Abstract
This blinded placebo‐controlled crossover study evaluated the acute effects of an orally disintegrating lozenge that generates nitric oxide (NO) in the oral cavity on blood pressure (BP) response, endothelial function, and vascular compliance in unmedicated hypertensive patients. Thirty patients with clinical hypertension were recruited and enrolled in a blinded placebo‐controlled clinical trial in an outpatient setting. Average baseline BP in 30 patients was 144±3/91±1 mm Hg. NO supplementation resulted in a significant decrease of 4 mm Hg in resting systolic BP (P<.003) and a significant decrease of 5 mm Hg in diastolic BP (P<.002) from baseline and placebo after 20 minutes. In addition, there was a further statistically significant reduction by 6 mm Hg in both systolic and diastolic pressure after 60 minutes (P<.0001 vs baseline). After a half hour of a single dose, there was a significant improvement in vascular compliance as measured by augmentation index and, after 4 hours, a statistically significant improvement in endothelial function as measured by the EndoPAT (Itamar Medical, Franklin, MA). A single administration of an oral active NO supplement appears to acutely lower BP, improve vascular compliance, and restore endothelial function in patients with hypertension.
In the United States, about 77.9 million (1 of every 3) adults have high blood pressure (BP) (hypertension). Despite major advances in understanding the pathophysiology of hypertension and availability of antihypertensive drugs, suboptimal BP control is still the most important risk factor for cardiovascular (CV) mortality. According to the American Heart Association's (AHA's) 2013 Statistics Fact Sheet, although 75% of people who know they have hypertension and are under current treatment, only about 52% of those have it controlled. Because BP remains elevated in approximately 50% of all treated hypertensive patients,1, 2 novel and cost‐effective therapeutic strategies are urgently required for the treatment of this condition.
Naturally produced and found in many different kinds of cells and organ systems, nitric oxide (NO) is an integral molecule in regulating BP and maintaining a healthy CV system.3 NO is produced naturally in the body and serves as a cell‐signaling molecule in mammalian physiology.4 Specifically, NO is the endothelium‐derived relaxing factor first described by Furchgott in 1980.5, 6 NO is produced endogenously from the 5‐electron oxidation of the guanidino nitrogen of l‐arginine by the enzyme isoform endothelial NO synthase (eNOS). NO produced or generated in the vasculature then diffuses into the underlying smooth muscle causing these muscles to relax. This results in vasodilation, causing a reduction in systemic BP and an increase in blood flow and oxygen delivery to specific vascular beds. Emerging evidence shows that endothelial dysfunction and subsequent NO deficiency are critically associated with the development of hypertension and other forms of CV disease.7 The concept of endothelial dysfunction arises from variations in blood flow observed in patients with atherosclerosis compared with healthy patients. In healthy patients, activation of eNOS causes vasodilation in both muscular conduit vessels and resistance arterioles. In contrast, in patients with atherosclerosis, similar stimulation yields attenuated vasodilation in peripheral vessels and causes paradoxical vasoconstriction in coronary arteries, thus indicating a decrease in the production and/or bioavailability of NO.8, 9 Interestingly, endothelial dysfunction can be demonstrated in patients with risk factors for atherosclerosis in the absence of atherosclerosis itself.10, 11 These observations lend credence to the concept that endothelial dysfunction is integral to the development and progression of disease. Therefore, treatment modalities that increase NO production and/or availability may have important implications in better management of hypertension and ultimately preventing and treating CV disease. Strategies designed around dietary supplementation with l‐arginine as a means to enhance NO production have proven to be largely ineffective in chronic studies.12, 13
The objective in the current clinical study was to determine the acute effects of a single administration of the oral NO supplementation on BP response, endothelial function, and vascular elasticity in patients with clinical hypertension.
Methods
Study Design
Given that this study involves human participation, the institutional review board (IRB) (RCRC IRB approval protocol number Neo 11‐2012 HypHouston, Acute Effects of Neo40, a nitric oxide dietary supplement on hypertension; Neogenis Labs, Austin, TX) approved the study to commence the clinical phase of the acute effects of oral NO supplementation on functional capacity and BP in hypertensive, healthy adults not currently taking any antihypertensive medications. All patients were informed of the study and gave informed consent. All procedures were in accordance with institutional guidelines. Patients were considered eligible for the study if they met the following inclusion criteria:
BP >130/90 mm Hg on two occasions.
