data Research Center, College of Public Health, Taipei Medical University, Taipei, Taiwan.
Data Availability
In regards to data availability, our study used National Health Insurance Research Data, a healthcare claims data that provided by the Health and Welfare Science Data Center (HWDC), Ministry of Health and Welfare in Taiwan. The HWDC is a third-party organization. Researchers can submit application to HWDC in order to have access to several health-related databases. Due to legal restrictions imposed by the government of Taiwan in relation to the Personal Information Protection Act, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the HWDC with an IRB approval letter. The contact information of Taipei Medical University Joint IRB is tmujirb@gmail.com. All data were fully anonymized before we access them. In addition, these data can only be access and analyzed in an independent operating area in the HWDC. Only statistical results can be brought out from the operating area. Therefore, original data cannot be shared publicly due to legal restrictions.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Stamellou E, Floege J. Novel oral anticoagulants in patients with chronic kidney disease and atrial fibrillation. Nephrol Dial Transplant. 2018;33(10):1683–9. 10.1093/ndt/gfx322 . [DOI] [PubMed] [Google Scholar]
- 2.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, et al. Chronic Kidney Disease Is Associated With the Incidence of Atrial Fibrillation The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2011;123(25):2946–U81. 10.1161/CIRCULATIONAHA.111.020982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, et al. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis. 2005;46(5):897–902. 10.1053/j.ajkd.2005.07.044 . [DOI] [PubMed] [Google Scholar]
- 4.Wizemann V, Tong L, Satayathum S, Disney A, Akiba T, Fissell RB, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77(12):1098–106. 10.1038/ki.2009.477 . [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Fang MC, Udaltsova N, Chang Y, Pomernacki NK, Borowsky L, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119(10):1363–9. 10.1161/CIRCULATIONAHA.108.816082 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuno T, Takagi H, Ando T, Sugiyama T, Miyashita S, Valentin N, et al. Oral Anticoagulation for Patients With Atrial Fibrillation on Long-Term Hemodialysis. J Am Coll Cardiol. 2020;75(3):273–85. 10.1016/j.jacc.2019.10.059 . [DOI] [PubMed] [Google Scholar]
- 7.Mavrakanas TA, Garlo K, Charytan DM. Apixaban versus No Anticoagulation in Patients Undergoing Long-Term Dialysis with Incident Atrial Fibrillation. Clin J Am Soc Nephrol. 2020;15(8):1146–54. Epub 2020/05/24. 10.2215/CJN.11650919 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marinigh R, Lane DA, Lip GY. Severe renal impairment and stroke prevention in atrial fibrillation: implications for thromboprophylaxis and bleeding risk. J Am Coll Cardiol. 2011;57(12):1339–48. 10.1016/j.jacc.2010.12.013 . [DOI] [PubMed] [Google Scholar]
- 9.Dahal K, Kunwar S, Rijal J, Schulman P, Lee J. Stroke, Major Bleeding, and Mortality Outcomes in Warfarin Users With Atrial Fibrillation and Chronic Kidney Disease: A Meta-Analysis of Observational Studies. Chest. 2016;149(4):951–9. 10.1378/chest.15-1719 . [DOI] [PubMed] [Google Scholar]
- 10.Nochaiwong S, Ruengorn C, Awiphan R, Dandecha P, Noppakun K, Phrommintikul A. Efficacy and safety of warfarin in dialysis patients with atrial fibrillation: a systematic review and meta-analysis. Open Heart. 2016;3(1):e000441. 10.1136/openhrt-2016-000441 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129(11):1196–203. 10.1161/CIRCULATIONAHA.113.004777 . [DOI] [PubMed] [Google Scholar]
- 12.Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6(11):2662–8. 10.2215/CJN.04550511 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha JT, Neuen BL, Cheng LP, Jun M, Toyama T, Gallagher MP, et al. Benefits and Harms of Oral Anticoagulant Therapy in Chronic Kidney Disease: A Systematic Review and Meta-analysis. Ann Intern Med. 2019;171(3):181–9. Epub 2019/07/16. 10.7326/M19-0087 . [DOI] [PubMed] [Google Scholar]
- 14.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. 10.1056/NEJMoa0905561 . [DOI] [PubMed] [Google Scholar]
- 15.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104. 10.1056/NEJMoa1310907 . [DOI] [PubMed] [Google Scholar]
- 16.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. 10.1056/NEJMoa1107039 . [DOI] [PubMed] [Google Scholar]
- 17.Hori M, Matsumoto M, Tanahashi N, Momomura S-i, Uchiyama S, Goto S, et al. Rivaroxaban vs. Warfarin in Japanese Patients With Atrial Fibrillation. Circulation Journal. 2012;76(9):2104–11. 10.1253/circj.cj-12-0454 [DOI] [PubMed] [Google Scholar]
- 18.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. 10.1056/NEJMoa1009638 . [DOI] [PubMed] [Google Scholar]
- 19.Pokorney SD. American College of Cardiology Webpage, AHA 2019 Presentation Slides for the RENAL-AF Trial.
