Abstract
Nondipping blood pressure (BP) is associated with increased morbidity and mortality. This study examines the relationship of “dipping” in 24‐hour ambulatory BP monitoring (ABPM) with awake and sleeping urinary norepinephrine (NE) and epinephrine (EPI), and that of urinary NE and EPI with urinary sodium (UNa). Fifty nondippers and 65 dippers were included in the present study. Collected data included age, sex, body mass index, history of hypertension, current antihypertensive treatment, ABPM data, and NE, EPI, and UNa values. Hierarchical multiple regression analysis with the night‐to‐day ratio (NDR) of systolic BP as a dependent variable showed that the composite term of the NDRs of urinary NE and EPI was a significant predictor for dipping. Results also show a differential role of NE and EPI in circadian UNa excretion in dippers and nondippers. These results indicate that the sympathetic nervous system is involved in the regulation of circadian BP variations and UNa excretion.
Ambulatory blood pressure (BP) monitoring (ABPM) may be used to identify diurnal variations in BP and to differentiate between so‐called dippers, defined as individuals with a ≥10% decrease in systolic BP (SBP) during nighttime, and nondippers, defined as individuals who lack this fall in BP.1, 2
Absence of the normal nocturnal fall in SBP is associated with increased morbidity and mortality,3 and poses a substantial risk even after antihypertensive treatment.4 Hence, restoration of the physiological circadian rhythm may be an aspect of crucial importance in the care of hypertensive patients independent of normalization of elevated BP values.5
The underlying mechanisms responsible for the normal fall in SBP, as well as its absence, are incompletely understood. Based on the seminal work of Guyton and colleagues6, 7 who first drew attention to high urinary sodium (UNa) excretion in hypertensive individuals compared with normotensives, numerous studies have since established a prominent role of sodium balance for the maintenance of a normal circadian BP profile.8, 9, 10, 11, 12, 13
On the other hand, a loss of circadian changes in BP and heart rate (HR) is common in conditions with persistent sympathetic‐parasympathetic imbalance such as after traumatic brain injury.14 In addition, diminished nighttime decreases in systolic and diastolic BP are associated with blunted reductions of nocturnal HR in hypertensive patients,15 and HR variability is reduced in patients with nondipping resistant hypertension.16 Therefore, an impaired sympathetic withdrawal during sleep might be another explanation for nondipping BP characteristics.17, 18
The aim of the present study was to establish a possible relationship between diurnal variations in SBP as determined by 24‐hour ABPM and changes in urinary catecholamine excretion in a population of normotensive and mildly (stage 1) hypertensive volunteers. We also measured UNa in order to find out whether there is a link between urinary catecholamines and UNa excretion in this study population.
Methods
Patients
A total of 115 consecutive normotensive (BP <140/90 mm Hg; n=43) and mildly (stage 1) hypertensive (SBP 140–159 mm Hg or diastolic BP 90–99 mm Hg, or both; n=72) volunteers were enrolled in the present study according to resting office BP readings. The data collected included age, sex, body mass index (BMI), history of hypertension, and current antihypertensive medication. The study was conducted as an extension of the ongoing PMT study and was approved by the local review board and ethics committee of the University Hospital Carl Gustav Carus at the Technical University of Dresden, Dresden. Informed consent was obtained from all study participants.
Ambulatory BP Monitoring
ABPM was obtained with the 90207‐2Q device (Spacelabs Healthcare, Snoqualmie, WA). Daytime and nighttime systolic and diastolic BP as well as HR were recorded at intervals of 15 minutes and 30 minutes each, respectively. Dipping BP characteristics were defined as a night‐to‐day ratio (NDR) of SBP <0.9. Sleep quality was not routinely assessed to authenticate nondipping.
Clinical Chemistry
Urine was collected into two separate boxes for daytime and nighttime samples. Participants were instructed to start daytime collection with the second morning urine and to stop with the last voiding before going to bed. Nighttime collections included all overnight voidings and the first morning urine. Urinary free norepinephrine (NE) and epinephrine (EPI) concentrations were determined by high‐performance liquid chromatography tandem mass spectrometry.19 Day and night UNa and creatinine concentrations were measured according to standard procedures in the Institute of Clinical Chemistry and Laboratory Medicine at the University of Dresden (standard procedures on Modular PPE 9000 from Roche Diagnostics Deutschland GmbH, Mannheim, Germany).
