Abstract
Obstructive sleep apneas syndrome (OSAS) is associated with nocturnal hypertension with higher sleep blood pressure (BP) and its variability, both of which increase cardiovascular risk. In this crossover design study, the effect of nighttime single‐dose administration of vasodilating (nifedipine 40 mg) vs sympatholytic (carvedilol 20 mg) antihypertensive agents on sleep BP in 11 hypertensive OSAS patients was evaluated. The authors recently developed a trigger sleep BP monitor with an oxygen‐triggered function that initiates BP measurement when oxygen desaturation falls. The BP‐lowering effects of nifedipine on the mean (P<.05) and minimum sleep systolic BPs (SBPs) (P<.01) were stronger than those of carvedilol. Sleep SBP surge (difference between the hypoxia‐peak SBP measured by oxygen‐triggered function and SBPs within 30 minutes before and after the peak SBP) was only significantly reduced by carvedilol (P<.05). The nighttime dosing of both vasodilating and sympatholytic antihypertensive drugs is effective to reduce sleep BP but with different BP‐lowering profiles.
Obstructive sleep apnea syndrome (OSAS) is the most frequent cause of secondary hypertension and resistant hypertension, and it is also a risk factor for cardiovascular events, particularly those that occur during sleep.1, 2, 3, 4, 5 Population‐based and clinical studies have demonstrated that sleep blood pressure (BP) and sleep BP variability are more important for predicting cardiovascular events than awake BP and awake BP variability.6, 7 Patients with hypertensive OSAS are likely to exhibit a nondipper/riser pattern of nocturnal hypertension, often with increased sleep BP variability.2
Ambulatory BP monitoring (ABPM) has historically been the gold standard for measuring BP during sleep. However, self‐measured home BP monitoring can also be used to evaluate sleep BP, with results that are comparable to those of ABPM.8 The drawback of both monitoring methods is that they use fixed‐interval measurement, and thus cannot specifically detect OSAS‐related BP variability.9 Recently, we developed a trigger sleep BP monitoring (TSP) method, which is based on the automated fixed‐interval measurement function with an additional oxygen‐triggered function that initiates BP measurement when oxygen desaturation falls below a set variable threshold continuously monitored by pulseoximetry.2, 10, 11 A previous study of noninvasive continuous BP monitoring using a Finapres device (Amsterdam, Netherland) demonstrated a sleep BP surge just after each episode of sleep apnea BP in patients with OSAS.9 TSP is more convenient than Finapres for the clinical assessment of different sleep BP profiles, including mean sleep BP, hypoxia‐related peak sleep BP, basal (minimum) sleep BP, and sleep BP surge in patients with OSAS. Neither previous home BP monitoring nor ABPM could detect the peak sleep BP or the sleep BP surge, both of which have been specifically related to hypoxia caused by individual sleep apnea episodes. Exaggerated sleep BP surge may trigger sleep‐onset cardiovascular events, including wake‐up stroke, in patients with OSAS.12, 13
In this crossover study using the TSP, we evaluated the effects of bedtime dosing of vasodilating (nifedipine, a calcium channel blocker [CCB]) vs sympatholytic (carvedilol, a nonselective β‐blocker/α1‐blocker) antihypertensive agents on the sleep BP profile in patients with hypertensive OSAS.
Methods
Study Design
The Effects of Vasodilating vs Sympatholytic Antihypertensives on Sleep Blood Pressure in Hypertensive Patients With Sleep Apnea Syndrome (VASSPS) was conducted in a prospective, randomized, parallel‐group crossover design (Figure 1). After a 2‐ to 8‐week run‐in period after baseline overnight TSP and polysomnography, a single‐dose of nifedipine (slow‐release) 40 mg or carvedilol 20 mg was randomly administered after dinner at 6 pm, and overnight TSP and polysomnography were performed. After a 2 week‐washout period, the first drug was changed to a drug from a different class, and the TSP and polysomnography were conducted in the same fashion.
Figure 1.
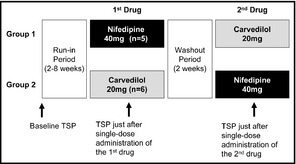
Crossover design of this study. A single dose of nifedipine 40 mg or carvedilol 20 mg was randomly administered at 6 pm, and trigger sleep blood pressure monitoring (TSP) and polysomnography were initiated at 7:30 pm. After a 2‐week washout period, the first drug was changed to a drug from a different class, and the TSP and polysomnography were conducted in the same fashion.
