Abstract
Background/Objectives:
Chronic kidney disease (CKD) is associated with frailty. Fibroblast growth factor 23 (FGF23) is elevated in CKD and associated with frailty among non-CKD older adults and individuals with human immunodeficiency virus. Whether FGF23 is associated with frailty and falls in CKD is unknown.
Design:
Cross-sectional and longitudinal observational study
Setting:
Systolic Blood Pressure Intervention Trial (SPRINT), a randomized trial evaluating standard (SBP<140 mmHg) vs. intensive (SBP<120 mmHg) blood pressure lowering on cardiovascular and cognitive outcomes among older adults without diabetes.
Participants:
2376 participants with CKD (estimated glomerular filtration rate (eGFR)<60 mL/min/1.73m2).
Measurements:
The exposure variable was intact FGF23. We used multinomial logistic regression to determine the cross-sectional association of intact FGF23 with frailty and Cox proportional hazards analysis to determine the longitudinal association with incident falls. Models were adjusted for demographics, comorbidities, randomization group, antihypertensives, eGFR, mineral metabolism markers, and frailty.
Results:
After adjustment, the odds ratio for prevalent frailty vs. non-frailty per two-fold higher FGF23 was 1.34 (95% CI, 1.01–1.77). FGF23 levels in the highest quartile vs. the lowest quartile demonstrated >2-fold increased fall risk (hazard ratio 2.32 (95% CI, 1.26–4.26)), and the hazard ratio per two-fold higher FGF23 was 1.99 (95% CI, 1.48–2.68).
Conclusions:
Among SPRINT participants with CKD, FGF23 was associated with prevalent frailty and falls.
Keywords: fibroblast growth factor 23, falls, frailty, chronic kidney disease, biomarkers
INTRODUCTION
Fibroblast growth factor (FGF23) is a bone-derived hormone that promotes phosphate excretion into urine and inhibits 1-α-hydroxylase reducing serum 1,25-dihydroxyvitamin D (1,25(OH)2D) levels to suppress phosphate absorption from the intestine.1 FGF23 also inhibits parathyroid (PTH) synthesis2 limiting bone resorption of calcium and phosphate. Thus, FGF23 induces negative phosphate balance.3
Extracellular phosphate is necessary to allow mineralization of bone matrix, while intracellular phosphate plays an important role in energy production in the form of phosphocreatine and adenosine triphosphate (ATP),4 which are needed for muscles to properly function. Hypophosphatemic rickets is characterized by excess FGF23 along with frequent fractures and muscle weakness that severely affect activities of daily living.5 Experimental studies have shown that inhibition of FGF23 through injections of FGF23 neutralizing antibodies increased serum phosphate and 1,25(OH)2D levels as well as grip strength and spontaneous movements in hypophosphatemic mice.6 Consequently, FGF23 may play an important role not only in bone mineralization but also in the maintenance of muscle mass and function given its interplay with vitamin D and the critical role of phosphate in energy (ATP).
Chronic kidney disease (CKD) is associated with an increased risk of frailty.7 Circulating FGF23 levels are elevated in CKD, increase as kidney disease progresses,8 and are associated with adverse clinical outcomes.9–13 Among community-dwelling older adults without kidney disease and individuals with human immunodeficiency virus (HIV), FGF23 is associated with frailty.14,15 Frailty increases risk of falls,16,17 and risk of falling is higher among those with CKD compared to those without kidney disease.18 An important consequence of falling is fracture. Not only do individuals with CKD have an increased risk of fracture compared to the general population,19 but fracture in those with CKD is costly20 and associated with a high mortality risk.21 A biomarker to identify individuals with CKD at risk for frailty and falls, who would benefit from monitoring and early intervention, may improve these outcomes. Whether serum FGF23 levels are associated with an increased risk of falls in CKD is unknown. We hypothesized that higher serum intact FGF23 concentrations would be associated with prevalent frailty and greater risk of falls in CKD. To test this hypothesis, we conducted an analysis of participants with CKD (baseline eGFR <60 mL/min.1.73m2) from the Systolic Blood Pressure Intervention Trial (SPRINT).
