Abstract
The authors aimed to quantify end‐digit and threshold biases in blood pressure (BP) measurement with manual and digital sphygmomanometers. In a 3‐year follow‐up, end‐digit and threshold biases were investigated and a new index, called the deviation index, was used to quantify measurement bias. The distribution of systolic and diastolic BPs became close to normal after implementation of digital sphygmomanometers. The appearance of zero end digits decreased from 97% to 30% (P<.0001). The deviation index decreased from 97% to 20% (P<.0001). Mean systolic and diastolic BPs increased immediately after implementation of automated sphygmomanometers (124.22±0.83 vs 132.90±0.78 and 74.38±0.50 vs 80.43±0.51, respectively; P<.0001 for both) but showed a linear decreasing trend during follow‐up (systolic −3.59 mm Hg per year; 95% confidence interval, −5.57 to −1.61 [P<.0001]; diastolic: −2.52 mm Hg per year; 95% confidence interval, −3.78 to −1.26 [P<.0001]). Threshold bias decreased from 12.94% to 6.68% (P<.0001). Replacing manual sphygmomanometers with digital devices decreased end‐digit and threshold biases in BP measurement. The deviation index can be used to quantify the magnitude of measurement bias.
Blood pressure (BP) is recorded in nearly each medical visit. Various kinds of error could arise in this process, originating from uncalibrated devices, improper technique, or examiner error.1, 2 Rounding BP values to the nearest 10 mm Hg is a known source of bias in recording BP in the clinical setting.3, 4, 5 This is called end‐digit bias and leads to the appearance of zeros as end digits more frequently than would be expected by chance alone. Another kind of bias is threshold bias, defined as selection of particular values near treatment cut‐offs.6 Introduction of automated digital sphygmomanometers have made a difference in proper BP measurement and may help decrease both end‐digit and threshold biases.7 While monitoring for quality assurance is not routine in general practice, several studies have focused on how and when a flawed BP recording system should be suspected in the clinical setting.
From a statistical perspective, we propose a simple index to assess the validity of BP measurement systems. In this study, we investigated a quantitative method by which one could examine the degree of deviation of recorded BP measurements from expected values. We then compared manual and automated sphygmomanometers in terms of end‐digit and threshold biases in a real clinical setting and applied our index to quantify the magnitude of end‐digit bias in this context.
Methods
Study Design
We propose a 5‐year historical cohort in which two methods of BP measurements were compared in terms of end‐digit rounding error, and a “deviation index” was applied in a real‐world clinical setting. Systolic and diastolic BP recordings were investigated in an outpatient diabetes clinic in Vali‐Asr Hospital, which is affiliated with Tehran University of Medical Sciences, from 2009 to 2013. In 2009 and 2010, BP measurements were performed with standard mercury auscultatory sphygmomanometers by trained nurses. Data during these 2 years were retrospectively gathered from patients' documents. In 2011, mercury sphygmomanometers were replaced with automated BP measurement devices (Omron M7 HEM‐780‐E, Kyoto, Japan; approved by the British Hypertension Society). The same nurses were trained to use the new devices. The prospective phase of this study began in 2011 and lasted for 3 years.
Training of Operators
Operators (nurses) were trained according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) guidelines for appropriate BP measurement.8 The appropriate technique for BP measurement (according to JNC 7 guidelines) was demonstrated. Nurses were tested for the standardized technique by an internist to review the method of BP measurement. Adherence to the suggested method was periodically (at 1‐month periods) re‐evaluated. At the time of implementation of automated sphygmomanometers, the operators were trained in the appropriate method for using the automated devices.
BP Measurement
BP measurements were performed by the same nurses and immediately before a medical visit with a physician. BP recordings were used for medical decision‐making and patients were qualified as normotensive or hypertensive according to the recorded values. Measurements were taken while the patient was seated quietly for at least 5 minutes in a chair with feet on the floor and arm supported at heart level. A cuff with a bladder that encircled at least 80% of the arm circumference was chosen for each measurement. Measurements were made in the right arm while the patient was in the sitting position. Two measurements were made at each visit. Demographic, anthropometric, clinical, and biochemical values were recorded.
