Abstract
The authors tested the hypothesis that low‐salt diet education by nutritionists would lower blood pressure (BP) levels in treated hypertensive patients. The amount of urinary salt excretion and clinic, home, and ambulatory BP values at baseline and at 3 months were measured in 95 patients with hypertension. After randomization to a nutritional education group (E group, n=51) or a control group (C group, n=44), the C group received conventional salt‐restriction education and the E group received intensive nutritional education aimed at salt restriction to 6 g/d by nutritionists. From baseline to the end of the study, 24‐hour urinary sodium excretion was significantly lowered in the E group compared with the C group (6.8±2.9 g/24 h vs 8.6±3.4 g/24 h, P<.01). Morning home systolic BP tended to be lowered in the E group (P=.051), and ambulatory 24‐hour systolic BP was significantly lowered in the E group (−4.5±1.3 mm Hg) compared with the C group (2.8±1.3 mm Hg, P<.001). Intensive nutritional education by nutritionists was shown to be effective in lowering BP in treated hypertensive patients.
The association between excessive salt intake and blood pressure (BP) elevation is well‐known, and some interventional studies such as the International Study of Electrolyte Excretion and Blood Pressure (INTERSALT) demonstrated that the amount of salt intake was associated with BP levels.1 In an international study of 101,945 individuals from 17 countries, it was found that the estimated sodium intake of 3 g/d to 6 g/d was associated with lower incidences of cardiovascular events and death compared with higher or lower levels of salt intake.2 In a study of a mildly hypertensive population, clinic and ambulatory BP levels were significantly lowered by low salt intake compared with those in the control group.3 In the same study, lower salt intake was associated with lower excretion of urinary albumin and a lower pulse wave velocity (a measure of arterial stiffness) compared with those in the control group. Taken together, these findings support the importance of salt restriction for the improved control of BP and protection from end‐organ damage, provided that the salt restriction is successfully performed.
In the 2014 guidelines from the Japanese Society of Hypertension (JSH), salt restriction to <6 g/d is recommended for all hypertensive populations.4 However, this recommendation is mostly based on observational studies5 or interventional studies in which the diets of patients were completely controlled, sometimes under hospitalized conditions.6 For example, in the Dietary Approaches to Stop Hypertension (DASH) trial,7 a low‐salt diet was given to the patients during the study period. Few studies have examined whether intensive nutritional education in an outpatient clinic, especially education on dietary salt restriction, can lower not only clinic BP but also home and ambulatory BP levels. Thus, in the present study, we tested the hypothesis that intensive nutritional education focused on salt restriction and provided by nutritionists in an outpatient clinic lowers clinic, home, and ambulatory BP in treated hypertensive patients.
Methods
This study was a prospective, randomized, and open‐label study. All of the patients were Japanese hypertensives. The study was conducted from September 2012 to May 2014 in the outpatient hypertension clinic of Jichi Medical University Hospital in Japan. The ethics committee of the internal review board at Jichi Medical University, Tochigi, Japan, approved the protocol. Written informed consent was obtained from each patient at the time of enrollment in this study.
The protocol of this study has been registered on the University Hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR) Web site under the trial number UMIN000014935. At the time of the study, all patients were taking antihypertensive treatment including nonpharmacologic treatments (eg, a conventional diet and exercise therapy); 82 patients (86%) had been taking antihypertensive medications and 14% were undergoing only nonpharmacologic treatments. The patients were randomized using a random number table in the research office of Jichi Medical University.
Measurements of the amount of urinary salt excretion and the monitoring of clinic, home, and ambulatory BP values were performed in all patients at baseline. The methods used to estimate urinary salt excretions were based on 24‐hour urine collections; whole urine samples collected in large bottles were used. The self‐measured home BP was taken using a validated upper arm cuff oscillometric device (HEM‐5001; Omron, Kyoto, Japan).8 Home BP was measured in the morning and evening, and is expressed as morning BP, evening BP, and morning‐evening (ME) average BP. Details of the home BP monitoring methods used are described in our recent publication.9 Ambulatory BP monitoring (ABPM) was performed every 30 minutes using a validated monitor with an upper arm cuff as described.10 After being randomized to either the control (C group) or the intensive nutritional education for salt‐restriction group (E group), all patients visited the clinic every 4 weeks for 3 months. At the end of the 3‐month study period, the examinations performed at baseline were repeated.