METHODS
Study Population
SPRINT22 was a randomized, controlled, open-label trial conducted at 102 clinical sites in the United States and Puerto Rico. SPRINT tested the hypothesis that a systolic blood pressure goal <120 mm Hg would reduce cardiovascular disease events more than a standard goal (<140 mm Hg) among 9361 hypertensive participants. Study-group assigned blood pressure targets were achieved by adjusting baseline antihypertensive regimens.22 At enrollment, participants were ≥50 years, had a systolic blood pressure of 130 to 180 mm Hg and an increased risk of cardiovascular events defined by ≥1 of the following: clinical or subclinical cardiovascular disease other than stroke; CKD with a Modification of Diet in Renal Disease eGFR of 20–60 mL/min/1.73m2; a 10-year Framingham cardiovascular risk score ≥15%; or an age ≥75 years. Major exclusion criteria were diabetes, prior stroke, polycystic kidney disease, proteinuria >1 g/day, or any solid organ transplant. For this FGF23 ancillary study, serum cystatin C (Gentian, Moss, Norway) was measured in participants to identify the subpopulation with CKD defined as eGFR <60 mL/min/1.73m2, using the CKD Epidemiology Collaboration combined creatinine and cystatin C equation;23 2486 participants met these criteria and baseline serum FGF23 levels were measured in this subset. SPRINT was approved by the institutional review board at each participating study site and this FGF23 ancillary study was approved at the Veterans Affairs San Diego Healthcare System.
Serum FGF23
Serum samples from the baseline study visit were stored at −70°C; a first-thaw aliquot was used to measure intact FGF23 in 2015 with a two-site ELISA (Kainos Laboratories, Tokyo, Japan), with an interassay coefficient of variation of 8.6% at 22.5 pg/mL and 3.2% at 85.1 pg/mL, with an analytic range of 2.2–800 pg/mL.24,25 All measurements were made in duplicate in each sample and averaged to provide a single value for each participant. Measurements were at the SPRINT Central Laboratory (University of Minnesota, Minneapolis, MN).
Frailty
A deficit accumulation approach was used to construct a measure of frailty, the frailty index, in SPRINT using 36 total items and is described elsewhere26 and in Supplemental Table 1 and Supplemental Material. We excluded the renal component of the frailty for this analysis. Among participants ≥75 years, gait speed was measured based on a 4-meter walk test and was included in the frailty index, yielding 37 items in this subset. The frailty index was calculated as the sum of the score for each deficit, divided by the total number of non-missing items. Each item was weighed equally and participants who had 30 or more non-missing items were included. Participants were classified as non-frail (frailty index ≤ 0.10), pre-frail (0.10 < frailty index ≤ 0.21), or frail (0.21 < frailty index).26–29
Falls
Injurious falls were pre-specified as a safety event of interest in SPRINT.30 Site staff asked participants about falls at quarterly study visits using a standardized data collection form. Site staff may have also learned about serious adverse events (SAE) at as-needed visits or in other ways, such as participant-initiated contact, investigator involvement in participant care, or electronic medical record notifications. A fall was defined as a sudden, unintentional change in position in which the participant came to rest on the ground, floor, or a lower level not as the result of syncope or overwhelming external force. A fall due to syncope was not counted as a fall, because syncope was captured separately. An injurious fall was defined as a SAE if it was fatal or life threatening, resulted in significant or persistent disability, required hospitalization, or investigators judged it to represent a significant hazard or harm to the participant that might require intervention to prevent a subsequent event. Falls defined as SAEs were included in this analysis.
Other measurements
Participant age, sex, race, past medical history, and smoking status were obtained by questionnaire at the baseline visit. Blood pressure was measured three times after a 5-minute seated rest using an automated BP device (Model 907; Omron Healthcare), and the mean value was used. Body mass index was calculated as weight in kilograms divided by height in meters squared. Serum PTH was measured using an intact PTH immunoassay (e411 analyzer; Roche, Indianapolis, IN), with an interassay coefficient of variation of 4.9% at 35.1 pg/mL and 2.5% at 210.4 pg/mL, with an analytic measurement range of 1.2–5000 pg/mL.31 Fasting serum cholesterol, high density lipoprotein (HDL) cholesterol, creatinine, urine albumin, and urine creatinine were measured at the Central Laboratory on a Roche c501 instrument.32 The CKD Epidemiology Collaboration combined creatinine and cystatin C equation23 was used to estimate GFR.