Ability to be evaluated by a noninvasive EndoPAT (Itamar Medical, Franklin, MA) measurement.
Ability to sign informed consent.
Patients were excluded from the study if they met the following exclusion criteria:
Not considered medically stable.
Currently taking any antihypertensive prescription medication.
Known allergy to ingredients of lozenge.
Lactating or pregnant.
Known coronary artery disease, heart failure, or history of chronic medical or cardiac history including cancer and uncontrolled diabetes.
Unwilling or unable to provide informed consent.
Only English‐speaking patients were recruited. A complete medical history and focused physical examination were conducted and findings were documented. Thirty individuals were recruited from an outpatient clinic at the Hypertension Institute of Nashville without any significant chronic medical or cardiac history. These patients were referred for either cardiac clearance for an elective surgical procedure or for BP readings at the primary care physicians’ request or self‐referrals for general CV evaluation. Patients were screened and enrolled by Mark Houston, MD, and/or his delegated research staff upon patient consult. Patients were informed of the purpose of the study including risks, benefits, and alternative treatments to the study. After the potential participants had been given the opportunity to ask questions and have their questions answered, they were asked to sign an informed consent prior to any study‐specific procedures being performed. The participant's confidentiality was maintained at all times by the principal investigator and research staff. Data were collected on case report forms and all medical records and case report forms were retained in research files that are kept in a secure location in the research area. Only those individuals authorized by the IRB to participate in the study had access to the study records.
BP readings were conducted by Dr Houston and his trained staff. Triplicate readings were recorded for each measurement. Patients were sitting upright without their legs crossed and their arms were supported at the level of the heart. A standard wall mount mercury sphygmomanometer was used for all BP readings. The 30 study participants were first administered the NO‐donating lozenge after a complete medical workup. After a 3‐week wash‐out period, 10 of the patients were administered the placebo and the study protocol was repeated. The following parameters were analyzed:
Baseline BP (mm Hg): had 3 baseline BP readings while sitting, then administered a single dose. BP measurements were in accordance with the AHA's most recent guidelines for BP measurement.14 BP was measured again 3 times after 20 minutes and 4 readings after 1 hour.
Baseline endothelial function test: EndoPAT is a Food and Drug Administration (FDA)–cleared medical device for noninvasive endothelial function assessment. Baseline measurements were performed and then again at 4 hours after a single dose of the NO‐donating lozenge. Because we know that NO plays an important role in the dilation of arteries,5, 6, 15 endothelial NO production can be measured noninvasively through peripheral arterial tone. This technique has been shown to correlate with coronary artery disease (CAD) risk and the presence or absence of CAD.16 EndoPAT (www.itamar-medical.com) assesses digital flow‐mediated dilation during reactive hyperemia using measurements from both arms (occluded side and control side). A patient's responding vasodilatation can be a measure of their endothelial function.
Internal diameter of left common carotid by ultrasound (Sonosite, Bothell, WA). Baseline internal diameter was recorded and then remeasured 10 minutes after a single dose of the NO‐donating lozenge. Using the Sonosite portable ultrasound instrument, baseline diameter of the left common carotid artery was determined at the level just above the bifurcation. CardioRisk, Inc (South Jordan, UT) provided a trained and licensed sonographer with national certification by the American Registry of Radiologic Technologists and the American Registry of Diagnostic Medical Sonographers. All data were sent blindly to readers at CardioRisk for analysis and statistics.
Pulse wave velocity and augmentation index: a noninvasive measure of vascular compliance using Mobilograph by Cardiograde, Inc (Fremont, CA), was recorded at baseline and again at 30 minutes after a single dose of the NO‐donating lozenge. This instrument determines central aortic BP, cardiac output, total vascular resistance, and augmentation index.
The study and protocol design is illustrated in Figure 1.
Figure 1.
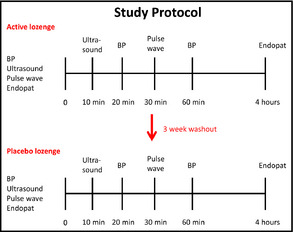
Study design. BP indicates blood pressure.