In the E group, intensive nutritional education was performed by nutritionists a total of five times (each session was 20 minutes or more) at 0, 2, 4, 8, and 12 weeks. All of the nutritionists had national qualifications and had experience providing training in salt restriction. Two nutritionists provided nearly all of the education sessions, and each patient was seen by the same assigned nutritionist, generally. The nutritional education sessions were held at the same time as the patients’ visits to their assigned physicians, except for the second session with the nutritionists, which was performed 2 weeks after baseline. At the time of the first and the fifth nutritional education sessions, each patient completed the Food Frequency Questionnaire (FFQ, version 3.0)11 in addition to a survey to determine their amount of salt intake (salt preference survey).12
The nutritional education sessions were personalized based on the individual results of the questionnaire and survey. The goal of salt restriction was set as no more than 6 g of salt per day based on the JSH 2014 guidelines.4 In the C group, the patients visited the outpatient clinic every 4 weeks, and a conventional nutritional education session was provided by the assigned physicians at every clinic visit. The physicians’ instruction was only a conventional and brief explanation of the causes and consequences of a high‐salt diet. By contrast, intensive nutritional education was performed by nutritionists a total of five times during a 3‐month period. The nutritional education sessions were personalized based on the individual results of the questionnaire and survey. Thus, dietary salt education that was customized to each patient was provided. A physician's counsel during each clinical visit was not likely to provide as much information and personalized attention as these five sessions. The participants were also instructed to not alter their physical activities, including daily exercise.
The use of diuretics was more frequent in the C group than the E group (although the difference was not significant), and the C group had taken significantly more oral antihypertensive drugs compared with the E group. These differences seem to be due to chance, because the patients were randomized using a random number table in the research office of Jichi Medical University, but the data regarding the use of antihypertensive drugs were not stratified at this randomization.
Statistical Analyses
All statistical analyses were performed with SPSS software, version 19.0 (IBM, Armonk, NY, USA). Data are expressed as mean±standard deviation (SD) or percentage. The chi‐square test was used to evaluate differences in prevalence rates. Unpaired t tests were used to compare mean values between the C and E groups, and paired t tests were used to test within‐group temporal changes in mean values. The extent of changes in BP was compared using the general linear model adjusting for significant covariates. Differences with a P value <.05 (two‐tailed) were considered significant.
Results
The initial enrollment in the study was 101 hypertensive patients, and a total of 95 completed the protocol. The flow chart of the study is shown in Figure. The mean age was 58.7±13.4 years (range 26–87 years), and the sex distribution was 59 women/36 men. The baseline characteristics of the patients in the E group (n=51) and C group (n=44) are shown in Table 1. There were no significant differences in baseline characteristics between the E and C groups except for the significantly higher percentages of calcium channel blockers and α‐blockers in the C group compared with the E group.
Figure 1.
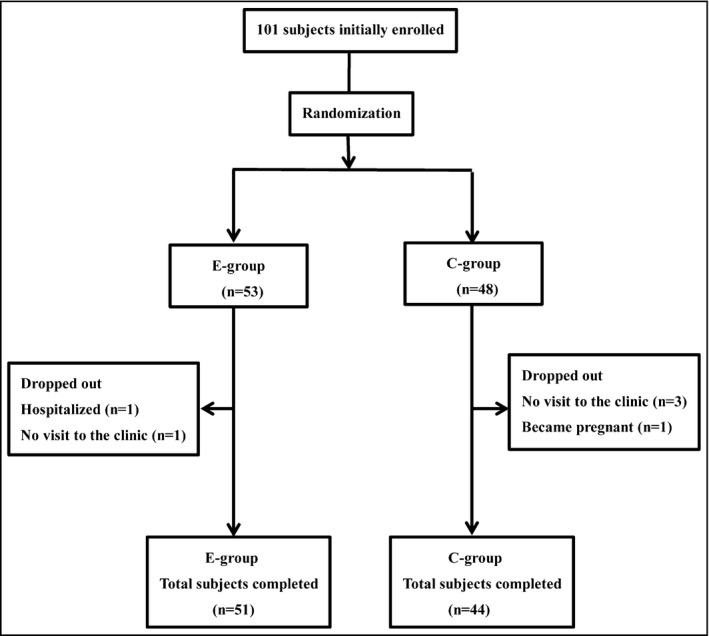
Flow chart of the study patients. E group indicates nutritional education group; C group, control group.
Table 1.