Statistical Analysis
Baseline characteristics were compared across FGF23 quartiles. To examine whether FGF23 was independently associated with prevalent frailty, we used multinomial logistic regression with non-frailty as the reference and pre-frailty and frailty as the alternative outcomes. FGF23 was treated as a continuous variable, using log2 transformation, such that coefficients could be interpreted as “per two-fold higher.” Cox proportional hazards models examined the association between baseline FGF23 and falls. We assessed the proportional hazards assumption for each covariate by including the interaction of the covariate and time in the model. If the interaction covariate did not have a significant coefficient, then we considered the proportional hazards assumption met for the covariate and removed the interaction from the final model. We did not find evidence of violation of the assumption of our analysis. Quartiles of FGF23 were evaluated, using the lowest FGF23 quartile as the reference group. Because no clinically meaningful cut-point for FGF23 levels has been established in CKD, we evaluated FGF23 in quartiles similar to previous publications examining FGF23 in SPRINT and to allow for better understanding of the relationship between FGF23 and outcomes across a range of FGF23 levels.33 Similar to the multinomial regression models, the Cox model was also fit with FGF23 as a continuous variable (log2) to allow for interpretation as “per two-fold higher.” A sequence of multivariable models were evaluated for each analysis. Model 1 adjusted for age, sex, race (white vs. other), and randomized treatment arm. Model 2 added smoking (never, former, current), cardiovascular disease, congestive heart failure, body mass index (BMI), systolic and diastolic blood pressures, eGFR, urine albumin-to-creatinine ratio (UACR), number of antihypertensive medications at baseline, and baseline serum calcium, phosphorus, and PTH. The frailty index was added to the Cox proportional hazards model 3 as a continuous variable to examine whether FGF23 was associated with falls independent of frailty. Analyses were repeated using data from a subset of participants ≥75 years because gait speed was included in the frailty index only for this group and they experienced over 80% of falls. Finally, we tested the interaction between log2 FGF23 and randomization to intensive blood pressure lowering in order to determine if treatment randomization modified the association of FGF23 and falls. All statistical analyses were performed with SAS software version 9.4 (SAS Institute, Cary, NC). P-values <0.05 were considered statistically significant for all analyses including the interaction terms.
RESULTS
Among the 2486 SPRINT participants with an eGFR <60 mL/min/1.73m2 at the baseline study visit, 110 participants had missing baseline data and were not included in this analysis. Of the 110 participants who were excluded, 19 were missing BMI, 3 were missing serum calcium levels, 3 were missing serum phosphorus levels, 1 was missing PTH level, 77 were missing urine albumin-to-creatinine ratio, and 11 were missing frailty index calculations. The baseline characteristics of the 110 excluded participants did not differ from the final analytic cohort. Baseline characteristics of the final analytic cohort (N = 2376) are presented in Table 1. The mean age was 73 ± 9 years, 40% were female, and 67% were white. Median baseline FGF23 [IQR] was 66 [52–88] pg/mL. Thirty-six percent of the participants were frail, 53% were pre-frail, and 11% were non-frail. Participants with FGF23 in the highest quartile were more likely to be female, had greater history of heart failure, lower eGFR, higher UACR and PTH, and were more likely to be frail. The proportion of participants randomized to the standard vs. intensive arms of the trial were similar across FGF23 quartiles.