Deviation Index
End‐digit bias was quantified with an error tool called the deviation index. We assumed that in an unbiased BP measurement system, the frequency of reporting each end digit is 1/10. The deviation index yields the percent deviation of recorded BP values from this expectation. In the case of our study, we wanted to check the amount of zero‐rounding end‐digit bias; therefore, the proposed formula was:
In which “|BP – nearest 10 mm Hg number|” gives the absolute difference of each BP from its nearest 10 mm Hg rounded number. Theoretical basis and assumptions behind this formula are presented in Appendix S1.
Ethics
The local ethics review committee of Tehran University of Medical Sciences approved the study protocol (ethics committee approval number: 139073021) and the study complied with the principles of the Declaration of Helsinki.
Statistical Analysis
Continuous variables are presented as mean±standard error of the mean. Categorical variables are presented as number and percent. Kolmogorov‐Smirnov (K‐S) test was used to test the normality of distribution. Chi‐square, independent samples t, and analysis of variance tests were used for group comparisons. Linear regression was used for data modeling. A P value <.01 was considered statistically significant. The Stata (version 11.2 for Windows; Stata Corp, College Station, TX) program was used for data analysis.
Results
Study Population
Baseline characteristics of participants are presented in Table, stratified into retrospective (manual sphygmomanometer) and prospective (automated sphygmomanometer) arms of the study population. A total of 1366 patients were studied, including 587 in the manual group and 779 in the automated sphygmomanometer group. Except for antihypertensive drugs (P<.0001), no measured variable was significantly different between the two groups.
Table 1.
Baseline Characteristics of Study Population
| Manual Device | Automated Device | P Value | |
|---|---|---|---|
| Female, No. (%) | 347 (59.2) | 446 (61.3) | .48 |
| Age, y | 56.14±0.45 | 55.34±0.54 | .25 |
| Weight, kg | 74.71±0.57 | 73.39±0.62 | .12 |
| BMI, kg/m2 | 27.86±0.19 | 27.96±0.23 | .74 |
| Waist, cm | 95.34±0.51 | 95.24±0.56 | .89 |
| Duration of diabetes, y | 8.19±0.30 | 7.76±0.32 | .33 |
| FBS, mg/dL | 178.26±2.83 | 187.69±4.12 | .05 |
| PPG, mg/dL | 255.27±4.96 | 278.66±9.51 | .02 |
| HbA1c, % | 8.32±0.08 | 8.43±0.12 | .49 |
| Triglycerides, mg/dL | 180.96±4.99 | 187.70±7.99 | .45 |
| Total cholesterol, mg/dL | 183.10±2.07 | 187.33±2.95 | .23 |
| LDL‐C, mg/dL | 103.32±1.82 | 103.33±2.46 | .99 |
| HDL‐C, mg/dL | 44.30±0.59 | 44.94±1.09 | .57 |
| Uric acid, mg/dL | 4.88±0.12 | 5.09±0.23 | .41 |
| Urea, mg/dL | 31.54±0.64 | 29.88±0.81 | .12 |
| Creatinine, mg/dL | 1.00±0.02 | 1.01±0.02 | .89 |
| Microalbuminuria, mg/24 h | 8.41±0.7 | 8.33±1.13 | .90 |
| eGFR, mL/min | 75.03±1.03 | 73.40±1.25 | .33 |
| AST, U/L | 23.52±0.93 | 23.93±1.59 | .81 |
| ALT, U/L | 25.39±0.86 | 27.75±2.49 | .26 |
| TSH | 3.1±0.69 | 3.3±0.65 | .85 |
| Myocardial infarction, % | 5.6 | 5.8 | .92 |
| Glucose‐lowering drug, % | |||
| Oral hypoglycemic drug | 79.2 | 77 | .42 |
| Insulin | 20.8 | 23 | |
| Blood pressure drug, % | |||
| ACE inhibitor | 23.6 | 28.4 | <.0001 |
| ARB | 33.4 | 48.2 | |
| β‐Blocker | 21.4 | 13.7 | |
| CCB | 0.8 | 4.3 | |
| Combined | 20.5 | 5.1 | |
Abbreviations: ACE, angiotensin‐converting enzyme; ALT, alanine aminotransferase; ARB, aldosterone receptor blocker; AST, aspartate aminotransferase; BMI, body mass index; CCB, calcium channel blocker; eGFR, estimated glomerular filtration rate; FBS, fasting blood sugar; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; PPG, post‐prandial glucose; TSH, thyroid‐stimulating hormone.