The ethics committee of the internal review board of the Jichi Medical University School of Medicine, Tochigi, Japan, approved the protocol, and written informed consent was obtained from all patients who enrolled in the study. The study protocol was registered on a clinical trials registration site (University Hospital Medical Information Network Clinical Trials Registry: #UMIN000010600).
Study Patients
The 11 hypertensive patients with OSAS (apnea hypopnea index [AHI] >15 per hour) were recruited between July 2009 and November 2012 and met all of the following criteria: (1) previous diagnosis by polysomnography, (2) unwillingness to receive continuous positive airway pressure (CPAP), and (3) sleep BP (measured by ABPM) ≥120/70 mm Hg. Exclusion criteria were treatment with sympatholytic agents, treatment with bedtime dosing of antihypertensive drug, and malignancy, renal failure (serum creatinine >2.0 mg/dL), or severe liver dysfunction. The patients' characteristics are shown in Table 1. All patients were taking ≥1 antihypertensive drug except sympatholytic drugs (α‐ or β‐blockers).
Table 1.
Baseline Characteristics of 11 Study Hypertensive Patients With Obstructive Sleep Apnea Syndrome
| Characteristic | Mean±Standard Deviation or % |
|---|---|
| Age, y | 64.8±12.5 |
| Men, % | 72.7 |
| BMI, kg/m2 | 25.8±2.4 |
| Current smokers, % | 18.2 |
| Habitual drinkers, % | 45.5 |
| Hyperlipidemia, % | 54.4 |
| Diabetes, % | 18.2 |
| History of angina, % | 18.2 |
| History of myocardial infarction, % | 18.2 |
| History of stroke, % | 18.2 |
| Total cholesterol, mg/dL | 180.7±20.2 |
| HDL cholesterol, mg/dL | 52.8±8.2 |
| Glucose, mg/dL | 104.7±30.0 |
| Creatinine, mg/dL | 0.83±0.17 |
| eGFR, mL/min/1.73 m2 | 69.0±13.6 |
| Antidiabetic drug, % | 9.1 |
| Lipid‐lowering drug, % | 27.3 |
| Statin, % | 27.3 |
| Antihypertensive drugs | |
| Number of antihypertensive drugs | 1.72±1.0 |
| Calcium channel blockers (dihydropyridines), % | 54.4 |
| Angiotensin‐converting enzyme inhibitors, % | 18.2 |
| Angiotensin II receptor blockers, % | 72.7 |
| Diuretics, % | 36.4 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein.
Trigger Sleep BP Monitoring
Simultaneous TSP and full polysomnography were conducted in the sleep laboratory of the Washiya Hospital (Utsunomiya, Japan). TSP can evaluate different sleep BP profiles, including mean sleep BP, hypoxia‐related peak sleep BP, basal (minimum) sleep BP, and sleep BP surge in patients with OSAS. Briefly, TSP measures BP on the basis of (1) the automated fixed‐interval (30 minutes) measurement function (the same function as ABPM) to calculate mean sleep BPs, and (2) the oxygen‐triggered function that initiates BP measurement when oxygen desaturation falls below a set variable oxygen threshold continuously monitored by pulseoximetry to calculate hypoxia‐related peak sleep BP. Detailed information on TSP, including the algorithm used to calculate the variable threshold, is given elsewhere.10, 11
Home Information Technology–Based TSP
Finally, we introduced TSP techniques into the home information technology (IT)–based TSP (HITS) with a 3G data communication system (Figure S1). The HITS system is a cloud computing–based composite management and analysis system for data sent directly from the HITS device at the patient's home.
Definition of Sleep BP Parameters
We defined the sleep systolic BP (SBP) surge as the difference between the maximum SBP measured by an oxygen‐triggered function (hypoxia‐peak SBP) and the average of the SBPs measured by a fixed‐interval function within 30 minutes before and after the hypoxia‐peak SBP (Figure 2). Mean sleep BP was the average of the sleep BPs measured only by the fixed‐interval function. Basal SBP was the lowest SBP among all the sleep BPs measured by both oxygen‐triggered and fixed‐interval functions. Calculation of these variables was performed without knowledge of the treatment that each patient had received.
Figure 2.