Table 1.
Baseline characteristics of SPRINT participants with CKD
| Total Cohort N = 2376 | FGF23 Q1 N = 591 | FGF23 Q2 N = 594 | FGF23 Q3 N = 595 | FGF23 Q4 N = 596 | |
|---|---|---|---|---|---|
| FGF23, pg/mL | 66.2 [51.7–87.6] | <52.8 | 52.8–67.2 | 67.3–89.0 | ≥89.1 |
| Age, years | 73 ± 9 | 73 ± 9 | 74 ± 8 | 73 ± 9 | 73 ± 10 |
| Female | 948 (40) | 216 (37) | 220 (37) | 245 (41) | 267 (45) |
| Race, White | 1559 (67) | 357 (60) | 415 (70) | 403 (68) | 384 (64) |
| Treatment randomization | |||||
| Standard | 1168 (49) | 278 (47) | 281 (47) | 306 (51) | 303 (51) |
| Intensive | 1208 (51) | 313 (53) | 313 (53) | 289 (49) | 293 (49) |
| Prevalent CVD | 605 (26) | 136 (23) | 157 (26) | 158 (27) | 154 (26) |
| Prevalent heart failure | 153 (6) | 35 (6) | 35 (6) | 32 (5) | 51 (9) |
| eGFR-CrCys, mL/min/1.73m2 | 49 ± 11 | 53 ± 10 | 51 ± 9 | 49 ± 11 | 42 ± 12 |
| UACR, mg/g | 87 ± 253 | 71 ± 247 | 58 ± 172 | 80 ± 224 | 139± 334 |
| SBP, mmHg | 140 ± 16 | 141 ± 17 | 140 ± 16 | 139 ± 17 | 139 ± 17 |
| DBP, mmHg | 74 ± 12 | 76 ± 13 | 74 ± 12 | 74 ± 13 | 73 ± 13 |
| Antihypertensive medication, number | 2.2 ± 1.0 | 2.0 ± 1.0 | 2.1 ± 1.0 | 2.2 ± 1.0 | 2.3 ± 1.0 |
| Smoking | |||||
| Never | 1075 (45) | 251 (43) | 283 (48) | 269 (45) | 272 (47) |
| Former | 1087 (46) | 260 (44) | 274 (46) | 282 (47) | 271 (46) |
| Current | 214 (9) | 80 (14) | 37 (6) | 44 (7) | 53 (9) |
| Body mass index, kg/m2 | 30 ± 6 | 29 ± 6 | 29 ± 5 | 30 ± 6 | 30 ± 6 |
| Serum Calcium, mg/mL | 9.6 ± 0.5 | 9.5 ± 0.4 | 9.6 ± 0.5 | 9.6 ± 0.6 | 9.6 ± 0.6 |
| Serum Phosphate, mg/mL | 3.6 ± 0.5 | 3.4 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.5 | 3.7 ± 0.6 |
| Serum PTH, pg/mL | 56 ± 33 | 50 ± 24 | 50 ± 25 | 55 ± 32 | 68 ± 45 |
| Frailty (w/o GFR) | |||||
| Non-frail | 265 (11) | 69 (12) | 90 (15) | 58 (10) | 48 (8) |
| Pre-frail | 1247 (53) | 320 (54) | 321 (54) | 316 (53) | 290 (48) |
| Frail | 864 (36) | 202 (34) | 183 (31) | 221 (37) | 258 (43) |
Data presented as N (%), mean ± SD, or median [IQR]
Abbreviations: FGF23, fibroblast growth factor 23; CVD, cardiovascular disease; eGFR-CrCys, estimated glomerular filtration rate; UACR, urine albumin-to-creatinine ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; PTH, parathyroid hormone
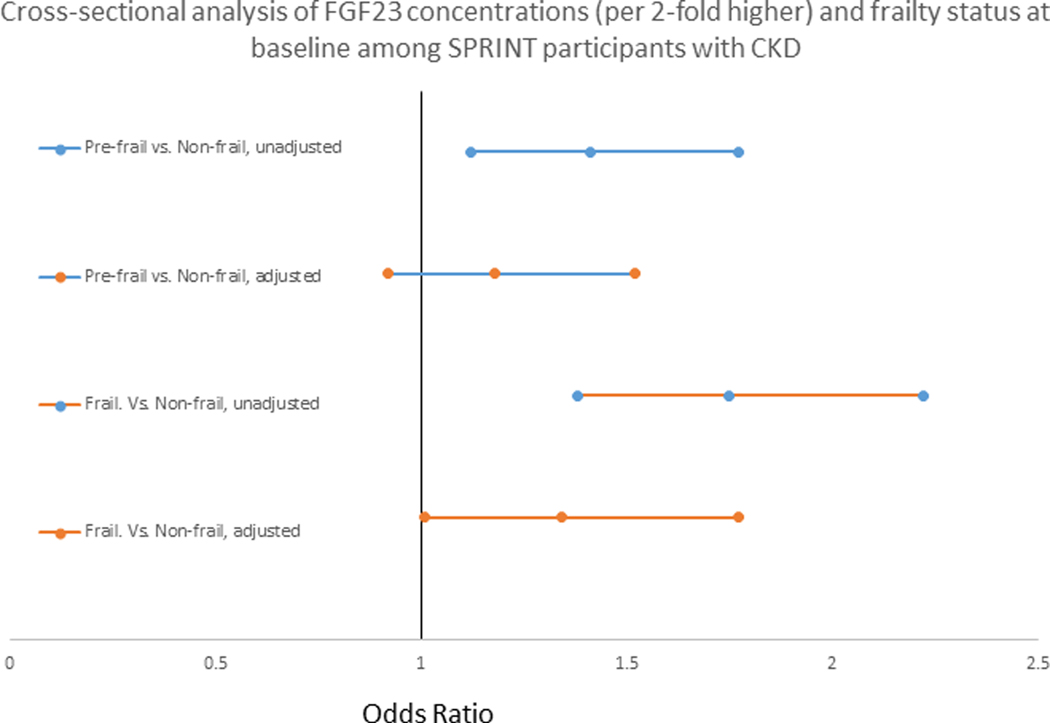
Higher baseline serum FGF23 was associated with prevalent frailty. In the unadjusted model, for every two-fold higher baseline serum FGF23 the odds of frailty compared to non-frailty was 1.75 (95% CI, 1.38–2.22). The association was somewhat attenuated after adjustment for demographics, cardiovascular disease and kidney function parameters, and markers of mineral metabolism an odds ratio of 1.34 (95% CI,1.01–1.77). In the unadjusted model, for every two-fold higher FGF23, the odds of pre-frailty compared to non-frailty was 1.41 (95% CI, 1.12–1.77). However, after full adjustment the association was attenuated (Figure 1). Neither sex nor race had an effect on the association of FGF23 and frailty (p-value for interaction terms >0.05).