Statistical Analyses
All variables were examined using the Shapiro test for normal distribution and equality of variance using F test. For comparison between dippers and nondippers, Pearson's chi‐squared, Student's t, and Wilcoxon rank tests were used for paired and unpaired data, as appropriate. For correlations, Spearman's correlation coefficient was applied. “Dips” were defined as the respective NDRs. Hierarchical linear models were constructed by backward selection and analyzed using “R”. For model comparison, analysis of variance and Bayesian information criterion20 were used, as required. Two‐tailed statistical tests with a threshold P value of .05 were applied for all analyses and the method of Bonferroni was used for adjustment in subgroup analyses (Ns=2).
Results
Clinical and Laboratory Characteristics of the Study Population
For the whole study population (N=115), mean SBP decreased from day to night from 132±11 mm Hg to 117±10 mm Hg, diastolic BP from 83±9 mm Hg to 70±9 mm Hg, and HR from 78±10 to 66±7 beats per minute (Table 1). Urinary NE and EPI decreased from 16±8 μmol/mol to 8±5 μmol/mol creatinine and from 3.9±2.4 μmol/mol to 0.9±0.5 μmol/mol creatinine. UNa decreased from 103±62 mmol to 52±37 mmol. All of these differences were statistically significant (P<.001) (Table 2).
Table 1.
Population Characteristics With Calculated Night‐to‐Day Ratios
| Total (N=115) | Dipper (n=65) | Nondipper (n=50) | P Value | |
|---|---|---|---|---|
| Age, y | 50±9 | 49±9 | 50±9 | NS |
| Female, % | 60 | 55 | 66 | NS |
| BMI, kg/m² | 27±5 | 26±4 | 28±6 | NS |
| Treated for hypertension, % | 50 | 46 | 56 | NS |
| 24‐h ABPM | ||||
| SBP, mm Hg | 128±13 | 130±15 | 125±11 | NS |
| DBP, mm Hg | 80±11 | 81±12 | 78±9 | NS |
| HR, beats per min | 75±11 | 76±12 | 74±11 | NS |
| Day ABPM | ||||
| SBP, mm Hg | 132±11 | 135±12 | 127±11 | <.01 |
| DBP, mm Hg | 83±9 | 85±9 | 80±9 | .015 |
| HR, beats per min | 78±10 | 79±10 | 76±10 | NS |
| Night ABPM | ||||
| SBP, mm Hg | 117±10 | 113±11 | 120±9 | <.005 |
| DBP, mm Hg | 70±9 | 69±9 | 72±8 | NS |
| HR, beats per min | 66±7 | 66±7 | 66±7 | NS |
| Night‐to‐day ratios | ||||
| Ratio SBP | 0.89±0.06 | 0.84±0.04 | 0.95±0.03 | <.001 |
| Ratio DBP | 0.85±0.07 | 0.81±0.05 | 0.90±0.05 | <.001 |
| Ratio HR | 0.85±0.08 | 0.84±0.08 | 0.87±0.06 | .0470 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; BMI, body mass index; DBP, diastolic blood pressure; HR, heart rate; NS, not significant; SBP, systolic blood pressure. Values are expressed as means±standard deviation.
Table 2.