- 20.Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131(11):972–9. 10.1161/CIRCULATIONAHA.114.014113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman CI, Kreutz R, Sood NA, Bunz TJ, Eriksson D, Meinecke AK, et al. Rivaroxaban Versus Warfarin in Patients With Nonvalvular Atrial Fibrillation and Severe Kidney Disease or Undergoing Hemodialysis. Am J Med. 2019;132(9):1078–83. 10.1016/j.amjmed.2019.04.013 . [DOI] [PubMed] [Google Scholar]
- 22.Miao B, Sood N, Bunz TJ, Coleman CI. Rivaroxaban versus apixaban in non-valvular atrial fibrillation patients with end-stage renal disease or receiving dialysis. Eur J Haematol. 2020. 10.1111/ejh.13383 [DOI] [PubMed] [Google Scholar]
- 23.Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, et al. Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation. 2018;138(15):1519–29. 10.1161/CIRCULATIONAHA.118.035418 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation—the J-ROCKET AF study. Circ J. 2012;76(9):2104–11. Epub 2012/06/06. 10.1253/circj.cj-12-0454 . [DOI] [PubMed] [Google Scholar]
- 25.Mueck W, Lensing AW, Agnelli G, Decousus H, Prandoni P, Misselwitz F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50(10):675–86. Epub 2011/09/08. 10.2165/11595320-000000000-00000 . [DOI] [PubMed] [Google Scholar]
- 26.Ogata K, Mendell-Harary J, Tachibana M, Masumoto H, Oguma T, Kojima M, et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol. 2010;50(7):743–53. Epub 2010/01/19. 10.1177/0091270009351883 . [DOI] [PubMed] [Google Scholar]
- 27.Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64(3):292–303. Epub 2007/05/18. 10.1111/j.1365-2125.2007.02899.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace. 2018;20(8):1231–42. Epub 2018/03/22. 10.1093/europace/euy054 . [DOI] [PubMed] [Google Scholar]
- 29.Lin YC, Chien SC, Hsieh YC, Shih CM, Lin FY, Tsao NW, et al. Effectiveness and Safety of Standard- and Low-Dose Rivaroxaban in Asians With Atrial Fibrillation. J Am Coll Cardiol. 2018;72(5):477–85. 10.1016/j.jacc.2018.04.084 . [DOI] [PubMed] [Google Scholar]
- 30.Chao TF, Liu CJ, Tuan TC, Chen SJ, Wang KL, Lin YJ, et al. Comparisons of CHADS2 and CHA2DS2-VASc scores for stroke risk stratification in atrial fibrillation: Which scoring system should be used for Asians? Heart Rhythm. 2016;13(1):46–53. Epub 2015/08/19. 10.1016/j.hrthm.2015.08.017 . [DOI] [PubMed] [Google Scholar]
- 31.O’Brien EC, Simon DN, Thomas LE, Hylek EM, Gersh BJ, Ansell JE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. 2015;36(46):3258–64. Epub 2015/10/02. 10.1093/eurheartj/ehv476 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–4. 10.1111/j.1538-7836.2005.01204.x . [DOI] [PubMed] [Google Scholar]
- 33.Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res. 2017;26(4):1654–70. Epub 2015/05/03. 10.1177/0962280215584401 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, et al. Comparison of Propensity Score Methods and Covariate Adjustment: Evaluation in 4 Cardiovascular Studies. J Am Coll Cardiol. 2017;69(3):345–57. Epub 2017/01/21. 10.1016/j.jacc.2016.10.060 . [DOI] [PubMed] [Google Scholar]
- 35.Fox KA, Piccini JP, Wojdyla D, Becker RC, Halperin JL, Nessel CC, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32(19):2387–94. 10.1093/eurheartj/ehr342 . [DOI] [PubMed] [Google Scholar]
- 36.Hussain S, Siddiqui AN, Baxi H, Habib A, Hussain MS, Najmi AK. Warfarin use increases bleeding risk in hemodialysis patients with atrial fibrillation: A meta-analysis of cohort studies. J Gastroen Hepatol. 2019;34(6):975–84. 10.1111/jgh.14601 [DOI] [PubMed] [Google Scholar]
- 37.Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50(4):309–15. 10.1016/j.jacc.2007.01.098 . [DOI] [PubMed] [Google Scholar]
- 38.Apostolakis S, Guo Y, Lane DA, Buller H, Lip GY. Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: the AMADEUS trial. Eur Heart J. 2013;34(46):3572–9. Epub 2013/08/24. 10.1093/eurheartj/eht328 . [DOI] [PubMed] [Google Scholar]
- 39.Sumi A, Yamanaka-Hanada N, Bai F, Makino T, Mizukami H, Ono T. Roles of coagulation pathway and factor Xa in the progression of diabetic nephropathy in db/db mice. Biol Pharm Bull. 2011;34(6):824–30. Epub 2011/06/02. 10.1248/bpb.34.824 . [DOI] [PubMed] [Google Scholar]
- 40.De Vriese AS, Caluwe R, Bailleul E, De Bacquer D, Borrey D, Van Vlem B, et al. Dose-finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis. 2015;66(1):91–8. 10.1053/j.ajkd.2015.01.022 . [DOI] [PubMed] [Google Scholar]
- 41.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20(10):2223–33. Epub 2009/08/29. 10.1681/ASN.2009030319 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen JJ, Lin LY, Yang YH, Hwang JJ, Chen PC, Lin JL. Anti-platelet or anti-coagulant agent for the prevention of ischemic stroke in patients with end-stage renal disease and atrial fibrillation—a nation-wide database analyses. Int J Cardiol. 2014;177(3):1008–11. Epub 2014/12/03. 10.1016/j.ijcard.2014.09.140 . [DOI] [PubMed] [Google Scholar]
- 43.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. 10.1093/eurheartj/ehw210 . [DOI] [PubMed] [Google Scholar]