Baseline Characteristics of the Hypertensive Patients
| E Group (n=51) | C Group (n=44) | P Value | |
|---|---|---|---|
| Men/women, No. (%) | 16/35 (31/69) | 20/24 (45/55) | .16 |
| Age, y | 57.5±13.7 | 60.1±13.1 | .35 |
| Body mass index, kg/m2 | 25.2±3.9 | 25.1±3.7 | .87 |
| Body weight, kg | 63.9±12.6 | 64.8±10.1 | .70 |
| Diabetes mellitus, No. (%) | 3 (6) | 3 (7) | .85 |
| Chronic kidney disease, No. (%) | 10 (20) | 9 (20) | .92 |
| Myocardial infarction, No. (%) | 0 (0) | 1 (2) | .28 |
| Antihyperlipidemic drugs, No. (%) | 8 (16) | 12 (27) | .17 |
| Antihyperglycemic drugs, No. (%) | 0 (0) | 3 (7) | .06 |
| Antihypertensive medications, No. (%) | |||
| Diuretics | 9 (18) | 15 (34) | .07 |
| Calcium channel blockers | 25 (49) | 33 (75) | .01 |
| ARBs | 25 (49) | 24 (55) | .60 |
| ACE inhibitors | 2 (4) | 5 (11) | .17 |
| β‐Blockers | 5 (10) | 4 (9) | .91 |
| α‐Blockers | 1 (2) | 7 (16) | .02 |
| α‐β‐blockers | 2 (4) | 2 (5) | .88 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARBs, angiotensin II receptor blockers; C group, control group; E group, nutritional education group. Data are expressed as mean±standard deviation or number (percentage).
The patients’ estimated salt excretion and laboratory test results at baseline and 12 weeks are shown in Table 2. At baseline, the urinary salt excretions assessed using 24‐hour urine samples were similar between the groups. At the end of the study period, the urinary salt excretion assessed by 24‐hour urine was significantly lower in the E group compared with the C group (P<.01). The extent of the changes in 24‐hour urinary salt excretion from baseline were significantly larger in the E group compared with the C group (−1.8±3.8 vs 0.5±3.3 g/24 h, P<.01), respectively. The changes in the other laboratory parameters were similar between the groups except for a minor difference in the alanine aminotransferase and glycated hemoglobin levels.
Table 2.
Laboratory Test Results and Body Weight at Baseline and 12 Weeks
| Week 0 | Week 12 | P Value | ||
|---|---|---|---|---|
| 24‐Hour urine NaCl, g/24 h | E group | 8.6±3.2 | 6.8±2.9a | .002 |
| C group | 8.1±2.9 | 8.6±3.4 | .34 | |
| 24‐Hour urine K, g/24 | E group | 1.8±0.7 | 1.6±0.5 | .02 |
| C group | 1.7±0.6 | 1.5±0.6 | .04 | |
| Body weight, kg | E group | 63.9±12.6 | 63.7±12.6 | .32 |
| C group | 64.8±10.1 | 65.2±10.9 | .41 | |
| RBC, ×104/μL | E group | 435±41.5 | 432±38.0 | .21 |
| C group | 441±40.1 | 443±37.8 | .70 | |
| WBC, /mm3 | E group | 5435±1468 | 5486±1413 | .76 |
| C group | 6013±1687 | 5968±1632 | .82 | |
| Hemoglobin, g/dL | E group | 13.4±1.5 | 13.3±1.3 | .27 |
| C group | 13.7±1.3 | 13.7±1.2 | .98 | |
| Total cholesterol, mg/dL | E group | 199±31 | 192±37 | .18 |
| C group | 198±28 | 201±31 | .42 | |
| LDL cholesterol, mg/dL | E group | 116±25 | 114±23 | .57 |
| C group | 115±26 | 119±32 | .22 | |
| Glycated hemoglobin, % | E group | 5.3±0.4 | 5.4±0.4 | .006 |
| C group | 5.4±0.6 | 5.5±0.6 | <.001 | |
| BUN, mg/dL | E group | 15±4 | 15±4 | .64 |
| C group | 16±12 | 14±4 | .24 | |
| Creatinine, mg/dL | E group | 0.73±0.28 | 0.74±0.25 | .31 |
| C group | 0.73±0.27 | 0.76±0.26 | .19 | |
| eGFR, mL/min per 1.73 m2 | E group | 77.2±21.4 | 75.1±19.7 | .06 |
| C group | 77.0±19.7 | 76.5±20.9 | .74 | |
| Na, mEq/L | E group | 142±2 | 141±2 | .43 |
| C group | 142±2 | 142±2 | .57 | |
| K, mEq/L | E group | 4.3±0.4 | 4.2±0.4 | .56 |
| C group | 4.2±0.4 | 4.2±0.4 | .44 | |
| AST, U/L | E group | 22±7 | 21±6 | .015 |
| C group | 22±8 | 23±11 | .49 | |
| ALT, U/L | E group | 25±14 | 21±11 | .003 |
| C group | 21±11 | 22±14 | .31 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, serum urea nitrogen; C group, control group; eGFR, estimated glomerular filtration rate; E group, nutritional education group; K, potassium; LDL, low‐density lipoprotein; Na, sodium; NaCl, sodium chloride; RBC, red blood cell count; WBC, white blood cell count. Data are expressed as mean±standard deviation. a P<.01 vs data in the C group at 12 weeks.