Urine Catecholamine and Sodium Values With Calculated Night‐to‐Day Ratios
| Total (N=115) | Dipper (n=65) | Nondipper (n=50) | P Value | |
|---|---|---|---|---|
| Urine | ||||
| Day‐NE, μmol/mol creatinine | 16±8 | 17±8 | 15±7 | NS |
| Night‐NE, μmol/mol creatinine | 8.0±5 | 7.9±5 | 8.0±5 | NS. |
| Day‐EPI, μmol/mol creatinine | 3.9±2.4 | 4.3±2.3 | 3.2±2.5 | <.005 |
| Night‐EPI, μmol/mol creatinine | 0.9±0.5 | 0.9±0.5 | 0.9±0.6 | NS |
| Day‐total sodium, mmol | 103±62 | 104±64 | 100±61 | NS |
| Night‐total sodium, mmol | 52±37 | 48±36 | 56±39 | NS |
| Day‐sodium/creatinine, mol/mol crea | 14±8a | 13±6b | 15±10c | NS |
| Night‐sodium/creatinine, mol/mol crea | 12±9a | 11±7b | 14±11c | NS |
| vs daytime | ||||
| Night‐to‐day ratios | ||||
| Ratio NE/creatinine | 0.51±0.19 | 0.47±0.17 | 0.54±0.21 | .023 |
| Ratio EPI/creatinine | 0.26±0.16 | 0.23±0.12 | 0.31±0.20 | .022 |
| Ratio total sodium | 0.61±0.47 | 0.56±0.42 | 0.69±0.53 | NS |
| Ratio sodium/creatinine | 0.97±0.64 | 0.89±0.52 | 1.07±0.77 | NS |
Abbreviations: EPI, epinephrine; NE, norepinephrine. Values are expressed as means±standard deviation. a P<.005. b P<.001. cNot significant (NS).
Dippers and Nondippers
Following the results of ABPM, study participants were divided into 65 dippers and 50 nondippers. There were no differences in age, sex, BMI, mean 24‐hour systolic and diastolic BP, and HR between the two groups of patients. The proportions of hypertensive patients and patients treated for hypertension were also not different (65% and 60% [P=.75] and 46% and 56% [P=.39] in dippers and nondippers, respectively). Diuretics (2 vs 4; P=.24), β‐blockers (7 vs 14; P=.018), calcium channel blockers (9 vs 9; P=.54), and angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (21 vs 19; P=.53) accounted for 95% and 96%, respectively, of antihypertensive drugs used. No significant differences could be detected in the average number of antihypertensive drugs per patient in dippers compared with nondippers (1.4 vs 1.7; P=.21). Awake systolic and diastolic BP values were significantly higher in dippers than in nondippers, whereas nighttime SBP was significantly higher in nondippers (Table 1). Daytime urinary EPI was significantly higher in dippers. A circadian pattern of sodium excretion was demonstrated both in dippers and nondippers, but nighttime decrease in UNa was significant only in dippers (P<.001), indicating enhanced nighttime excretion of sodium in nondippers (Table 2).
Relationship Between the NDRs of NE, EPI, and SBP
A stepwise multiple linear regression model was fitted by backwards selection with the NDR of the SBP as the dependent variable and waking and sleeping SBP; NDRs of HR, NE, EPI; and UNa as independent variables. The NDR of diastolic BP was not considered because of a high correlation with the NDR of SBP (Rs=0.79; P<.001). In this model, a composite term of the NDRs of NE and EPI proved to be a powerful predictor of dipping (P<.001), and a highly significant correlation could be established between these variables (Rs=.338; P<.001) (Figure 1). Moreover, a highly significant correlation could be demonstrated between the NDRs of EPI and SBP (Rs=0.338; P<.0002), whereas the NDRs of NE and SBP were only weakly correlated (Rs=0.213; P<.022) (not shown).
Figure 1.
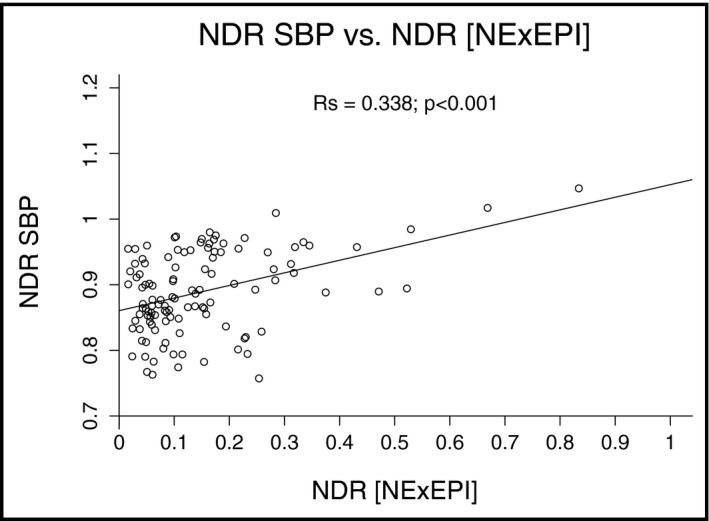
Relationship of the dip in systolic blood pressure (NDR SBP) with the dip in the composite term of norepinephrine and epinephrine (NDR [NE×EPI]). Significance of Spearman's coefficient of correlation is denoted.