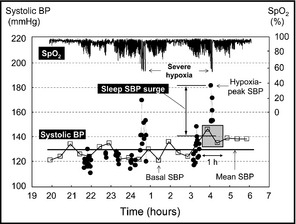
Definition of sleep blood pressure (BP) parameters measured by trigger sleep BP monitoring. SpO2 indicates oxygen saturation monitored by pulse oximetry; SBP, systolic blood pressure. Closed circles indicate SBP measured by oxygen‐triggered function, and open boxes indicate SBP measured by the fixed‐interval function. Sleep SBP surge=difference between the maximum SBP measured by oxygen‐triggered function (hypoxia‐peak SBP) and fixed‐interval function‐measured SBP measured within 30 minutes before and after the hypoxia‐peak SBP. Mean sleep BP=the average of the sleep BPs measured only by the fixed‐interval function. Basal SBP=the lowest SBP among all the sleep BPs measured by both triggered and fixed‐interval functions. The hypoxia‐peak SBPs were detected at the time of the severe hypoxic episodes <60% of SpO2.
Statistical Analysis
Data were expressed as means±standard deviations (SDs) or percentages. Paired t tests were used for comparison of the mean values between two phases. Associations/differences with a P value <.05 (two‐tailed) were considered to be statistically significant. All statistical analyses were performed with SPSS version 15 (IBM, Armonk, NY).
In this study, we used the maximum single dose of each drug (40 mg for nifedipine [slow‐release] and 20 mg for carvedilol) permitted in Japan. Power calculations were originally based on an expected average difference of SBP fall between two drugs of 10 mm Hg (SD 10 mm Hg), because one comparative study demonstrated that the SBP‐lowering effect of slow‐release nifedipine 20 mg to 40 mg was comparable to that of carvedilol 25 mg to 50 mg.14 The half‐lives of nifedipine (slow‐release) and carvedilol are 8.1 hours and 7.7 hours, respectively. Both are relatively short‐acting and their pressure‐lowering effect cover during the nighttime period. We needed a total of 10 patients to have 80% power for SBP at P≤.05.
Results
At baseline, hypoxia‐related peak sleep SBP (maximum SBP measured by an oxygen‐triggered function) was markedly higher (by 27.4 mm Hg) than mean sleep SBP (the average of sleep SBPs measured by a fixed‐interval function) (Table 2).
Table 2.
Trigger Sleep Pressure Monitoring‐Measured Blood Pressure Parameters and Polysomnography Parameters at the Baseline and Carvedilol‐ or Nifedipine‐Administered Nights in Hypertensive Patients With Obstructive Sleep Apnea Syndrome
| Baseline | Carvedilol Added | Nifedipine Added | |
|---|---|---|---|
| Blood pressure parameters | |||
| Nighttime | |||
| Fixed‐interval function | |||
| Mean SBP, mm Hg | 137.3±9.1 | 121.8±8.3a | 112.8±10.7a,b |
| SD of SBP, mm Hg | 11.5±2.2 | 10.8±2.5 | 11.0±2.2 |
| Mean DBP, mm Hg | 81.1±7.4 | 72.5±5a | 66.6±6.3a,c |
| Mean PR, beats per min | 59.8±9.4 | 57.2±7.8d | 62.4±8.1e |
| Oxygen‐triggered function | |||
| Maximum SBP, mm Hg | 164.7±15.2 | 143.0±12.7f | 138±22.4f |
| Mean SBP, mm Hg | 142.8±11.4 | 120.0±8.0a | 114.2±12.5a |
| SD of SBP, mm Hg | 10.3±4.3 | 9.8±3.4 | 9.4±3.2 |
| Mean DBP, mm Hg | 85.2±8.0 | 71.1±5.3a | 67.2±6.9a |
| Mean PR, beats per min | 63.0±8.7 | 59.1±7.7f | 64.5±7.4e |
| Sleep SBP surge, mm Hg | 30.8±12.7 | 18.6±7.8d | 22.1±16.8 |
| Minimum (basal) sleep SBP, mm Hg | 113.6±9.6 | 99.6±9.5a | 88.6±14.6a,c |
| Minimum sleep PR, beats per min | 53.4±8.1 | 49.5±7.1f | 54.8±7.5e |
| Morning | |||
| SBP, mm Hg | 150.8±12.3 | 137.4±10.5f | 118.2±15.7a,e |
| DBP, mm Hg | 86.4±10.6 | 79.9±6.6d | 69.9±9.2f,c |
| PR, beats per min | 61.8±9.6 | 57±8.2f | 64.7±10e |
| Polysomnography parameters | |||
| AHI (per h) | 26±13.1 | 28.5±13.4 | 33.1±12.2 |
| Apnea index (per h) | 9±9.7 | 7.6±5.6 | 8.8±6 |
| Arousal index (per h) | 25.7±7.3 | 22.1±7.9 | 24.2±13.9 |
| SpO2 <90%, % | 11.7±12.5 | 11±6.8 | 12.3±12.6 |
| Lowest SpO2, % | 75.5±6.7 | 72.5±6.9 | 73.6±10 |
Abbreviations: AHI, apnea hypopnea index; DBP, diastolic blood pressure; PR, pulse rate; SpO2, oxygen saturation. Date are shown as mean±standard deviation. Sleep blood pressure (BP) surge is defined as the difference between the maximum systolic BP (SBP) measured by oxygen‐triggered function (hypoxia‐peak SBP) and the average of BPs measured by the fixed‐interval function within 30 minutes before and after the hypoxia‐peak SBP. a P<.001, d P<.05, and f P<.01, vs baseline, by paired t test; b P<.05, c P<.01, and e P<.001, vs carvedilol‐added phase, by paired t test.