Statistics
Using Stata Statistical Software, Release 10 (StataCorp, College Station, TX), we assumed an alpha of 0.05, power of 0.80 and a 2‐tailed t test for the sample size calculation. An unequal/unbalanced randomization is sometimes used for a number of reasons,17, 18 and statistical software packages allow for unbalanced randomization ratios to be used in making sample size estimates and power calculations. This calculation was based on the average decrease in systolic BP detected in a primary care environment. According to power analyses from previous clinical studies, 30 patients were sufficient to detect a significant decrease in systolic BP. All baseline measurements were compared at different intervals post‐treatment as well as with placebo.
Product Formulation
Utilizing intellectual property developed out of the University of Texas Health Science Center in Houston (US patents 8,298,589; 8,303,995; and 8,435,570), Neogenis Laboratories has the exclusive license for this technology. The NEO40 Daily product ingredients list, and packaging was submitted to the FDA Office of Compliance by Neogenis Labs, Inc, for use as a dietary supplement made up of Beet root extract, Hawthorn Berry, vitamin C, L‐citrulline, and sodium nitrite. The lozenges utilize natural product chemistry activated by the saliva to generate authentic NO gas in the oral cavity.19, 20 This product's formulation was designed to be a quick dissolve that melts in the mouth within 4 to 5 minutes. Previously published data that incorporated in vitro biochemical assays, animal studies, and human clinical trials not only demonstrate a robust improvement in NO activity, but also that the formula is effective at modifying biomarkers of CV risk and replenishing NO biochemistry in at‐risk patients.20, 21, 22 The placebo looked and tasted the same as the active NO supplement. The patients were blinded to the treatment.
Results
Blood Pressure
The average BP at baseline for the 30 patients was 144±3/91±1 mm Hg. Twenty minutes after a single dose of the NO‐donating lozenge, both systolic and diastolic BPs significantly decreased to 140±2/86±1 mm Hg (P=.003 for systolic and P=.002 for diastolic pressures). BP measurements after 1 hour revealed a further statistically significant decrease in both systolic and diastolic BPs to 138±3/85±1 mm Hg (P=.0001 for systolic and P=.00001 for diastolic vs baseline and P=.04 for systolic and P=.04 for diastolic when compared with data at 20 minutes. Ten of the patients with the highest BPs were crossed‐over after a 3‐week washout period. After 3 weeks, baseline BP was 145±3/91±1 mm Hg, indicating that the acute effects of the NO lozenge were gone after 3 weeks. There was no significant change in systolic BP after 20 minutes (P=.089) or after 60 minutes (P=.067) of administration of a single placebo. Placebo also had no effect on diastolic BP after 20 minutes (P=.37), but did significantly reduce diastolic BP after 60 minutes 91±1 vs 89±0.9 mm Hg (P=.027). When compared with placebo, the active NO lozenge led to a nonstatistically significantly reduction in systolic BP after 20 minutes (P=.06) but a statistically significant difference at 60 minutes (P=.03). The NO active lozenge led to a statistically significant decrease in diastolic BP at 20 and 60 minutes compared with placebo (P=.01 for both). These data are illustrated in Figure 2.
Figure 2.
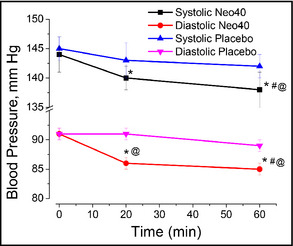
Administration of a single dose of the nitric oxide (NO)–donating lozenge significantly reduces both systolic and diastolic blood pressure after 20 and 60 minutes compared with baseline. *Statistical comparison compared with baseline; #statistical comparison between 20 and 60 minutes. Compared with placebo, the NO‐donating lozenge led to a significant reduction in diastolic blood pressure at 20 minutes and a significant reduction in systolic and diastolic blood pressure after 60 minutes. @Statistical comparison to placebo determined by two‐tailed t test. Data are presented as average±standard error of the mean of 30 patients in the Neo40 group and 10 in the crossover placebo group.
Ultrasound
As shown in Figure 3A, 10 minutes after the NO lozenge administration resulted in an average 8.5% increase in blood vessel diameter. Actual images from the above data demonstrate that within a period of 10 minutes, the NO released by the lozenge dilates carotid arteries as proven by an increase in the internal diameter of the artery. Understanding Poiseuille's Law, the radius of the blood vessel is inversely proportional to the rate of blood flow through the vessel. A 19% increase in vessel radius or a 38% increase in vessel diameter will cause a 100% increase in blood flow. In the representative image in Figure 3B, the internal diameter increases from 0.52 cm to 0.60 cm within 10 minutes. Using Poiseuille's Law, this 13.3% increase in blood vessel diameter causes a 34% increase in blood flow through these arteries in this representative example. Using an average of 8.5% from the total patients, the average increase in blood flow would be 21.7%.