BP Distribution
Scatter plots of systolic BP (SBP) and diastolic BP (DBP) are represented in Figure 1. For both SBP and DBP, the recorded values mainly gathered around 10 mm Hg rounded numbers in the manual group (before the dash line), while they scattered uniformly in the automated group (after the dash line). Variance of SBP and DBP increased from 399.20 mm Hg2 and 123.21 mm Hg2 in the manual group to 469.11 mm Hg2 and 207.01 mm Hg2 in the automated group, respectively. The distribution of SBP and DBP was not normal in the manual group (SBP: K‐S statistic 3.499, P<.0001; DBP: K‐S statistic 4.340, P<.0001). These distributions became close to normal in the automated group (SBP: K‐S statistic 1.243, P=.091; DBP: K‐S statistic 1.279, P=.076).
Figure 1.
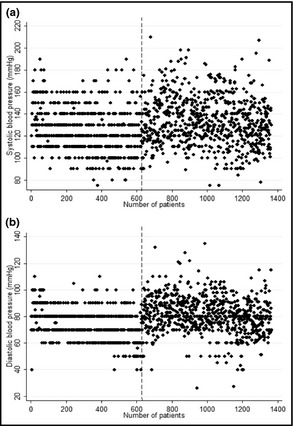
Scatter plot of systolic and diastolic blood pressure values in manual and automated sphygmomanometer groups. Note: the dash line separates manual and automated sphygmomanometers groups.
End Digits and Deviation Index
The frequency of appearance of each end digit in recorded BP numbers is represented in Figure 2. The probability of recording a zero end digit in SBP and DBP decreased from 97.7% and 98.5% in the manual group to 28.8% and 30.6% in the automated group, respectively. The deviation index decreased for both SBP and DBP after using the automated sphygmomanometer (SBP: 96.4%±0.8% vs 18.1%±2.4%, P<.001; DBP: 97.6%±0.8% vs 20.8%±2.4%, P<.001). Deviation indices for both SBP and DBP in the automated sphygmomanometers group were still significantly different from zero (P<.001 for both).
Figure 2.
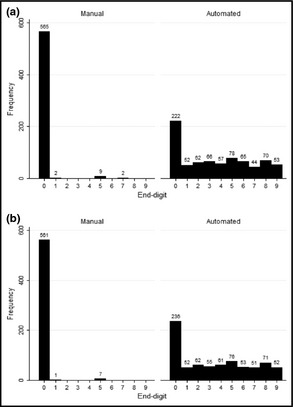
Frequency of systolic blood pressure (A) and diastolic blood pressure (B) end digits in manual and automated sphygmomanometers groups.
Systolic and Diastolic BPs
Mean SBP and DBP values in the study groups are presented in Figure 3A and 3B. For both SBP and DBP, means were significantly different between the manual and automated groups (SBP: 124.22±0.83 vs 132.90±0.78, P<.001; DBP: 74.38±0.50 vs 80.43±0.51, P<.001). There were significant associations between type of sphygmomanometer and systolic BP (beta=8.68, 95% confidence interval [CI], 6.42–10.94; P<.0001), diastolic BP (beta=6.05, CI, 4.58–7.51; P<.0001), deviation index of systolic BP (beta=78.2, CI, 72.5–84.3; P<.0001), and deviation index of diastolic BP (beta=76.4, CI, 70.8–82.5; P<.0001).