Figure 3 demonstrates the time‐trend data of oxygen saturation and sleep SBP measured by TSP in a patient with hypertensive OSAS. In addition to the mean sleep SBP, the excess sleep SBP surges around the period of the extended hypoxia were suppressed by nighttime dosing of both carvedilol and nifedipine.
Figure 3.
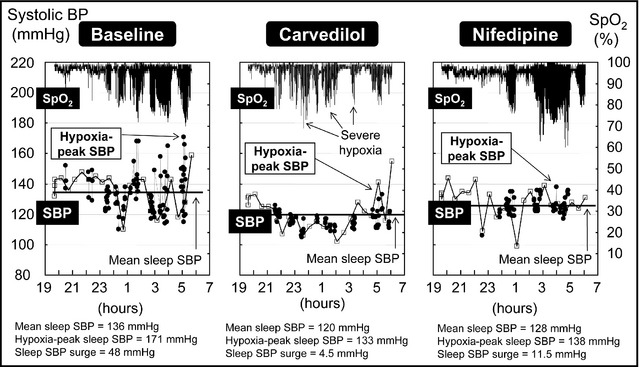
Time‐trend data of oxygen saturation and sleep systolic blood pressure measured by trigger sleep blood pressure monitoring in a hypertensive patient with sleep apnea syndrome. SpO2 indicates oxygen saturation monitored by pulse oximetry; SBP, systolic blood pressure. The closed circles indicate SBPs measured by an oxygen‐triggered function, and open boxes indicate SBPs measured by the fixed‐interval (30 minutes) function. In addition to the mean sleep SBP, the excess sleep SBP surge around the period of the extended hypoxia was suppressed by nighttime administration of a single‐dose of both carvedilol and nifedipine.
The nighttime single‐dose administration of both nifedipine and carvedilol markedly lowered both mean sleep SBP and hypoxia‐related peak sleep SBP, as well as morning BPs (the average of the 3 measures after awakening), while the AHI was not changed by either drug (Table 2).
The BP‐lowering effect of nifedipine on the mean sleep SBP (P<.05) and the basal (minimum) sleep SBP (P<.01) was greater than that of carvedilol (Table 2), while that on the hypoxia‐peak sleep SBP was comparable between the two drugs.
Sleep SBP surge (the difference between the hypoxia‐peak SBP and SBPs within 30 minutes before and after the hypoxia‐peak SBP) was reduced only by carvedilol (P<.05), and the difference in this parameter between the two drugs was not significant (Table 2). Sleep pulse rates measured both by fixed‐interval function and by oxygen‐triggered function and the minimum pulse rate were significantly reduced by carvedilol, while these measures were not changed by nifedipine (Table 2). There was no significant difference in the effect on these sleep BP indexes between the nifedipine first‐started group and the carvedilol first‐started group. Approximately half (54.4%) of study patients were already on morning administration of CCBs while no patient was taking β‐blockers; however, there was no difference in the effects of nifedipine on sleep BP indexes between patients with or without concomitant CCB administration (data not shown).
Figure 4 shows the sleep BPs measured by HITS in a hypertensive patient with OSAS. The results showed that HITS was effective for daily sleep BP monitoring and could detect repeated and day‐to‐day variability in mean sleep BP and the hypoxia‐peak sleep BP under different daily oxygen desaturation indexes (number per hour of oxygen desaturation >4%), of which would be extensively affected by daily environmental changes such as alcohol intake, daytime stress, and sleep quality.
Figure 4.