The BP parameters at baseline and at the 12th week are shown in Table 3. The baseline clinic, home, and ambulatory BP values were similar between the groups except for the E group's significantly higher values of awake SBP (139±13 mm Hg vs 133±11 mm Hg, P=.017) and 24‐hour SBP (134±12 mm Hg vs 128±11 mm Hg, P=.013) and lower values of home evening pulse rate (67±9 beats per min [bpm] vs 72±11 bpm, P=.04) and ambulatory morning pulse rate (71±11 vs 76±12 bpm, P=.04) compared with the C group.
Table 3.
BP and PR at Baseline and 12 Weeks
| Week 0 | Week 12 | P Value | ||
|---|---|---|---|---|
| Clinic BP | ||||
| SBP, mm Hg | E group | 136±12 | 132±14 | .048 |
| C group | 135±14 | 136±12 | .54 | |
| DBP, mm Hg | E group | 83±10 | 82±9 | .34 |
| C group | 82±12 | 83±11 | .59 | |
| PR, beats per min | E group | 71±10 | 71±10 | .79 |
| C group | 76±12 | 73±12 | .07 | |
| Home BP | ||||
| Morning SBP, mm Hg | E group | 134±9 | 130±12 | .006 |
| C group | 134±11 | 133±12 | .83 | |
| Morning DBP, mm Hg | E group | 80±8 | 79±8 | .13 |
| C group | 80±10 | 80±11 | .86 | |
| Morning PR, beats per min | E group | 66±9 | 66±9 | .72 |
| C group | 70±10 | 69±12 | .35 | |
| Evening SBP, mm Hg | E group | 129±10 | 126±12 | .09 |
| C group | 128±12 | 130±15 | .45 | |
| Evening DBP, mm Hg | E group | 76±8 | 75±8 | .48 |
| C group | 74±9 | 75±12 | .27 | |
| Evening PR, beats per min | E group | 67±9a | 68±9 | .18 |
| C group | 72±11 | 70±10 | .08 | |
| ME average SBP, mm Hg | E group | 131±9 | 128±11 | .012 |
| C group | 131±11 | 131±12 | .68 | |
| ME average DBP, mm Hg | E group | 78±8 | 77±7 | .24 |
| C group | 77±10 | 77±11 | .52 | |
| ME average PR, beats per min | E group | 67±8a | 67±9 | .37 |
| C group | 71±10 | 69±10 | .06 | |
| Ambulatory BP | ||||
| 24‐Hour SBP, mm Hg | E group | 134±12a | 129±11 | .001 |
| C group | 128±11 | 130±11 | .04 | |
| 24‐Hour DBP, mm Hg | E group | 81±9 | 79±8 | .01 |
| C group | 78±9 | 79±8 | .11 | |
| 24‐Hour PR, beats per min | E group | 71±8 | 71±8 | .60 |
| C group | 74±10 | 73±12 | .86 | |
| Morning SBP, mm Hg | E group | 141±13 | 134±13 | .01 |
| C group | 137±17 | 138±18 | .60 | |
| Morning DBP, mm Hg | E group | 86±9 | 84±9 | .13 |
| C group | 84±14 | 85±12 | .90 | |
| Morning PR, beats per min | E group | 71±11a | 73±9 | .18 |
| C group | 76±12 | 72±11 | .048 | |
| Awake SBP, mm Hg | E group | 139±13a | 134±12 | .002 |
| C group | 133±11 | 135±11 | .051 | |
| Awake DBP, mm Hg | E group | 84±9 | 82±8 | .02 |
| C group | 81±9 | 83±9 | .17 | |
| Awake PR, beats per min | E group | 74±9 | 74±9 | .44 |
| C group | 77±10 | 77±11 | .48 | |
| Sleep SBP, mm Hg | E group | 120±13 | 118±13 | .12 |
| C group | 116±13 | 119±12 | .048 | |
| Sleep DBP, mm Hg | E group | 73±10 | 71±8 | .20 |
| C group | 70±10 | 72±9 | .08 | |
| Sleep PR, beats per min | E group | 62±8 | 62±7 | .60 |
| C group | 65±10 | 62±10 | .011 | |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; ME, morning‐evening; PR, pulse rate; SBP, systolic blood pressure. Data are expressed as mean±standard deviation. a P<.05 vs data in the C group at 0 week.