Figure 3.
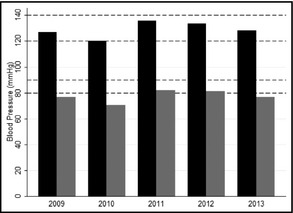
Mean systolic and diastolic blood pressures stratified according to study year.
Changes Over Time
During 3 years after implementation of automated sphygmomanometers, there was a significant linear decrease in the means of both SBP (−3.59 mm Hg per year; CI, −5.57 to −1.61 [P<.0001]) and DBP (−2.52 mm Hg per year; CI, −3.78 to −1.26 [P<.0001]). The deviation index for SBP was 21.92%±4.2%, 23.73%±3.95%, and 3.66%±4.84% in consecutive years of follow‐up (P<.001 for trend). The deviation index for DBP was 26.07%±4.19%, 18.83%±4.02%, and 16.23%±4.83% during 3 years of follow‐up (P<.001 for trend).
Threshold Bias
Changing the definition of hypertension from BP ≥140/90 mm Hg to BP >140/90 mm Hg decreased the proportion of hypertensive patients from 30.83% to 17.89% in the manual group (P<.0001). In the automated group, the proportion of hypertensive patients decreased from 46.6% to 39.92% after change in definition (P=.0078). The dependency of the number of hypertensive patients to this 1 mm Hg change in definition was more prominent in the manual group compared with the automated group (12.94% vs 6.68%, P<.0001).
Discussion
In this article, we proposed an index that enables the practitioner to quantitatively measure the amount of BP end‐digit bias in the clinical setting. We examined this index in two different BP measurement devices, manual and automated digital sphygmomanometers. The amount of end‐digit bias was significantly higher with the manual devices, evident by the appearance of zero end digits in nearly all recorded BP values. The frequency of zero end digits decreased from 97% to 98% to nearly 30% after changing the device. This corresponded to a decreased deviation index from 96% to 97% to nearly 20%. This means that even after using automated devices, there was still nearly 20% extra reporting of zero end digits compared with their expected 10% frequency, which may suggest a problem in training or education of operators measuring BPs or end‐digit preference during the documentation, and not measurement, of BP values.
The clinical importance of an accurate BP reading cannot be overemphasized. Faulty BP measurements may deprive hypertensive patients from the benefits of treatment or expose normotensive patients to unnecessary drugs and their potential side effects. End‐digit bias is a universal medical error and the frequency of recording a zero end digit ranged from 30% to more than 98% of BP measurements in previous studies.9, 10 Surprisingly, a similar pattern was also observed in hypertension specialty clinics with trained nurses and specialized physicians.5, 11 It is unclear how this imprecision has an impact on assessment or treatment of cardiovascular diseases, but some studies reported that even minor errors in BP recordings may double the number of hypertensive patients12, 13, 14 and increase cardiovascular mortality in those with near–cut‐off BP readings.15 Consistent with these reports, we observed that a 1 mm Hg change in hypertension threshold from BP ≥140/90 mm Hg to BP >140/90 mm Hg nearly halved the number of hypertension‐labeled patients whose BPs were measured by manual sphygmomanometers. Using automated sphygmomanometers considerably attenuated the threshold bias.
Replacement of manual devices with automated devices resulted in increased variance of BP, close to normal distribution, and decreased end‐digit bias in our study. Immediately after implementation of automated sphygmomanometers, mean SBP and DBP increased but steadily decreased during 3 years of follow‐up. Compared with mercury sphygmomanometers, automated devices were generally thought to underestimate BP in crossover studies,16 although this belief is still controversial.7, 17 In our study, an immediate increase in recorded BPs after implementation of automated devices may be attributed to eliminated end‐digit bias and the possibility that examiners were inclined to round down rather than up when using manual sphygmomanometers. The trend toward lower BP values over time after implementation of automated sphygmomanometers is one novel finding of this study and may be caused by adaptation of the examiners with the new technique. This is consistent with our finding about a linear decreasing trend for deviation index during years of follow‐up. Both of these findings may be missed when comparing manual and automated sphygmomanometers in crossover studies in research setting. These findings take on more importance when considering that the general tendency is to consider the BP of 135/85 mm Hg as the cut‐off point of defining hypertension when using automated sphygmomanometers.16, 18, 19, 20 These issues influence medical decision‐making and must be considered when substituting manual devices with automated devices in real‐world settings.