Relationship Between the NDRs of NE and EPI and UNa
Further analysis showed a significant correlation between the NDRs of total NE and UNa both in dippers (Rs=0.536; P<.0001) and in nondippers (Rs=0.368; P<.01) (Figure 2A and 2B). In addition, the NDRs of total EPI and UNa were significantly correlated in dippers (Rs=0.540; P<.0001). In contrast, there was no significant correlation between the NDRs of total EPI and UNa in nondippers (Figure 2C and 2D).
Figure 2.

Relationship of the night‐to‐day ratio of total sodium excretion (NDR total Na) with the night‐to‐day ratio of total norepinephrine (NDR total NE) (A and B) and epinephrine (NDR total EPI) (C and D) excretion for dippers (A and C) and nondippers (B and D), respectively. Significances of Spearman's coefficients of correlation are denoted.
Relationship Between Urinary NE and EPI and 24‐Hour UNa
A significant correlation could also be established between 24‐hour urinary NE and 24‐hour UNa in dippers and a trend correlation in nondippers, which, however, became insignificant after adjustment of significance levels for subgroup analyses (Ns=2) according to Bonferroni (Figure 3A and 3B). Values of 24‐hour urinary EPI and 24‐hour UNa were significantly correlated in dippers but not in nondippers (Rs=0.455; P<.0001) (Figure 3C and 3D).
Figure 3.
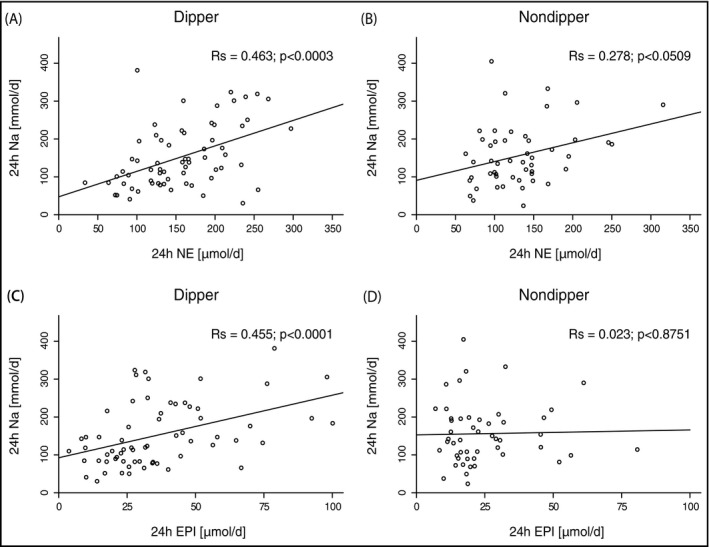
Relationship of the 24‐hour total sodium excretion (24h Na [mmol/d]) with the 24‐hour total NE (24h NE [μmol/d]) (A and B) and EPI (24h EPI [μmol/d]) (C and D) excretion for dippers (A and C) and nondippers (B and D), respectively. Significances of Spearman's coefficients of correlation are denoted.
Discussion
In the present study, we set out to explore the impact of the sympathetic nervous system (SNS) on circadian BP variations, as assessed by changes in urinary NE and EPI. We hypothesized that excess activation of the SNS might be responsible, at least in part, for the loss of the ability in certain individuals (ie, nondippers) to appropriately lower their nighttime SBP compared with their normal counterparts (dippers).
In support of our hypothesis, we found significantly lower NDRs of NE and EPI in our group of normotensive and mildly hypertensive dippers than in the group of nondippers. Furthermore, nighttime UNa excretion was significantly lower in dippers compared with nondippers, thus raising the possibility of major differences between dippers and nondippers not only in the neural mechanisms underlying BP regulation but also renal sodium handling.