In terms of the changes in clinic SBP values between the groups, the E group tended to show greater clinic SBP reductions compared with the C group (−3.6±1.8 mm Hg vs 1.1±1.8 mm Hg, P=.066). With regard to home BP, the E group also tended to show greater morning SBP reductions compared with the C group but not significantly: −3.5±1.2 mm Hg vs −0.2±1.1 mm Hg (P=.051).
Our comparison of the changes in ambulatory BP parameters revealed that the E group's ambulatory 24‐hour (−4.5±1.3 mm Hg vs 2.8±1.3 mm Hg, P<.001), awake (−5.0±1.5 mm Hg vs 2.8±1.4 mm Hg, P<.001), and morning SBP (−6.1±2.3 mm Hg vs 1.6±3.0 mm Hg, P=.04) values were significantly more reduced compared with the C group. Regarding DBP values, the 24‐hour DBP (−2.2±0.9 mm Hg vs 1.3±0.8 mm Hg, P=.003) and awake DBP (−2.1±0.9 mm Hg vs 1.2±0.9 mm Hg, P=.08) values tended to be lower in the E group compared with the C group, but the extent of the changes in sleep SBP and DBP were not significantly different between the two groups.
Because the baseline BP values were somewhat different between the groups, we compared the extent of the changes in BP by adjusting for the baseline BP values using the general linear model. The changes in BP before and after corrections are shown in Table 4. Even after adjusting for the baseline values, the ambulatory 24‐hour and awake SBP were significantly more reduced in the E group compared with the C group.
Table 4.
Changes in BP Before and After Corrections
| E Group | C Group | P Value | |
|---|---|---|---|
| Ambulatory 24‐hour SBP | |||
| Before corrected changes of BP, mm Hga | −4.5±1.3 | 2.8±1.3 | <.001 |
| After corrected changes of BP, mm Hg | −3.5±1.2 | 1.7±1.3 | <.01 |
| Ambulatory awake SBP | |||
| Before corrected changes of BP, mm Hgb | −5.0±1.5 | 2.8±1.4 | <.001 |
| After corrected changes of BP, mm Hg | −4.0±1.3 | 1.6±1.4 | <.01 |
Abbreviation: BP, blood pressure. Data are expressed as mean±standard error of the mean. aAdjusted by ambulatory 24‐hour systolic blood pressure (SBP) at baseline. bAdjusted by ambulatory awake SBP at baseline.
When we analyzed the patients in the E group separately by the use of diuretics, we observed that the patients with no diuretic use at baseline tended to show reductions in 24‐hour SBP to a greater extent than those who used diuretics (Table S1). This result suggests that the patients using diuretics are not always salt‐sensitive and that other reasons for the diuretic prescriptions, such as leg edema, are possible. Therefore, no further BP reduction was seen in the patients taking diuretics. However, the number of patients taking diuretics was small (n=9), and further research is needed on this issue.
As shown in Table S2, the patients in the E group who reached the 6 g/d salt intake goal presented significantly greater BP reductions than those who did not reach this goal.
Discussion
In the present study, intensive salt‐restriction education was effective in lowering ambulatory BP levels in treated hypertensive patients. This is the first study to show the favorable effect of dietary salt restriction by nutritional education on ambulatory BP in treated hypertensive patients.