Figure 1.
The cross-sectional analysis of FGF23 concentrations (per 2-fold higher) and frailty status at baseline among SPRINT participants with CKD. The adjusted model included age, sex, race, randomized treatment arm, smoking, cardiovascular disease, congestive heart failure, body mass index, systolic and diastolic blood pressures, estimated glomerular filtration rate, urine albumin to creatinine ratio, number of antihypertensive medications at baseline, and serum concentrations of calcium, phosphorus, and parathyroid hormone.
During a median follow-up of 39 [31–46] months, there were 102 falls (Table 2) and 5 deaths. Median [IQR] baseline FGF23 level was higher (80 [59–102] pg/mL) in the fall group compared to the group without falls (66 [52–87] pg/mL), while there was no difference between groups in baseline calcium, phosphate, and PTH levels (Supplemental Table 2). In the unadjusted analysis, the hazard ratio (HR) for falls among participants with baseline FGF23 levels in the highest quartile vs. the lowest quartile was 2.22 (95% CI, 1.29–3.84). This association was not attenuated after adjusting the model for demographics, cardiovascular disease and kidney function parameters, markers of mineral metabolism, and frailty index with a 2.32 (95% CI, 1.26–4.26) hazard. In the fully adjusted model, for every two-fold higher FGF23, the risk of falls increased by nearly 2-fold (HR 1.99 (95% CI, 1.48–2.68)) (Table 2). There was no interaction between FGF23 and randomization to intensive blood pressure lowering (p = 0.8).
Table 2.
Association of FGF23 concentrations and risk of falls among SPRINT participants with CKD
| Events/Total | Unadjusted | Model 1 | Model 2 | |
|---|---|---|---|---|
| SPRINT participants with CKD (n = 2376) | ||||
| Log2 FGF23 | 102/2376 | 1.80 (1.39 – 2.31) | 1.99 (1.48 – 2.67) | 1.99 (1.48 – 2.68) |
| Quartile 1 | 20/591 | REFERENCE | REFERENCE | REFERENCE |
| Quartile 2 | 18/594 | 0.98 (0.52 – 1.86) | 1.03 (0.54 – 1.95) | 1.05 (0.55 – 1.99) |
| Quartile 3 | 25/595 | 1.25 (0.68 – 2.28) | 1.32 (0.71 – 2.46) | 1.34 (0.72 – 2.49) |
| Quartile 4 | 39/596 | 2.22 (1.29 – 3.84) | 2.31 (1.26 – 4.25) | 2.32 (1.26 – 4.26) |
| SPRINT participants ≥ 75 years and CKD (n = 1192) | ||||
| Log2 FGF23 | 81/1192 | 1.57 (1.16 – 2.11) | 1.80 (1.27 – 2.56) | 1.82 (1.28 – 2.60) |
| Quartile 1 | 18/298 | REFERENCE | REFERENCE | REFERENCE |
| Quartile 2 | 15/298 | 0.84 (0.43 – 1.68) | 0.96 (0.48 – 1.93) | 0.99 (0.49 – 1.99) |
| Quartile 3 | 20/297 | 1.14 (0.60 – 2.16) | 1.18 (0.61 – 2.30) | 1.19 (0.61 – 2.34) |
| Quartile 4 | 28/299 | 1.67 (0.92 – 3.02) | 1.90 (0.98 – 3.71) | 1.95 (1.00 – 3.80) |
Model 1: adjusted for age, gender, race, randomized treatment arm, smoking, CVD, CHF, body mass index, systolic and diastolic blood pressures, eGFR, UACR, # antihypertensive medications at baseline, and serum calcium, phosphorus, and PTH concentrations
Model 2: adjusted for covariables in model 1 plus frailty index (and gait speed among SPRINT participants ≥75 years)
Gait speed was measured among SPRINT participants ≥75 years and included in the frailty index. We repeated the analysis in this sub-cohort of participants ≥75 years with CKD (N = 1192) (baseline characteristics in Supplemental Tables 3 and 4) and results were similar. The HR for falls among participants ≥75 years with baseline FGF23 levels in the highest quartile vs. the lowest quartile was 1.95 (95% CI, 1.00–3.80) and for every 2-fold increase in FGF23 levels the HR for falls was 1.82 (95% CI, 1.28–2.60) in the fully adjusted models. Notably the addition of the frailty index including gait speed did not change the magnitude of the HR (Table 2).