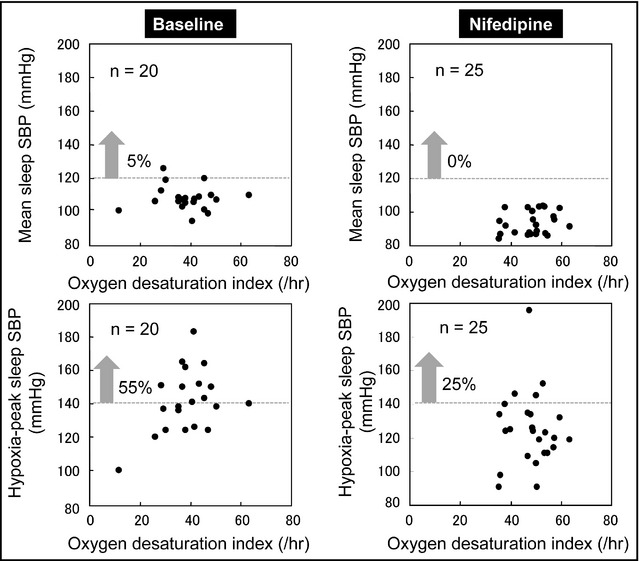
Association between the oxygen desaturation index and sleep blood pressure (BP) measured by home information technology‐based trigger sleep BP monitoring in a hypertensive patient with obstructive sleep apnea syndrome. Each plot shows the BP data and oxygen desaturation index of 20 nights at baseline and of 25 nights after nighttime dosing of nifedipine (40 mg/d). SBP indicates systolic blood pressure. Mean sleep SBP=the average of sleep BPs measured only by the fixed‐interval (30 minutes) function. Hypoxia‐peak sleep SBP=maximum SBP measured by the oxygen‐triggered function.
Discussion
Novelty of the New Device
In this study, we have newly developed the TSP with an oxygen‐triggered function, which could measure sleep BPs to characterize the drug pharmacokinetics in patients with OSAS. The TSP‐detected sleep BP variability in the patient with OSAS was more clear than conventional fixed‐interval BP measurements. By using this device, we defined the “sleep SBP surge” for the first time as the difference between the hypoxia‐related peak SBP and the average of SBPs within 30 minutes before and after the hypoxia‐peak SBP.
This crossover study using the newly developed device clearly demonstrated that the nighttime single‐dose administration of both vasodilating and sympatholytic antihypertensive drugs markedly lowered the mean sleep BP and the hypoxia‐related peak sleep BP in hypertensive patients with OSAS, while the AHI was not changed by either agent. There were several interesting differences in the sleep BP‐lowering effects between the two antihypertensive drugs. The BP‐lowering effect of nifedipine on the mean sleep SBP and the basal sleep SBP was stronger than that of carvedilol, while that on the hypoxia‐related peak sleep SBP was comparable between the two drugs. Finally, we found that only carvedilol reduced sleep SBP surge.
Mean Sleep BP
The mean sleep BP was markedly reduced by nighttime single‐dose administration of both nifedipine and carvedilol, but was more extensively lowered by nifedipine (by >20 mm Hg systolic) than carvedilol (by >15 mm Hg systolic). The mean sleep BP measured by the fixed‐interval (30 minutes in this study) function of TSP is the same as the gold standard sleep BP measured by conventional ABPM. Sleep BP measured by ABPM is well‐known to be more closely associated with cardiovascular risk than awake BP, particularly in medicated patients.7 In addition, nondipper/riser‐type nocturnal hypertension is closely associated with target organ damage and cardiovascular events in both community‐dwelling normotensive patients and hypertensive outpatients.7, 15, 16, 17, 18 Thus, our results support the idea that nighttime administration of either a vasodilating or sympatholytic antihypertensive drug might be recommended for the management of hypertensive patients with OSAS in order to reduce cardiovascular risk.
The previous results on the BP‐lowering effects of different classes of antihypertensive drugs are inconsistent.19, 20 One study demonstrated that a β‐blocker achieved significantly greater reductions in sleep SBP and diastolic BP than a CCB, an angiotensin‐converting enzyme inhibitor, or an angiotensin receptor blocker, but found no significant difference between the β‐blocker and diuretics.19 Monotherapy, including monotherapy with a β‐blocker, reduces daytime BP but is insufficient to control sleep BP.19 However, these studies employed morning administration of antihypertensive drugs.