Salt Restriction and BP
We observed that intensive salt‐restriction education by nutritionists was associated with a significant antihypertensive effect. To the best of our knowledge, there has been no such study evaluating home and ambulatory BP values in relation to the effect of salt‐restriction education at an outpatient clinic. Besides the JSH 2014 guideline, international hypertension guidelines reported that reduced salt intake is fundamental for the treatment of hypertension.13, 14 The effect of salt restriction on BP‐lowering in hypertensive patients has been reported previously15; in fact, a total of seven interventional studies have looked at the effect of salt restriction on BP lowering3, 7, 16, 17, 18, 19, 20 (Table S3). In real‐world settings, in which it may not always be easy to eat a low‐salt diet, it is crucially important for individual patients to recognize the importance of salt restriction on their health. In addition, none of the above studies showed that salt‐restriction education by nutritionists resulted in successful BP reduction in hypertensive patients, and thus the present study is the first to demonstrate the importance and effectiveness of patient education for the management of hypertension.
According to a meta‐analysis of studies of Asian hypertensive populations,15 the reduction of the amount of urinary salt was −4.0 g and the corresponding SBP/DBP reductions were −5.41/−2.17 mm Hg. Our present findings, ie, −1.8 g of urinary salt reduction, −5/−2 mm Hg reduction of 24‐hour BP, and −4/−1 mm Hg reduction of clinic BP, thus seem reasonable.
According to the results of the salt preference survey in the present study, miso soup and soy sauce were the patients’ major sources of salt intake, which is expected because the patients were Japanese. The salt‐restriction education was thus designed to target these components. The salt intake differed among the individual patients, but since customized education sessions were provided, we suggest that it was the behavioral modification regarding salt restriction that resulted in the patients’ successful BP reduction.
In the present study, in contrast to the conventional education by doctors, salt‐restriction education by nutritionists was shown to alter the behavior of the participants, based on the actual data of their salt intake. In most of the previous studies, a crossover design was used to evaluate the effect of salt restriction on BP. However, we felt that a crossover design was not desirable for the present study because nutritional education directly intervenes in the patients’ lifestyle and the effects can carry over.
We contend that the results of our study can be applied for nonhypertensive individuals such as those with prehypertension and those with a history of cardiovascular disease. Indeed, when we analyzed only the patients with baseline 24‐hour BP <130/80 mm Hg, the extent of BP reduction was significantly higher in the E group than the C group (Table S4). In other words, the increase in 24‐hour BP seen in the C group was suppressed in the E group. Therefore, even in the normotensive range, a BP‐lowering effect of salt‐restriction education can be expected.
We propose that salt‐restriction education by a nutritionist should be provided for all hypertensive patients––especially high‐risk patients such as those with drug‐resistant hypertension, cardiovascular complications, and chronic kidney disease (CKD). In addition, salt‐restriction education is cost‐effective because it can reduce the use of hypertensive drugs and lower the rate of cardiovascular events.
Regarding the validity of the results of the present study, we believe that our findings are applicable to other cultures and societies. In any group of patients, the individuals’ salt preferences and the corresponding amount of salt intake can be analyzed by a questionnaire, and salt‐restriction education can be performed based on the data obtained. Salt‐restriction education can thus be performed by modifying the questionnaire according to the food culture of the population being investigated.
Clinic, Home, and Ambulatory BP
Although the changes in home BP were marginally significant, the significant effect of the salt‐restriction education on the patients’ ambulatory BP highlights the real and robust antihypertensive effect of this education. ABPM has been shown to be the most accurate tool for the evaluation of drug efficacy. The JSH hypertension guidelines specifically emphasize that ABPM detects smaller BP changes than clinic BP; furthermore, ABPM can be used for the evaluation of nonpharmacologic antihypertensive therapy (ie, lifestyle adjustment).
In the E group at 12 weeks, ambulatory morning SBP, awake SBP, and awake DBP were significantly reduced compared with the C group. Sleep SBP and DBP in the E group were not significantly changed. There are two possible explanations for the finding that morning and awake BP but not sleep BP were reduced by salt restriction. Because the patients were relatively young, the awake BP level remained high, reflecting the higher daytime activity level expected in younger patients. However, the elevated diurnal BP level was more subject to age‐independent control. Second, the sleep BP level was lower than the awake BP level (ie, nondippers accounted for only 31% of the total patients), reflecting the low prevalence of diabetes and CKD. The well‐known finding that salt restriction converts nondipper‐type patients to dipper‐type hypertensive patients was reported in patients with diabetes or CKD.21
Reached the Goal of 6 g/d
Japanese people are well‐known for their high salt intake. In comparison with prior studies that provided a low‐salt diet to patients during the study period, in the present study we attempted to correct the patients’ high‐salt‐intake lifestyle by providing nutritional education. However, in their daily lives, although almost half of the participants attained the salt‐restriction goal, the other half did not. In the E group, the individuals who did not reach the goal of 6 g/d tended to have higher BMI values compared with those who did achieve the goal. We speculate that individuals with large body sizes may eat more food and thus consume more salt even when they are attempting to follow a low‐salt diet.