Study Strengths
Several strengths of this study merit consideration. Its prospective design, large sample size, and real clinical setting give this investigation a unique position among similar studies. Introduction of the deviation index was an attempt to quantify end‐digit bias and to make it possible to measure the magnitude of each kind of measurement bias in the process of BP recording. Its strength is that the applicability of its core idea is not limited to “zero reporting” bias. In fact, every biased measurement of BP, which is otherwise supposed to be normally distributed, results in a non‐zero deviation index (with some modification in formula) and the magnitude of this index corresponds to the amount of measurement bias. The usage of the deviation index can be generalized to quantify each kind of bias. In this study, we used the known example of end‐digit rounding bias to show a sensible interpretation of this index. Three‐year follow‐up of BP recordings after implementation of automated sphygmomanometers was rarely done before.
Study Limitations
As a notable limitation, extremely high prevalence of zero end digits with manual sphygmomanometers may point to the fact that training was not adequately given to nurses. Digital devices can obviate some mistakes of untrained operators, but training problem was not considered as a contributing variable in this study and hence is one of our limitations. Our study population was confined to patients with type 2 diabetes, although our results were compatible with previous findings in this group.21 It can be argued that increased use of angiotensin‐converting enzyme inhibitors and aldosterone receptor blockers in diabetic patients during these years was responsible for the observed decreasing trend in BP values after implementation of automated sphygmomanometers. It is notable that this trend occurred during the prospective phase of the study and no major changes in target cut‐points for BP control were made in this period. In addition, the study population was relatively stable during these years, as evidenced by similar baseline characteristics in two study arms. In addition, when introducing the deviation index, we made one important assumption that should be noted. We assumed that each end digit had the same probability to be recorded with an unbiased BP measurement device. Although roughly true, the validity of this assumption depends on the distribution of BP in the population under study. It can be statistically proven that in a hypothetical population with completely normal distribution of BPs and a mean value terminating to end digit 0 or 5, the deviation index can be calculated using the introduced formula. On the other hand, a distribution with a mean BP of 122 mm Hg, for example, makes a slight difference, because end digit 2 is more probable than other values to be recorded in each 10 mm Hg interval. It can also be argued that with a BP measurement system reporting all BPs in 2.5 end digits (eg, 112.5, 122.5), the deviation index yields a zero result. Complex calculations for a modified valid formula in such instances are beyond the scope of this paper, although it is provable that the underlying idea for the deviation index still holds in these situations. In routine general practice with zero end‐digit preference as the most common type of bias, the final result would be so close to our basic formula that for practical purposes it is reasonable to make these assumptions to simplify the model.
Conclusions
Implementation of automated sphygmomanometers decreases end‐digit and threshold biases in the clinical setting. In a prospective design, we also found a linear trend toward lower blood pressures after replacing manual devices with digital ones. Deviation index can be used to quantify the amount of measurement bias in blood pressure recording.
Disclosures
None declared.
Supporting information
Appendix S1. Theoretical basis.
J Clin Hypertens. 2014;16:716–721. DOI: 10.1111/jch.12400. © 2014 Wiley Periodicals, Inc.