Hypoxia‐Related Peak Sleep BP and Sleep BP Surge
In addition, nighttime dosing of either nifedipine or carvedilol markedly reduced hypoxia‐related peak sleep SBP, with both reductions being ≥20 mm Hg. The hypoxia‐related peak SBP was markedly higher by 27.4 mm Hg than the mean sleep SBP measured by conventional sleep BP measurement. However, the sleep SBP surge (hypoxia‐related peak SBP minus the average of sleep BPs measured as fixed‐interval function BPs before and after the hypoxia‐peak SBP) was only reduced by carvedilol and not by nifedipine, although there was no significant difference in the change between the two drugs. Thus, hypoxia‐related sleep SBP surge may be predominantly determined by the increased sympathetic tonus induced by sleep apnea episodes. Because exaggerated morning BP surge is a stroke risk independent of age and 24‐hour BP level in elderly hypertensive patients,21, 22 the exaggerated sleep BP surge found in hypertensive patients with OSAS may be a risk for cardiovascular events, and, particularly cardiovascular events with sleep‐onset, which are frequently found in patients with OSAS.12, 13 Although there are no definitive data for establishing the threshold and the target level of hypoxia‐related peak sleep SBP, a reduction of maximum sleep SBP in addition to a reduction of mean sleep SBP would be more effective in reducing target organ damage and sleep‐onset cardiovascular events.
Basal (Minimum) Sleep BP
Nighttime dosing of both nifedipine and carvedilol markedly reduced basal (minimum) sleep SBPs; however, the reduction was significantly greater by nifedipine than by carvedilol. This may indicate the minimum (basal) sleep BP is essentially determined not by sympathetic tonus, but by other factors such as structural change of the small resistance arteries or circulating volume. The previous papers demonstrated that nondippers could be reversed to dippers by reducing the circulating volume using sodium restriction, diuretics, and aldosterone blockers.23, 24
TSP may separately measure sleep BPs with different pathogenic and clinical implications in OSAS.
Proposal of Management of Hypertension in OSAS
As hypertensive patients with OSAS have increased risk of target organ damage and future cardiovascular events, more strict BP control over the course of 24 hours, ie, including the sleep period, is recommended.2, 3 The CPAP effectively reduces sleep BP surge as well as mean sleep BP to reduce cardiovascular risk in patients with OSAS. However, the adherence to CPAP use is poor, particularly in asymptomatic patients, even those with severe OSAS. The patients in this study were hypertensive with moderate/severe OSAS, who declined to be treated by CPAP therapy.
Antihypertensive medication may not diminish all the OSAS‐related cardiovascular risks, such as hypoxia and negative intrathoracic pressure, but nighttime dosing of antihypertensive drugs may significantly reduce the cardiovascular risk related to nocturnal hypertension in OSAS patients.2, 3 In this study, nighttime dosing of both vasodilating and sympatholytic antihypertensive drugs reduced mean sleep SBP to around 110 mm Hg to 120 mm Hg and hypoxia‐peak sleep SBP to around 140 mm Hg. This study demonstrated that nighttime dosing of vasodilating and sympatholytic antihypertensive drugs has the potential to achieve a mean sleep BP <120/70 mm Hg (the threshold of sleep BP for nocturnal hypertension) even in patients with OSAS who were already taking morning administration of ≥1 antihypertensive drugs.
Sleep Pulse Rate and AHI
The extensive sleep BP reduction by nifedipine did not cause an additive increase in pulse rate in the hypertensive patients with increased sympathetic tonus by OSAS. On the other hand, carvedilol significantly reduced sleep pulse rates measured by both oxygen‐triggered and fixed‐interval functions of TSP compared with nifedipine. As recent studies have demonstrated that the sleep pulse rate is synergistically associated with cardiovascular risk, this reduction of sleep pulse rate may have a beneficial effect in patients with OSAS.25, 26 There was no significant reduction of minimum sleep pulse rate even by treatment with nighttime dosing of carvedilol.
In this study, AHI was not changed by nighttime dosing with either nifedipine or carvedilol. Diuretics may be effective in improving AHI by reducing edema of the upper body in patients who have OSAS with heart failure.27 In addition, in patients with resistant hypertension, spironolactone,28 lower body negative pressure,29 and renal denervation30 have been shown to be effective in reducing not only sleep BP but also AHI, but these studies employed few patients and no control group.
Limitations
The hypoxia‐related peak sleep SBP was measured by our newly developed method of cuff inflation–based TSP. Thus, it may have been underestimated compared with the actual peak sleep BP triggered by each episode of sleep apnea. However, compared with fixed‐interval ABPM, the TSP could detect markedly higher BPs during the cluster of severe episodes of sleep apnea and could be used for the assessment of antihypertensive medication on the sleep BP profile, including hypoxia‐related peak SBP and sleep BP surge, in clinical practice. A previously reported beat‐by‐beat BP study using the Finapres device demonstrated a sleep apnea‐related BP surge ranging from 30 mm Hg to 50 mm Hg.9 In our study, the sleep BP surge detected by our TSP was approximately 30 mm Hg.