Figure 3.
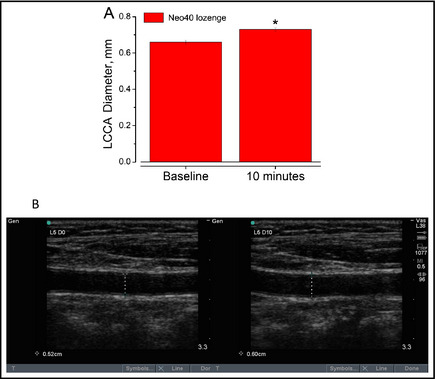
(A) Administration of a single nitric oxide–donating lozenge causes a statistically significant increase in luminal diameter of the left common carotid artery (LCCA; P=.00001). (B) Representative ultrasound of the increase in LCCA at baseline and 10 minutes after a single lozenge. Data are presented as average±standard error of the mean in 10 patients.
Vascular Compliance
Using the Mobilograph instrument (Cardiograde, Inc), baseline measurements were collected and then repeated after 30 minutes of taking the NO‐donating lozenge. The NO lozenge is designed to dissolve in 4 to 6 minutes. We were interested in determining any effects on vascular compliance after the lozenge had dissolved. Half an hour after taking the lozenge, there was a decrease in mean arterial pressure, pulse pressure, and central aortic systolic pressure. There was also a significant improvement in augmentation pressure, augmentation index, and pulse wave velocity. These data are shown in the Table. Arterial stiffness can be measured via augmentation index, which is a sensitive marker of arterial status and has been shown to be a predictor of adverse CV events in a variety of patient populations.23 The results described above indicate that the NO lozenge can improve arterial compliance within half an hour after a single lozenge.
Table 1.
Administration of the NO‐Donating Lozenge on Vascular Compliance and Reduced Central Pressure After 30 Minutes
| Baseline | After NO Lozenge | P Value | |
|---|---|---|---|
| Mean arterial pressure, mm Hg | 114±3 | 108±3 | .003 |
| Heart rate, beats per min | 77.4±2.4 | 76.2±2.0 | .26 |
| Pulse pressure, mm Hg | 44±3 | 38±2 | .007 |
| Central systolic BP, mm Hg | 127±3 | 122±3 | .01 |
| Central diastolic BP, mm Hg | 95±2 | 94±3 | .25 |
| Central pulse pressure, mm Hg | 31.4±1.9 | 28.7±1.6 | .07 |
| Cardiac output, L/min | 5.4±0.2 | 5.1±0.2 | .07 |
| Total vascular resistance, s*mm Hg/mL | 1.3±0.03 | 1.3±0.03 | .65 |
| Augmentation pressure, mm Hg | 6.2±0.8 | 4.3±0.6 | .02 |
| Augmentation index @75 | 21.2±2.5 | 15.4±1.7 | .01 |
| Pulse wave velocity, m/s | 8.6±0.4 | 8.3±0.4 | .005 |
Abbreviations: BP, blood pressure; NO, nitric oxide. Data are expressed as the average of 20 patients at baseline and a half hour after a single lozenge.
Endothelial Function
EndoPAT scores were recorded at baseline and then repeated 4 hours after a single dose of the NO‐donating lozenge. As shown in Figure 4, there was a statistically significant improvement in endothelial function. These data reveal that the NO‐donating lozenge not only has acute effects on the NO release but also appears to promote endothelial NO production.
Figure 4.
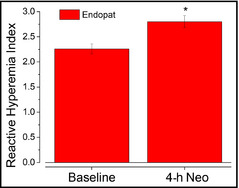
Administration of a single nitric oxide–donating lozenge improved endothelial function after 4 hours. Data are presented as average±standard error of the mean in 30 patients. *P<.05.