In order to unravel these mechanisms in more detail we at first fitted a linear regression model using waking and sleeping SBP and the NDRs of NE and EPI either as two independent variables or as a composite term, to take account of the synergistic actions of these catecholamines. In this model, the composite term of NE and EPI proved to be a powerful predictor of dipping. Moreover, significant correlations could be established between the NDRs of SBP and NE and EPI, strongly in favor of our main hypothesis.
Subgroup analysis revealed that the NDRs of urinary NE and UNa were closely related in dippers and nondippers. In addition, 24‐hour urinary NE was significantly correlated with 24‐hour UNa in dippers and trend correlated in nondippers. By contrast, both NDRs of EPI and UNa and 24‐hour urinary EPI and 24‐hour UNa were significantly correlated only in dippers.
Thus, beyond a close relationship between the nighttime decreases in urinary NE and EPI and the magnitude of nighttime drops in SBP in our study population, a significant relationship became apparent between NE and EPI and UNa in dippers, but only a weak relationship was found between NE and UNa and no relationship was found between EPI and UNa in nondippers. Given the known synergistic effects of NE and EPI on total peripheral resistance and the prime role of EPI in renal sodium handling,21 these results fit well with the notion of a prevailing regulation by NE of diurnal BP variations and UNa excretion in dippers and an increase in the weight of the effects of EPI in nondippers as reflected by the noticeable lack of correlation between EPI and UNa in these patients. The reason for this is not clear, but it may be the result of an enhanced recruitment of renin‐secreting cells in response to the attenuated nighttime decrease of EPI found in nondippers with ensuing upregulation of the renin‐angiotensin‐aldosterone system or determined genetically. However, since neither renin activity nor aldosterone levels were measured in the present study, these explanations remain speculative.
Our results are in line with findings that show a parallel decrease of sympathetic activity and UNa excretion in patients with mild to moderate essential hypertension on a long‐term low‐sodium–based diet.22 They are also consistent with studies describing recovery of a normal circadian BP rhythm in hypertensive nondippers upon treatment with diuretics.23, 24 These drugs are known to exert their short‐term BP‐lowering effect via volume and sodium depletion but their long‐term hypotensive action mainly by lowering total peripheral resistance.23, 25, 26
It should be clarified, however, that no firm conclusions can be drawn concerning the primacy of the SNS in the pathogenesis of nondipping BP characteristics. Although our results are consistent with the notion of enhanced sympathetic activity in nondippers in the first place, they would also appear to be compatible with available evidence of a lead role of the kidneys in the development of hypertension and abnormal BP diurnal variability.22, 27, 28
Study Limitations
Our study has several limitations. First, classification of dippers and nondippers relied on a single ABPM recording, and neither sleep records nor diaries were routinely kept to authenticate nondipping. However, conflicting evidence exists on the impact of poor sleep quality on nocturnal BP dipping.29, 30 Similarly, there is no consistent support in favor of more than one ABPM recording for satisfactory assessment of a diurnal BP rhythm,31 and there is also no indication for the preferential misclassification of patients into one group or the other.5 Although, occasional misclassifications cannot therefore be ruled out, this should not have affected the overall findings of our study. Another limitation relates to the use of urinary catecholamines as a surrogate for sympathetic activity.32, 33, 34, 35 Yet, measurement of urinary catecholamines has been successfully applied to delineate altered sympathetic activity in various health conditions and ethnicities.36, 37, 38, 39, 40 Therefore, we are confident that the differences in NDRs of urinary catecholamines observed between dippers and nondippers in the present study do reflect real differences in sympathetic activity between these two study populations.
Conclusions
The results of the present study provide evidence for the involvement of the SNS in the regulation of circadian BP variations and are in support of different contributions of NE and EPI to circadian BP regulation and UNa excretion in dippers and nondippers. However, the results do not allow conclusions to be drawn concerning the primacy of the SNS or the kidneys in the development of a nondipping BP pattern.
Acknowledgments
We thank Dr V. Neumeister for measurements of sodium and creatinine in urine collections; Ms D. Pelzel for data collection; and R.A. Wesley, PhD, for providing biostatistics services.
Disclosure
This research was supported by a research grant from the German‐Israeli Foundation. Otherwise, the authors report no specific funding in relation to this research and no conflicts of interest to disclose.