The other limitation includes drug dose. In this study, we used a single‐dose of nifedipine (slow‐release) 40 mg and carvedilol 20 mg, because one comparative study demonstrated that the SBP‐lowering effect of slow‐release nifedipine 20 mg to 40 mg was comparable to that of carvedilol 25 mg to 50 mg.14 The highest doses of nifedipine and carvedilol permitted in Japan are 40 mg and 20 mg, respectively; therefore, we used these as the highest doses. However, there is no reassurance that drug doses used are equivalent in reducing sleep BP, and a higher dose of carvedilol may have a more clear, specific effect on sleep BP parameters. In addition, as almost all patients were taking renin‐angiotensin system (RAS) inhibitors (RASi). The findings found in this study may partly be attributable to a higher efficacy of RASi‐CCB combination compared with a RASi–β‐blocker combination.
Finally, the major limitation of this study is small sample size. This study only included 11 patients and may not be able to adequately assess the potential hazards of nighttime dosing including excessive nighttime BP reduction potentially causing cardiovascular events. Therefore, additional studies in large population of patients with OSAS are needed. The Japanese patients with OSAS in the study were not severely obese. Our finding should be confirmed in Western obese patients with OSAS, who may also have different drug pharmacokinetics.
Conclusions
This study suggests that nighttime dosing of a vasodilating or a sympatholytic antihypertensive drug may be an effective option for controlling sleep BP in hypertensive patients with OSAS. Our recently developed method of oxygen‐triggered sleep BP monitoring (TSP) could detect different effects of antihypertensive drugs on sleep BP. We introduced TSP into an IT‐based BP management system (HITS), which could detect day‐by‐day variability of mean sleep BP and hypoxia‐related peak sleep BP in daily life. Using this system, we started the prospective Sleep Pressure and Disordered Breathing in Resistant Hypertension and Cardiovascular Disease (SPREAD) Registry to evaluate the clinical implications of different sleep BP profiles in high‐risk patients with resistant hypertension, which is likely to accompany OSAS. Further larger studies will be needed to evaluate the long‐term effects of different antihypertensive drugs, including diuretics and aldosterone blockers, in hypertensive patients with OSAS.
Sources of Funding
This research was supported by a Grant‐in‐Aid for Scientific Research (B) (21390247) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, to K.K., 2009–2013, and by MEXT‐Supported Program for the Strategic Research Foundation at Private Universities, 2011–2015, and by the Foundation for Development of the Community and the Department of Sleep and Circadian Cardiology, Jichi Medical University School of Medicine.
Disclosures
None.
Supporting information
Figure S1. Home information technology‐based trigger sleep blood pressure monitoring system (HITS).
Acknowledgments
We gratefully acknowledge Ms Nahoko Tomitani, Ms Noriko Harada, and Ms Haruna Hamasaki for coordination and data management of this study, and Ms Ayako Okura for editorial support on this paper.
J Clin Hypertens (Greenwich). 2014;16:459–466. DOI: 10.1111/jch.12327. ©2014 Wiley Periodicals, Inc.