Discussion
This blinded placebo‐controlled cross‐over study evaluated the acute effects of NO supplementation using a commercially available NO lozenge on systemic and central BP response, blood vessel diameter, arterial compliance, and endothelial function in a small number of patients with clinically untreated hypertension in an outpatient setting. The association of hypertension with CV morbidity and mortality is well established. Abundant epidemiological data have shown that the risk of CV disease rises with increasing BP levels, starting at ≥115/75 mm Hg, in a strong, independent, graded, and continuous manner.24 Lowering diastolic BP by 5 mm Hg reduces the risk of stroke by an estimated 34% and ischemic heart disease by 21% from any pretreatment level; there is currently no threshold.25 This study reveals that the single administration of an NO lozenge resulted in the following:
Significant reduction of systemic BP at 20 and 60 minutes (on average 6 mm Hg systolic and 6 mm Hg diastolic).
Demonstration that the NO released from the lozenge is bioactive as determined by dilation of the left common carotid artery measured by ultrasound.
Significantly improved vascular compliance and augmentation index after 30 minutes.
Significantly improved endothelial function at 4 hours after a single dose of the NO‐donating lozenge.
Our data are consistent with a recent case report using the NO lozenge to reduce BP in a pediatric patient with argininosuccinic aciduria with resistant hypertension.26
It is evident that loss of endogenous NO production and homeostasis is at least partly responsible for hypertension and is associated with most CV risk factors.27, 28, 29, 30, 31, 32 Diet and lifestyle are critically important in controlling and combatting hypertension. Moderate physical exercise promotes endothelial NO production.33 The Dietary Approaches to Stop Hypertension (DASH) diet is proven to moderately lower BP. The DASH diet lowered systolic BP significantly in untreated patients, with systolic BP <160 mm Hg, and diastolic BP 80 to 95 mm Hg by 5.5/3.0 mm Hg.34 This diet as well as the Mediterranean diet is thought to be effective at lowering BP because of its inorganic nitrate content of green leafy vegetables that can be utilized to regenerate NO.35, 36, 37 Recently published data reveal that the same NO lozenge was effective at taking prehypertensive patients and making them normotensive after 30 days (under review). This supplementation approach may be useful to support vasodilation and may help normalize BP in hypertensive individuals and reduce the need for anti‐hypertensive medications or polypharmacy.
Study Strengths and Limitations
This study is important because the NO lozenge investigated in this study, which utilizes natural product chemistry and Generally Recognized as Safe (GRAS) ingredients, provides an acute source of authentic NO as well as promotes endothelial production of NO. This may provide enormous therapeutic advantages over chronic use of organic nitrate therapy, the primary therapeutic option for NO delivery to patients. The beneficial pharmacologic effects of nitrovasodilators are severely limited by the rapid development of tolerance to their vasodilatory effects.38 There is no tolerance development with the use of inorganic nitrite,39 and no tolerance has been observed in the chronic use of the NO active lozenge used in this study.26 This study reveals that administration of the NO‐donating lozenge leads to a significant reduction in systolic and diastolic BP, dilates arteries, and improves vascular stiffness and endothelial function in unmedicated hypertensive patients. The limitation of this investigation is that it is an acute phase study with a limited number of participants. Therefore, the findings might not be easily extrapolated to larger populations.
Conclusions
Additional longitudinal studies are necessary to determine the long‐term effects of NO supplementation in more complex cases.
Disclosures
The study was sponsored by a grant from Neogenis Laboratories.
J Clin Hypertens (Greenwich). 2014;16:524–529. ©2014 Wiley Periodicals, Inc.