J Clin Hypertens (Greenwich). 2016;18:921–926. DOI: 10.1111/jch.12791. © 2016 Wiley Periodicals, Inc.
References
- 1. O'Brien E, Sheridan J, O'Malley K. Dippers and non‐dippers. Lancet. 1988;2:397. [DOI] [PubMed] [Google Scholar]
- 2. Pickering TG. The clinical significance of diurnal blood pressure variations. Dippers and nondippers. Circulation. 1990;81:700–702. [DOI] [PubMed] [Google Scholar]
- 3. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. [DOI] [PubMed] [Google Scholar]
- 4. Ben‐Dov IZ, Kark JD, Ben‐Ishay D, et al. Predictors of all‐cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension. 2007;49:1235–1241. [DOI] [PubMed] [Google Scholar]
- 5. Schillaci G, Battista F, Settimi L, et al. Antihypertensive drug treatment and circadian blood pressure rhythm: a review of the role of chronotherapy in hypertension. Curr Pharm Des. 2015;21:756–772. [DOI] [PubMed] [Google Scholar]
- 6. Evans RG, Majid DS, Eppel GA. Mechanisms mediating pressure natriuresis: what we know and what we need to find out. Clin Exp Pharmacol Physiol. 2005;32:400–409. [DOI] [PubMed] [Google Scholar]
- 7. Guyton AC, Coleman TG, Cowley AV Jr, et al. Arterial pressure regulation. Overriding dominance of the kidneys in long‐term regulation and in hypertension. Am J Med. 1972;52:584–594. [DOI] [PubMed] [Google Scholar]
- 8. Bankir L, Bochud M, Maillard M, et al. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension. 2008;51:891–898. [DOI] [PubMed] [Google Scholar]
- 9. Sachdeva A, Weder AB. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension. 2006;48:527–533. [DOI] [PubMed] [Google Scholar]
- 10. Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. [DOI] [PubMed] [Google Scholar]
- 11. Uzu T, Ishikawa K, Fujii T, et al. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1997;96:1859–1862. [DOI] [PubMed] [Google Scholar]
- 12. Ando K, Fujita T. Abnormal renal hemodynamics in salt‐sensitive patients with essential hypertension. Jpn Circ J. 1985;49:984–989. [DOI] [PubMed] [Google Scholar]
- 13. Schmidlin O, Forman A, Tanaka M, et al. NaCl‐induced renal vasoconstriction in salt‐sensitive African Americans: antipressor and hemodynamic effects of potassium bicarbonate. Hypertension. 1999;33:633–639. [DOI] [PubMed] [Google Scholar]
- 14. Pattoneri P, Tirabassi G, Pela G, et al. Circadian blood pressure and heart rate changes in patients in a persistent vegetative state after traumatic brain injury. J Clin Hypertens (Greenwich). 2005;7:734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ben‐Dov IZ, Kark JD, Ben‐Ishay D, et al. Blunted heart rate dip during sleep and all‐cause mortality. Arch Intern Med. 2007;167:2116–2121. [DOI] [PubMed] [Google Scholar]
- 16. Salles GF, Ribeiro FM, Guimaraes GM, et al. A reduced heart rate variability is independently associated with a blunted nocturnal blood pressure fall in patients with resistant hypertension. J Hypertens. 2014;32:644–651. [DOI] [PubMed] [Google Scholar]
- 17. Mancia G. Autonomic modulation of the cardiovascular system during sleep. N Engl J Med. 1993;328:347–349. [DOI] [PubMed] [Google Scholar]
- 18. Somers VK, Dyken ME, Mark AL, et al. Sympathetic‐nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. [DOI] [PubMed] [Google Scholar]
- 19. Peitzsch M, Pelzel D, Glöckner S, et al. Simultaneous liquid chromatography tandem mass spectrometric determination of urinary free metanephrines and catecholamines, with comparisons of free and deconjugated metabolites. Clin Chim Acta. 2013;418:50–58. [DOI] [PubMed] [Google Scholar]
- 20. Schwarz G. Estimating the dimension of a model. Ann Statist. 1978;6:461–464. [Google Scholar]
- 21. Goldstein DS. Adrenaline and noradrenaline eLS. National Institutes of Health, Bethesda, Maryland: 2001. [Google Scholar]