Similar to the total cohort and the subgroup ≥75 years, SPRINT participants <75 years (N = 1184) with FGF23 levels in the highest quartile had a greater risk of fall compared to those with FGF23 levels in the lowest quartile and a 4-fold greater risk of fall for every doubling of FGF23 in the fully adjusted models (Supplemental Table 5).
DISCUSSION
In this analysis of SPRINT participants with baseline CKD (eGFR <60 mL/min/1.73m2), higher serum intact FGF23 levels were independently associated with greater prevalence of frailty. There was also a strong association of higher FGF23 with risk of falls that remained even after accounting for baseline frailty status. These findings are consistent with the reports in community-living older adults and individuals with HIV predominantly without CKD,14,15 and extend similar findings to persons with moderate to severe CKD.
Sarcopenia, the loss of muscle mass with aging or chronic disease, and frailty, reduced homeostatic reserves, which increases risk for negative health-related events, both contribute to each other, are often studied in parallel, and share a unique core condition, which is physical function impairment.33 Sarcopenia is prevalent in CKD and thus contributes to frailty. Indeed, only 11% of the current CKD cohort was non-frail compared to 19% non-frail in the total SPRINT cohort.26 Numerous mechanisms, including inflammation, increased oxidative stress, and mitochondrial dysfunction, are linked to the development and progression of sarcopenia.35,36 FGF23 is also associated with energy molecule signaling37 and mitochondrial function,38 and along with phosphorus is associated with sarcopenia.39,40 Similar to non-CKD older community-dwelling adults and individuals with HIV,14,15 we found a significant and independent relationship between FGF23 levels and prevalent frailty among SPRINT participants with CKD. The relationship was somewhat attenuated by addition of comorbidities and markers of mineral metabolism to our statistical models. While FGF23 may simply be a marker of disease severity, it may also contribute to frailty via effects on muscle strength as suggested by the strong and independent relationship with falls demonstrated in our analysis and the association with slow walking speed in non-CKD older community-dwelling adults.14
The association between elevated FGF23 and falls was not attenuated, and in fact increased slightly in strength, after adjustment for demographics, clinical factors including eGFR and comorbidities, markers of mineral metabolism, and the frailty index. While the pathophysiology of falls is complex, impaired balance related to the ability to efficiently contract lower extremity muscles and muscle weakness, certainly plays a role in fall risk.41 In preclinical studies, FGF23 has been described in energy molecule signaling37 and it reduces reactive oxygen species in skeletal muscle.38 These preclinical studies and our findings support a potential role for FGF23 in muscle function independent of phosphorus, other markers of mineral metabolism, and frailty. Further, FGF23 may play an important role in sensory neurons, specifically nociceptive signaling; FGF23 enhances hyperalgesia in rodent models and inhibition of FGF receptor 1 attenuates allodynia in rats.42,43
The timed 4-meter walk test (gait speed) is a widely used measure of physical performance, which is inversely associated with inflammation,44 atherosclerosis,45 and mortality.46 Low 25(OH)D levels are associated with both proximal and distal muscle weakness, but 25(OH)D supplementation only improves proximal and not distal muscle strength. Therefore, it was hypothesized that inhibition of active vitamin D by FGF23 may more strongly affect proximal muscle strength and walking speed compared to distal muscle and grip strength.47 Indeed, among non-CKD older community-dwelling adults, higher levels of FGF23 were associated with slow gait speed but not grip strength.14 Contrary to this observation, we found that addition of gait speed (i.e., timed 4-meter walk test) to the final model did not attenuate the relationship between FGF23 and falls among SPRINT participants ≥75 years with CKD. In our analysis, gait speed was added to frailty index while among non-CKD older community-dwelling adults gait speed was assessed as an individual component of frailty.
Our study has many strengths including a large cohort of individuals with CKD, standardized data collection, and a precise definition of falls. Despite these and other strengths, our study was limited by the lack of 25(OH)D and 1,25(OH)2D measurements; however, among non-CKD older community-dwelling adults, 25(OH)D did not attenuate the association between FGF23 and frailty.14 We also did not have the ability to adjust for inflammatory markers, which may also contribute to frailty and falls. Inflammation has been found to increase concentrations of inactive fragments of FGF23 in prior analyses, which are captured by “c-terminal” FGF23 assays but not the intact FGF23 assay used here. Thus, the use of the intact FGF23 assay provides an additional strength to our analysis, as it makes residual confounding by inflammatory stress a less likely explanation for our results. In SPRINT, only data on injurious falls were collected as SAEs; as such, participants may have experienced less severe falls that were not recorded. Because frailty was measured at baseline, we were only able to analyze the cross-sectional relationship between FGF23 and frailty, which makes reverse causality a possibility. Finally, individuals with diabetes and proteinuria >1 g/day were excluded from SPRINT and all participants were volunteers in a clinical trial, which limits the generalizability of our findings.