References
- 1. Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. [DOI] [PubMed] [Google Scholar]
- 2. McAlister FA, Straus SE. Evidence based treatment of hypertension. Measurement of blood pressure: an evidence based review. BMJ. 2001;322:908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Lusignan S, Belsey J, Hague N, Dzregah B. End‐digit preference in blood pressure recordings of patients with ischaemic heart disease in primary care. J Hum Hypertens. 2004;18:261–265. [DOI] [PubMed] [Google Scholar]
- 4. Nietert PJ, Wessell AM, Feifer C, Ornstein SM. Effect of terminal digit preference on blood pressure measurement and treatment in primary care. Am J Hypertens. 2006;19:147–152. [DOI] [PubMed] [Google Scholar]
- 5. Thavarajah S, White WB, Mansoor GA. Terminal digit bias in a specialty hypertension faculty practice. J Hum Hypertens. 2003;17:819–822. [DOI] [PubMed] [Google Scholar]
- 6. Patterson HR. Sources of error in recording the blood pressure of patients with hypertension in general practice. Br Med J (Clin Res Ed). 1984;289:1661–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nelson MR, Quinn S, Bowers‐Ingram L, et al. Cluster‐randomized controlled trial of oscillometric vs. manual sphygmomanometer for blood pressure management in primary care (CRAB). Am J Hypertens. 2009;22:598–603. [DOI] [PubMed] [Google Scholar]
- 8. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 9. Hessel PA. Terminal digit preference in blood pressure measurements: effects on epidemiological associations. Int J Epidemiol. 1986;15:122–125. [DOI] [PubMed] [Google Scholar]
- 10. Ayodele OE, Sanya EO, Okunola OO, Akintunde AA. End digit preference in blood pressure measurement in a hypertension specialty clinic in southwest Nigeria. Cardiovasc J Afr. 2012;23:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graves JW, Bailey KR, Grossardt BR, et al. The impact of observer and patient factors on the occurrence of digit preference for zero in blood pressure measurement in a hypertension specialty clinic: evidence for the need of continued observation. Am J Hypertens. 2006;19:567–572. [DOI] [PubMed] [Google Scholar]
- 12. Niyonsenga T, Vanasse A, Courteau J, Cloutier L. Impact of terminal digit preference by family physicians and sphygmomanometer calibration errors on blood pressure value: implication for hypertension screening. J Clin Hypertens (Greenwich). 2008;10:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joffres MR, Hamet P, Rabkin SW, et al. Prevalence, control and awareness of high blood pressure among Canadian adults. Canadian Heart Health Surveys Research Group. CMAJ. 1992;146:1997–2005. [PMC free article] [PubMed] [Google Scholar]
- 14. Wen SW, Kramer MS, Hoey J, et al. Terminal digit preference, random error, and bias in routine clinical measurement of blood pressure. J Clin Epidemiol. 1993;46:1187–1193. [DOI] [PubMed] [Google Scholar]
- 15. Wingfield D, Freeman GK, Bulpitt CJ. Selective recording in blood pressure readings may increase subsequent mortality. QJM. 2002;95:571–577. [DOI] [PubMed] [Google Scholar]
- 16. Myers MG, McInnis NH, Fodor GJ, Leenen FH. Comparison between an automated and manual sphygmomanometer in a population survey. Am J Hypertens. 2008;21:280–283. [DOI] [PubMed] [Google Scholar]
- 17. McManus RJ, Mant J, Hull MR, Hobbs FD. Does changing from mercury to electronic blood pressure measurement influence recorded blood pressure? An observational study Br J Gen Pract. 2003;53:953–956. [PMC free article] [PubMed] [Google Scholar]
- 18. Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord. 2005;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alcocer L, Novoa G, Sotres D. Digit preferences observed in the measurement of blood pressure: repercussions on the success criteria in current treatment of hypertension. Am J Ther. 1997;4:311–314. [DOI] [PubMed] [Google Scholar]
- 20. Myers MG. Replacing manual sphygmomanometers with automated blood pressure measurement in routine clinical practice. Clin Exp Pharmacol Physiol. 2014;41:46–53. [DOI] [PubMed] [Google Scholar]
- 21. Kim ES, Samuels TA, Yeh HC, et al. End‐digit preference and the quality of blood pressure monitoring in diabetic adults. Diabetes Care. 2007;30:1959–1963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Theoretical basis.