Effect of K Intake
We also observed that the amount of the urinary potassium (K) excretions assessed with 24‐hour urine samples at baseline was not different between the groups, and the K excretion in the urine after 12 weeks was not increased in either group. Therefore, although it is known that K‐rich food (eg, the DASH diet is not only rich in K but in alkaline) can reduce BP levels,22 in the present study it is unlikely that K contributed to the significant antihypertensive effects in the E group.23
Effect of Body Weight
As shown in Table 2, body weight was not significantly changed between baseline and the 12th week in any of the participants. It is well‐known that body weight is one of the important determinants of BP levels, and body weight reduction is an essential strategy for the management of hypertension. However, because the present study period was relatively short, the patients’ body weights were not changed by the salt‐restriction training given by the nutritionists, and thus although the significant antihypertensive effect in the E group was clearly related to salt restriction, it was unlikely to be related to calorie restriction per se.
Duration of the Effect of the Salt‐Restriction Intervention
We do not know how long the effect of the salt‐restriction intervention will continue. At 6 months after the end of this study, we checked all 51 of the individuals in the E group and found that none had experienced a CV event, but the E group's clinic SBP values at 6 months after the end of the study were significantly higher compared with those at the end of the study (Table S5). However, in 10 of the 51 patients (19.6%), the clinic SBP was still reduced at 6 months post‐study compared with that at the end of the study. We think that in similar future studies it will be necessary to monitor the patients and continue the salt‐restriction education at infrequent intervals (eg, 6 months).
Study Limitations
There were some notable limitations in this study. First, the number of patients was small. With a greater number of participants, a significant difference may have appeared in clinic BP values. According to our retrospective power analysis using the present clinic BP difference and the SD, 142 patients in each group are needed. Since there were relatively small numbers of patients (E group, n=51; C group, n=44), a clear dose‐response relationship between the amount of salt restriction and the BP‐lowering effect was not observed; however, as shown above, the patients who reached the goal of 6 g/d salt intake demonstrated significantly greater BP reductions than those who did not reach this goal.
Second, there is a possibility that visits to the nutritionists were more frequent in the E group compared with the C group. However, it is difficult to establish two truly equal groups, both of which have equal numbers of opportunities to visit nutritionists. Third, the Hawthorne effect cannot be denied. As mentioned above, the E group's clinic SBP values at 6 months after the end of the study were significantly higher than those at the end of the study, although 10 of these patients showed reduced clinic SBP at 6 months post‐study. Fourth, since our participants were much leaner than western populations, body weight reduction was not always necessary to achieve the beneficial effect of salt restriction. We did not measure bioimpedance in this study. Finally, the use of diuretics was more frequent in the C group than the E group, but the prescriptions of diuretics depended on the assigned physicians’ discretion, and salt sensitivity was not taken into account in the randomization. Similarly, the C group patients were more likely to be using oral antihypertensive drugs compared with the E group. This seems to be by chance since the patients were randomized using a random number table, but antihypertensive drug use was not stratified at randomization.
Conclusions
In treated hypertensive patients, 3‐month intensive salt‐restriction education by nutritionists resulted in a lowering of 24‐hour urinary salt excretions and the measures of ambulatory BP. Even within this short period of intervention, the significant effect of salt reduction was confirmed. These findings suggest the importance of nutritional education in hypertensive patients for the management of hypertension.
Supporting information
Table S1. Changes in ambulatory SBP among the E group patients using or not using prescription diuretics.
Table S2. Changes in ambulatory SBP in the E group among the patients who reached or did not reach the goal of <6 g/d salt intake.
Table S3. Trials regarding the effect of salt reduction on blood pressure.
Table S4. Changes in the 24‐hour blood pressure among the E and C group patients with baseline BP ≤130/80 mm Hg.
Table S5. Clinic blood pressure at the end of the study and 6 months later in the E group.