In conclusion, among SPRINT participants with CKD, higher intact FGF23 was significantly associated with prevalent frailty and increased risk of falls. Whether FGF23 is simply a marker of disease severity or contributes to frailty and falls through muscle energy use or through altered vitamin D metabolism requires further investigation. Because FGF23 is independently associated with frailty among both CKD and non-CKD older community-dwelling adults and among individuals with HIV,14,15 it could potentially be used as a marker for frailty in the clinical setting. However, these findings must be confirmed in other CKD and non-CKD cohorts, including individuals with diabetes.
Supplementary Material
1. Supplemental Table 1. Items and scoring system used to calculate the frailty index in SPRINT
2. Supplemental Table 2. Baseline characteristics among SPRINT participants with CKD by fall
3. Supplemental Table 3. Baseline characteristics among SPRINT participants ≥75 years with CKD
4. Supplemental Table 4. Baseline characteristics of SPRINT participants ≥75 years with CKD by fall
5. Supplemental Table 5. Association of FGF23 concentrations and risk of fall among SPRINT participants <75 years with CKD
ACKNOWLEDGEMENTS
Sponsor’s Role
Kidney Tubule Health Ancillary Study (NIDDK R01DK098234), SPRINT (NHLBI, NIDDK, NIA), Anna Jovanovich is supported by VA DCA (5IK2CX0010303-03), Alexandra K. Lee is supported by NIH/NIA T32AG000212. The sponsors did not have any role in the design, methods, data collection, analysis, or preparation of this manuscript.
Conflict of Interest Statement
Anna Jovanovich reports receiving study drug from Shire. Joachim H. Ix is supported by an investigator-initiated research grant from Baxter International.
REFERENCES
- 1.Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of FGF23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 2004;113:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest 2007;117:4003–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukumoto S. Physiological regulation and disorders of phosphate metabolism--pivotal role of fibroblast growth factor 23. Intern Med 2008;47:337–343. [DOI] [PubMed] [Google Scholar]
- 4.Crasto CL, Semba RD, Sun K, Cappola AR, Bandinelli S, Ferrucci L. Relationship of low-circulating “anti-aging” klotho hormone with disability in activities of daily living among older community-dwelling adults. Rejuvenation Res 2012;15:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet 1976;1:626–629. [DOI] [PubMed] [Google Scholar]
- 6.Aono Y, Hasegawa H, Yamazaki Y, et al. Anti-FGF-23 neutralizing antibodies ameliorate muscle weakness and decreased spontaneous movement of Hyp mice. J Bone Miner Res 2011;26:803–810. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr 2017;68:135–142. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez O, Isakova I, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 2005;16:2205–2215. [DOI] [PubMed] [Google Scholar]
- 9.Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK. Low Vitamin D and High Fibroblast Growth Factor 23 Serum Levels Associated with Infectious and Cardiac Deaths in the HEMO Study. J Am Soc Nephrol 2016;27:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011;22:1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008;359:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011;305:2432–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scialla JJ, Xie H, Rahman M, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 2014;25:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beben T, Ix JH, Shlipak MG. Fibroblast growth factor-23 and frailty in elderly community-dwelling individuals: the Cardiovascular Health Study. J Am Geriatr Soc 2016;64:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R, Shlipak MG, Ix JH, et al. Association of fibroblast growth factor-23 (FGF-23) with incident frailty in HIV-infected and HIV-uninfected individuals. J Acquir Immune Defic Syndr 2019;80:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng MH, Chang SF. Frailty as a risk factor for falls among community dwelling people: evidence from a meta-analysis. J Nurs Scholarsh 2017;49:529–536. [DOI] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, Suresh S, Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol 2013;14:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sink KM, Evans GW, Shorr RI, et al. Syncope, hypotension, and falls in the treatment of hypertension: results from the randomized clinical systolic blood pressure intervention trial. J Am Geriatr Soc 2018;66:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 2006;17:3223–3232. [DOI] [PubMed] [Google Scholar]
- 20.Schumock GT, Sprague SM. Clinical and economic burden of fractures in patients with renal osteodystrophy. Clin Nephrol 2007;67:201–208. [DOI] [PubMed] [Google Scholar]
- 21.Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis 2006;1:149–156. [DOI] [PubMed] [Google Scholar]
- 22.SPRINT Research Group. A Randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Maoche D, Dumitrescu CE, Andreopoulou P, et al. Stability and degradation of fibroblast growth factor 23 (FGF23): the effect of time and temperature and assay type. Osteoporos Int 2016;27:2345–2353. [DOI] [PubMed] [Google Scholar]
- 25.TECO Medical Group: Calcium metabolism. Available at: https://www.tecomedical.com/en/laboratory-ivd-kits-reagents/bone-and-cartilage-parameters/calcium-metabolism/FGF-23-Intact-Human-Kainos. Accessed November 1, 2017. [Google Scholar]
- 26.Pajewski NM, Williamson JD, Applegate WB, et al. Characterizing frailty status in the systolic blood pressure intervention trial. J Gerontol A Biol Sci Med Sci 2016;71:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr 2015;60:464–470. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong JJ, Andrew MK, Mitnitski A, Launer LJ, White LR, Rockwood K. Social vulnerability and survival across levels of frailty in the Honolulu-Asia Aging Study. Age Ageing 2015;44:709–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoover M, Rotermann M, Sanmartin C, et al. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep 2013;24:10–17. [PubMed] [Google Scholar]
- 30.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014;11:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roche Diagnostics USA: Cobas 4000 Analyzer Series, 2017. Available at: https://usdiagnostics.roche.com/en/core_laboratory/instrument/cobas-4000-analyzer-series.html#menu. Accessed August 1, 2017.