References
- 1. Somers VK, White DP, Amin R, et al. American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation . Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118:1080–1111. [DOI] [PubMed] [Google Scholar]
- 2. Kario K. Obstructive sleep apnea syndrome and hypertension: ambulatory blood pressure. Hypertens Res. 2009;32:428–432. [DOI] [PubMed] [Google Scholar]
- 3. Kario K. Obstructive sleep apnea syndrome and hypertension: mechanism of the linkage and 24‐h blood pressure control. Hypertens Res. 2009;32:537–541. [DOI] [PubMed] [Google Scholar]
- 4. Ogihara T, Kikuchi K, Matsuoka H, et al. Japanese Society of Hypertension Committee . The Japanese Society of hypertension guidelines for the management of hypertension (JSH 2009). Hypertens Res. 2009;32:3–107. [PubMed] [Google Scholar]
- 5. Parati G, Lombardi C, Hedner J, et al. European Respiratory Society, EU COST ACTION B26 members . Position paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (COoperation in Scientific and Technological research) ACTION B26 on obstructive sleep apnea. J Hypertens. 2012;30:633–646. [DOI] [PubMed] [Google Scholar]
- 6. Pringle E, Phillips C, Thijs L, et al. Syst‐Eur Investigators . Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251–2257. [DOI] [PubMed] [Google Scholar]
- 7. Boggia J, Li Y, Thijs L, et al. International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators . Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229. [DOI] [PubMed] [Google Scholar]
- 8. Ishikawa J, Hoshide S, Eguchi K, et al. Nighttime home blood pressure and the risk of hypertensive target organ damage. Hypertension. 2012;60:921–928. [DOI] [PubMed] [Google Scholar]
- 9. Planès C, Leroy M, Fayet G, et al. Exacerbation of sleep‐apnoea related nocturnal blood‐pressure fluctuations in hypertensive subjects. Eur Respir J. 2002;20:151–157. [DOI] [PubMed] [Google Scholar]
- 10. Shirasaki O, Yamashita S, Kawara S, et al. A new technique for detecting sleep apnea‐related “midnight” surge of blood pressure. Hypertens Res. 2006;29:695–702. [DOI] [PubMed] [Google Scholar]
- 11. Shirasaki O, Kuwabara M, Saito M, et al. Development and clinical application of a new technique for detecting ‘sleep blood pressure surges' in sleep apnea patients based on a variable desaturation threshold. Hypertens Res. 2011;34:922–928. [DOI] [PubMed] [Google Scholar]
- 12. Hsieh SW, Lai CL, Liu CK, et al. Obstructive sleep apnea linked to wake‐up strokes. J Neurol. 2012;259:1433–1439. [DOI] [PubMed] [Google Scholar]
- 13. Kuniyoshi FH, Garcia‐Touchard A, Gami AS, et al. Day‐night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall S, Prescott RI, Hallman RJ, et al. A comparative study of carvedilol, slow‐release nifedipine, and atenolol in the management of essential hypertension. J Cardiovasc Pharmacol. 1991;18(suppl 4):S35–S38. [PubMed] [Google Scholar]
- 15. Kario K, Matsuo T, Kobayashi H, et al. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–135. [DOI] [PubMed] [Google Scholar]
- 16. Kario K, Pickering TG, Matsuo T, et al. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. [DOI] [PubMed] [Google Scholar]
- 17. Hoshide S, Kario K, Hoshide Y, et al. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community‐dwelling normotensives. Am J Hypertens. 2003;16:434–348. [DOI] [PubMed] [Google Scholar]
- 18. Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24‐h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189. [DOI] [PubMed] [Google Scholar]
- 19. Kraiczi H, Hedner J, Peker Y, Grote L. Comparison of atenolol, amlodipine, enalapril, hydrochlorothiazide, and losartan for antihypertensive treatment in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1423–1428. [DOI] [PubMed] [Google Scholar]
- 20. Pelttari LH, Hietanen EK, Salo TT, et al. Little effect of ordinary antihypertensive therapy on nocturnal high blood pressure in patients with sleep disordered breathing. Am J Hypertens. 1998;11:272–279. [DOI] [PubMed] [Google Scholar]
- 21. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 22. Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56:765–773. [DOI] [PubMed] [Google Scholar]
- 23. Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–1638. [DOI] [PubMed] [Google Scholar]
- 24. Yano Y, Hoshide S, Tamaki N, et al. Efficacy of eplerenone added to renin‐angiotensin blockade in elderly hypertensive patients: the Jichi‐Eplerenone Treatment (JET) study. J Renin Angiotensin Aldosterone Syst. 2011;12:340–347. [DOI] [PubMed] [Google Scholar]
- 25. Kabutoya T, Hoshide S, Ishikawa J, et al. The effect of pulse rate and blood pressure dipping status on the risk of stroke and cardiovascular disease in Japanese hypertensive patients. Am J Hypertens. 2010;23:749–755. [DOI] [PubMed] [Google Scholar]
- 26. Palatini P, Reboldi G, Beilin LJ, et al. Predictive value of night‐time heart rate for cardiovascular events in hypertension. The ABP‐International study. Int J Cardiol. 2013;168:1490–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bucca CB, Brussino L, Battisti A, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. 2007;132:440–446. [DOI] [PubMed] [Google Scholar]
- 28. Gaddam K, Pimenta E, Thomas SJ, et al. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2010;24:532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friedman O, Bradley TD, Chan CT, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug‐resistant hypertension. Hypertension. 2010;56:1077–1082. [DOI] [PubMed] [Google Scholar]
- 30. Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Home information technology‐based trigger sleep blood pressure monitoring system (HITS).