Acknowledgments and disclosures
We thank Kimiyo Saito, Hideko Taguchi, and Kaori Kobayashi for the coordination and data management of this study; Ayako Okura for editorial assistance; and Yukiko Arakawa and Miho Murakoshi for assisting with the nutritional education. The first author (M.N.) and second author (K.E.) contributed equally to the writing process. The authors have no conflicts of interest to report.
J Clin Hypertens (Greenwich). 2016;18:385–392. 10.1111/jch.12770. © 2016 Wiley Periodicals, Inc.
References
- 1. INTERSALT Cooperative Research Group . INTERSALT: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ. 1998;294:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. [DOI] [PubMed] [Google Scholar]
- 3. He FJ, Marciniak M, Visagie E, et al. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension. 2009;54:482–488. [DOI] [PubMed] [Google Scholar]
- 4. Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension . The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37:253–390. [DOI] [PubMed] [Google Scholar]
- 5. MacGregor GA, Markandu ND, Sagnella GA, et al. Double‐blind study of three sodium intakes and long‐term effects of sodium restriction in essential hypertension. Lancet. 1989;2:1244–1247. [DOI] [PubMed] [Google Scholar]
- 6. Richards AM, Nicholls MG, Espiner EA, et al. Blood‐pressure response to moderate sodium restriction and to potassium supplementation in mild essential hypertension. Lancet. 1984;1:757–761. [DOI] [PubMed] [Google Scholar]
- 7. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH‐Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 8. Anwar YA, Giacco S, McCabe EJ, et al. Evaluation of the efficacy of the Omron HEM‐737 IntelliSense device for use on adults according to the recommendations of the Association for the Advancement of Medical Instrumentation. Blood Press Monit. 1998;3:261–265. [PubMed] [Google Scholar]
- 9. Hoshide S, Kario K, Yano Y, et al. Association of morning and evening blood pressure at home with asymptomatic organ damage in the J‐HOP Study. Am J Hypertens. 2014;27:939–947. [DOI] [PubMed] [Google Scholar]
- 10. Eguchi K, Hoshide S, Ishikawa S, et al. Aggressive blood pressure‐lowering therapy guided by home blood pressure monitoring improves target organ damage in hypertensive patients with type 2 diabetes/prediabetes. J Clin Hypertens (Greenwich). 2012;14:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takahashi K, Yoshimura Y, Kaimoto T, et al. Validation of food frequency questionnaire based on food groups for estimating individual nutrient intake. Jpn J Nutr. 2001;59:221–232. In Japanese. [Google Scholar]
- 12. Arakawa K, Matsushita Y, Matsuo H, et al. Examination of the efficiency of salt taste preference questionnaire in hypertensive patients. J Clin Ther Med. 2009;25:723–734. [Google Scholar]
- 13. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 14. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 15. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta‐analysis of randomised trials. BMJ. 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 16. Cappuccio FP, Markandu ND, Carney C, et al. Double‐blind randomised trial of modest salt restriction in older people. Lancet. 1997;350:850–854. [DOI] [PubMed] [Google Scholar]
- 17. Meland E, Laerum E, Aakvaag A, et al. Salt restriction: effects on lipids and insulin production in hypertensive patients. Scand J Clin Lab Invest 1997;57:501–505. [DOI] [PubMed] [Google Scholar]
- 18. Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41. [DOI] [PubMed] [Google Scholar]
- 19. Swift PA, Markandu ND, Sagnella GA, et al. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: a randomized control trial. Hypertension. 2005;46:308–312. [DOI] [PubMed] [Google Scholar]
- 20. Melander O, von Wowern F, Frandsen E, et al. Moderate salt restriction effectively lowers blood pressure and degree of salt sensitivity is related to baseline concentration of renin and N‐terminal atrial natriuretic peptide in plasma. J Hypertens. 2007;25:619–627. [DOI] [PubMed] [Google Scholar]
- 21. Uzu T, Ishikawa K, Fujii T, et al. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1997;96:1859–1862. [DOI] [PubMed] [Google Scholar]
- 22. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 23. Appel LJ, Brands MW, Daniels SR, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006;47:296–308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Changes in ambulatory SBP among the E group patients using or not using prescription diuretics.
Table S2. Changes in ambulatory SBP in the E group among the patients who reached or did not reach the goal of <6 g/d salt intake.
Table S3. Trials regarding the effect of salt reduction on blood pressure.
Table S4. Changes in the 24‐hour blood pressure among the E and C group patients with baseline BP ≤130/80 mm Hg.
Table S5. Clinic blood pressure at the end of the study and 6 months later in the E group.