- 32.Rocco MV, Chapman A, Chertow GM, et al. SPRINT Research Group: Chronic kidney disease classification in systolic blood pressure intervention trial: Comparison using modification of diet in renal disease and CKD-epidemiology collaboration definition. Am J Nephrol 2016;44:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ginsberg C, Craven TE, Chonchol MB, et al. PTH, FGF23, and intensive blood pressure lowering in chronic kidney disease participants in SPRINT. Clin J Am Soc Nephrol 2018;13:1816–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and Physical frailty: Two sides of the same coin. Front Aging Neurosci 2014;6:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moorthi RN, Avin KG. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens 2017;26:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao M, Jaber BL, Balakrishnan VS. Chronic kidney disease and acquired mitochondrial myopathy. Curr Opin Nephrol Hypertens 2018;27:113–120. [DOI] [PubMed] [Google Scholar]
- 37.Glosse P, Feger M, Mutig K, et al. AMP-activated kinase is a regulator of fibroblast growth factor 23 production. Kidney Int 2018;94:491–501. [DOI] [PubMed] [Google Scholar]
- 38.Li DJ, Fu H, Zhao T, Ni M, Shen FM. Exercise-stimulated FGF23 promotes exercise performance via controlling the excess reactive oxygen species production and enhancing mitochondrial function in skeletal muscle. Metabolism 2016;65:747–756. [DOI] [PubMed] [Google Scholar]
- 39.Nakatani T, Sarraj B, Ohnishi M, et al. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (FGF23)-mediated regulation of systemic phosphate homeostasis. FASEB J 2009;23:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.John GB, Cheng CY, Kuro-o M. Role of Klotho in aging, phosphate metabolism, and CKD. Am J Kidney Dis 2011;58:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berry SD, Miller R. Falls: epidemiology, pathophysiology, and relationship to fracture. Curr Osteoporos Rep. 2008;6:249–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujimaki H, Inoue G, Uchida K, et al. Elevation of microglial basic fibroblast growth factor contributes to development of neuropathic pain after spinal nerve ligation in rats. Spine 2016;41:E108-E115. [DOI] [PubMed] [Google Scholar]
- 43.Yamanaka H, Obata K, Kobauashi K, dai Y, Fukuoka T, Nogushi K. Activation of fibroblast growth factor receptor by axotomy, through downstream p38 in dorsal root ganglion, contributes to neuropathic pain. Neuroscience. 2008;150:202–211. [DOI] [PubMed] [Google Scholar]
- 44.Cesarai M, Pennix BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the In CHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. [DOI] [PubMed] [Google Scholar]
- 45.Elbaz A, Ripert M, Tavernier B, et al. Common carotid artery intima-media thickness, carotid plaques, and walking speed. Stroke. 2005;36:2198–2202. [DOI] [PubMed] [Google Scholar]
- 46.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2010;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stockton KA, Mengersen K, Pratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systemic review and meta-analysis. Osteoporos Int 2011;22:859–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. Supplemental Table 1. Items and scoring system used to calculate the frailty index in SPRINT
2. Supplemental Table 2. Baseline characteristics among SPRINT participants with CKD by fall
3. Supplemental Table 3. Baseline characteristics among SPRINT participants ≥75 years with CKD
4. Supplemental Table 4. Baseline characteristics of SPRINT participants ≥75 years with CKD by fall
5. Supplemental Table 5. Association of FGF23 concentrations and risk of fall among SPRINT participants <75 years with CKD