References
- 1. Wang YR, Alexander GC, Stafford RS. Outpatient hypertension treatment, treatment intensification, and control in Western Europe and the United States. Arch Intern Med. 2007;167:141–147. [DOI] [PubMed] [Google Scholar]
- 2. Cutler JA, Sorlie PD, Wolz M, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. [DOI] [PubMed] [Google Scholar]
- 3. Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147(suppl 1):S193–S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53(4 pt 1):503–514. [PubMed] [Google Scholar]
- 5. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetycholine. Nature. 1980;288:373–376. [DOI] [PubMed] [Google Scholar]
- 6. Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium‐derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32:1103–1108. [DOI] [PubMed] [Google Scholar]
- 8. Lieberman EH, Gerhard MD, Uehata A, et al. Flow‐induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214. [DOI] [PubMed] [Google Scholar]
- 9. Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. [DOI] [PubMed] [Google Scholar]
- 10. Creager MA, Cooke JP, Mendelsohn ME, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose‐related and potentially reversible impairment of endothelium‐dependent dilation in healthy young adults. Circulation. 1993;88(5 pt 1):2149–2155. [DOI] [PubMed] [Google Scholar]
- 12. Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L‐arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007;116:188–195. [DOI] [PubMed] [Google Scholar]
- 13. Schulman SP, Harada R, Nair N, Balasubramanian N, Cooke JP, et al. L‐arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295:58–64. [DOI] [PubMed] [Google Scholar]
- 14. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich). 2005;7:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′‐cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA. 1977;74:3203–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuvin JT, Mammen A, Mooney P, Alsheikh‐Ali AA, Karas RH. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007;12:13–16. [DOI] [PubMed] [Google Scholar]
- 17. Dumville JC, Hahn S, Miles JN, Torgerson DJ. The use of unequal randomisation ratios in clinical trials: a review. Contemp Clin Trials. 2006;27:1–12. [DOI] [PubMed] [Google Scholar]
- 18. Avins AL. Can unequal be more fair? Ethics, subject allocation, and randomised clinical trials J Med Ethics. 1998;24:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bryan NS. Application of nitric oxide in drug discovery and development. Expert Opin Drug Discov. 2011;6:1139–1154. [DOI] [PubMed] [Google Scholar]
- 20. Zand J, Lanza F, Garg HK, Bryan NS. All‐natural nitrite and nitrate containing dietary supplement promotes nitric oxide production and reduces triglycerides in humans. Nutr Res. 2011;31:262–269. [DOI] [PubMed] [Google Scholar]
- 21. Stokes KY, Dugas TR, Tang Y, et al. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1281–H1288. [DOI] [PubMed] [Google Scholar]
- 22. Bryan NS, Calvert JW, Elrod JW, et al. Dietary nitrite supplementation protects against myocardial ischemia‐reperfusion injury. Proc Natl Acad Sci USA. 2007;104:19144–19149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laurent S, Boutouyrie P. Arterial stiffness: a new surrogate end point for cardiovascular disease? J Nephrol. 2007;20(suppl 12):S45–S50. [PubMed] [Google Scholar]
- 24. Antonakoudis G, Poulimenos L, Kifnidis K, Zouras C, Antonakoudis H. Blood pressure control and cardiovascular risk reduction. Hippokratia. 2007;11:114–119. [PMC free article] [PubMed] [Google Scholar]
- 25. Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess. 2003;7:1–94. [DOI] [PubMed] [Google Scholar]
- 26. Nagamani SC, Campeau PM, Shchelochkov OA, et al. Nitric‐oxide supplementation for treatment of long‐term complications in argininosuccinic aciduria. Am J Hum Genet. 2012;90:836–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moncada S, Palmer RM, Higgs EA. The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension. 1988;12:365–372. [DOI] [PubMed] [Google Scholar]
- 28. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 suppl 1):III27–III32. [DOI] [PubMed] [Google Scholar]
- 29. Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long‐term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. [DOI] [PubMed] [Google Scholar]
- 30. Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 31. Bugiardini R, Manfrini O, Pizzi C, Fontana F, Morgagni G. Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation. 2004;109:2518–2523. [DOI] [PubMed] [Google Scholar]
- 32. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. [DOI] [PubMed] [Google Scholar]
- 33. Rassaf T, Lauer T, Heiss C, et al. Nitric oxide synthase‐derived plasma nitrite predicts exercise capacity. Br J Sports Med. 2007;41:669–673; discussion 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sacks FM, Appel LJ, Moore TJ, et al. A dietary approach to prevent hypertension: a review of the Dietary Approaches to Stop Hypertension (DASH) Study. Clin Cardiol. 1999;22(7 suppl):III6–III10. [DOI] [PubMed] [Google Scholar]
- 35. Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. [DOI] [PubMed] [Google Scholar]
- 36. Lundberg JO, Feelisch M, Björne H, Jansson EA, Weitzberg E. Cardioprotective effects of vegetables: is nitrate the answer? Nitric Oxide. 2006;15:359–362. [DOI] [PubMed] [Google Scholar]
- 37. Lundberg JO, Weitzberg E, Gladwin MT. The nitrate‐nitrite‐nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. [DOI] [PubMed] [Google Scholar]
- 38. Fung HL. Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved? Annu Rev Pharmacol Toxicol. 2004;44:67–85. [DOI] [PubMed] [Google Scholar]
- 39. Dejam A, Hunter CJ, Tremonti C, et al. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. [DOI] [PubMed] [Google Scholar]