- 22. Beckmann SL, Os I, Kjeldsen SE, et al. Effect of dietary counselling on blood pressure and arterial plasma catecholamines in primary hypertension. Am J Hypertens. 1995;8:704–711. [DOI] [PubMed] [Google Scholar]
- 23. Shah S, Khatri I, Freis ED. Mechanism of antihypertensive effect of thiazide diuretics. Am Heart J. 1978;95:611–618. [DOI] [PubMed] [Google Scholar]
- 24. Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–1638. [DOI] [PubMed] [Google Scholar]
- 25. Duarte JD, Cooper‐DeHoff RM. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide‐like diuretics. Expert Rev Cardiovasc Ther. 2010;8:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pickkers P, Hughes AD, Russel FG, et al. Thiazide‐induced vasodilation in humans is mediated by potassium channel activation. Hypertension. 1998;32:1071–1076. [DOI] [PubMed] [Google Scholar]
- 27. Koeners MP, Braam B, Joles JA. Blood pressure follows the kidney: perinatal influences on hereditary hypertension. Organogenesis. 2008;4:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Farmer CK, Goldsmith DJ, Cox J, et al. An investigation of the effect of advancing uraemia, renal replacement therapy and renal transplantation on blood pressure diurnal variability. Nephrol Dial Transplant. 1997;12:2301–2307. [DOI] [PubMed] [Google Scholar]
- 29. Manning G, Rushton L, Donnelly R, et al. Variability of diurnal changes in ambulatory blood pressure and nocturnal dipping status in untreated hypertensive and normotensive subjects. Am J Hypertens. 2000;13:1035–1038. [DOI] [PubMed] [Google Scholar]
- 30. Loredo JS, Ancoli‐Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in obstructive sleep apnea. Am J Hypertens. 2001;14:887–892. [DOI] [PubMed] [Google Scholar]
- 31. Staessen J, Bulpitt CJ, O'Brien E, et al. The diurnal blood pressure profile. A population study. Am J Hypertens. 1992;5:386–392. [DOI] [PubMed] [Google Scholar]
- 32. Saxena AR, Chamarthi B, Williams GH, et al. Predictors of plasma and urinary catecholamine levels in normotensive and hypertensive men and women. J Hum Hypertens. 2014;28:292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Folkow B, Di Bona GF, Hjemdahl P, et al. Measurements of plasma norepinephrine concentrations in human primary hypertension. A word of caution on their applicability for assessing neurogenic contributions. Hypertension. 1983;5:399–403. [DOI] [PubMed] [Google Scholar]
- 34. Schöfl C, Becker C, Prank K, et al. Twenty‐four‐hour rhythms of plasma catecholamines and their relation to cardiovascular parameters in healthy young men. Eur J Endocrinol. 1997;137:675–683. [DOI] [PubMed] [Google Scholar]
- 35. Esler M, Lambert G, Brunner‐La Rocca HP, et al. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand. 2003;177:275–284. [DOI] [PubMed] [Google Scholar]
- 36. O'Driscoll DM, Horne RS, Davey MJ, et al. Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep Med. 2011;12:483–488. [DOI] [PubMed] [Google Scholar]
- 37. Sherwood A, Steffen PR, Blumenthal JA, et al. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens. 2002;15:111–118. [DOI] [PubMed] [Google Scholar]
- 38. Sherwood A, Routledge FS, Wohlgemuth WK, et al. Blood pressure dipping: ethnicity, sleep quality, and sympathetic nervous system activity. Am J Hypertens. 2011;24:982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Berge‐Landry H, James GD, Brown DE. Relationships between diurnal changes in blood pressure and catecholamines among Filipino–American and European–American women. Am J Hum Biol. 2013;25:431–433. [DOI] [PubMed] [Google Scholar]
- 40. Snow AB, Khalyfa A, Serpero LD, et al. Catecholamine alterations in pediatric obstructive sleep apnea: effect of obesity. Pediatr Pulmonol. 2009;44:559–567. [DOI] [PubMed] [Google Scholar]


