Abstract
This study aimed to examine the association between obstructive sleep apnea (OSA) and morning blood pressure surge in never‐treated patients with essential hypertension. This prospective study included a total of 58 patients (mean age, 51.7 years; 55.2% men) with never‐treated essential hypertension. The patients were divided into non‐OSA (n=23, 49.3±12.7 years) and OSA (n=35, 53.2±9.8 years) groups. The OSA group was defined as having an apnea‐hypopnea index level >5 as measured by the Watch‐PAT 200. The authors collected 24‐hour ambulatory BP, plasma aldosterone concentration, and plasma renin activity data from all of the patients. The measured sleep‐trough morning systolic blood pressure (SBP) increases were higher in the OSA group than in the non‐OSA group (28.7±11.8 mm Hg vs 19.6±12.8 mm Hg, P=.008). The sleep‐trough morning SBP increase was inversely correlated with the lowest oxygen saturation (r=−0.272, P=.039). OSA known to be associated with increased daytime and nocturnal sympathetic activity was associated with significantly higher sleep‐trough morning SBP levels in this study.
Obstructive sleep apnea (OSA) is a common disorder characterized by episodes of upper airway obstruction during sleep. The prevalence of OSA (apnea±hypopnea index [AHI] ≥5) in adults 30 to 69 years is estimated at 17%, increasing to 23% to 35% in relatively unselected hypertensive populations.1, 2 OSA is associated with endothelial dysfunction, metabolic syndrome, atherosclerosis, and cardiovascular disorders.3, 4, 5, 6, 7 Morning blood pressure (BP) surge (MS) is a normal physiological phenomenon; however, extreme MS is a risk factor for stroke and cardiovascular mortality.8, 9, 10
Sympathetic activity is suspected to play a role as an underlying mechanism in OSA and MS.7, 11, 12, 13 Few studies have evaluated the association between OSA and MS.14, 15 Peripheral arterial tone (PAT) is based on the pulsatile plethysmographic signal that is measured on a finger, which could serve as a single noninvasive substitute for sympathetic activity.16 This study aimed to examine the association of sleep parameters with WATCH‐PAT 200 (WP200; (Itamar Medical Ltd, Caesarea, Israel) based on measurements of PAT variations and MS in never‐treated patients with essential hypertension.16, 17, 18, 19
Materials and Methods
This prospective cross‐sectional study included a total of 58 consecutive patients (mean age, 51.7 years; 55.2% men) with never‐treated essential hypertension between January 2013 and May 2014. All the enrolled patients underwent 24‐hour ambulatory BP monitoring (ABPM) and WP200 monitoring simultaneously. The patients were divided into non‐OSA (n=23, 49.3±12.7 years) and OSA (n=35, 53.2±9.8 years) groups. The OSA group was defined as having an AHI ≥5, measured with the WP200 system. WP200 is a diagnostic tool for OSA based on measurements of PAT variations coupled with pulse rate accelerations and desaturations in oximetry that has been validated with polysomnography.
The enrolled patients had a systolic BP (SBP) >135 mm Hg and a separate or simultaneous diastolic BP (DBP) >85 mm Hg based on ABPM.20 We excluded patients with the following disorders: secondary hypertension, history of peripheral vasculopathy or neuropathy, autonomic nervous system dysfunction, cardiac arrhythmia, lung disease, diabetes, or <80% valid BP readings while awake or asleep. Office BP was measured by a physician using a mercury sphygmomanometer with patients sitting and relaxed for ≥10 minutes. Three BP measurements were averaged for the analysis, and SBP and DBP were identified by Korotkoff phases I and V.
We collected the ABPM parameters, WP200 data, pulse wave velocity (PWV), ankle brachial index (ABI), plasma aldosterone concentration (PAC), and plasma renin activity (PRA) from all patients.
Ambulatory BP was monitored using an automatic oscillometric device (TM‐2430, A&D Company, Tokyo, Japan) at 30‐minute intervals during the night and at 15‐minute intervals during the day. Morning BP was defined as the mean SBP or DBP for 2 hours after waking. Sleep‐trough morning SBP increase was defined as the difference between the morning SBP and the average of three readings centered on the lowest SBP taken while the patients were asleep. Preawakening morning SBP increase was defined as the difference between the average of three readings centered on the lowest SBP taken while the patients were asleep and the average BP for 2 hours before awakening.9
All of the patients underwent overnight WP200 monitoring with a commercially available portable nonattended sleep recorder based on the PAT signal, pulse rate, actigraphy, and pulse oximetry.17 WP200 monitoring has been validated to accurately detect OSA, automatic arousals, and sleep/wake status.2, 17 The scoring and interpretation of sleep study data were performed with an automatic Watch‐PAT software algorithm (Itamar Medical Ltd, Caesarea, Israel). The AHI is a numerical measure that accounts for the number of pauses in a patient's breathing per hour of sleep. The oxygen desaturation index is the number of times per hour of sleep that the blood oxygen level drops by ≥3% or more from baseline. OSA was defined as an AHI ≥5, which is currently classified as having more than mild sleep apnea.
The PAC and PRA were measured using overnight fasting blood samples obtained between 7 am and 9 am after patients rested for 30 minutes in a sitting position. The PRA (1.3–3.95 ng/mL/h) and PAC (reference value, 4.0–31 ng/dL) values were determined by a radioimmunoassay using an r‐counter (Cobra; Packard, Meriden, CT). The PRA was measured using a DiaSorinLiaison immunochemiluminometric analyzer (DiaSorin Ltd, Wokingham, Berkshire, United Kingdom). The PAC was measured using a solid‐phase radioimmunoassay kit (Siemens Ltd, Camberley, Surrey, United Kingdom). Kidney function was assessed by estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease formula: GFR, in mL/min/1.73 m2=175 × SCr (exp[−1.154]) × age (exp[−0.203]) × (0.742 if female).21 Bilateral brachial‐ankle pulse wave velocity (baPWV) was measured in all patients using an automated device (form PWV/ABI, Colin Co. Ltd, Komaki, Japan). Form ABI/PWV is a device with four cuffs that can simultaneously measure BP levels in both arms and both legs and automatically calculate the ankle brachial pressure index. This device can also record pulse waves by sensors in the cuffs, and automatically compute and output the baPWV values by transmission time and distance.
Written informed consent was obtained from all patients. The protocol was approved by the ethics committee of Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Daejeon, and Republic of Korea (DC13DISI0029).
Statistical Analysis
Continuous variables are expressed as mean±standard deviation (SD), and categorical variables are expressed as the numbers of individuals and percentages. Non‐OSA and OSA patient variables were analyzed using a two‐sample t test or a Wilcoxon signed‐rank test as appropriate. Correlations between continuous variables were analyzed using Pearson's correlation tests.
Morning surge was the dependent variable and lowest oxygen saturation, 24‐hour SBP SD, 24‐hour DBP SD, night SBP SD, night DBP SD, and eGFR were independent variables. This model permitted us to assess the contribution of each variable alone to the variability in morning surge. We took all of the variables with significant (P<.05) contribution to the morning surge except for 24‐hour and night DBP SD and night SBP SD. Twenty‐four–hour SBP SD was selected representatively because of correlation among SD parameters as BP variety parameters. Multiple, stepwise, forward, linear regression analysis was performed to identify those variables that have an independent contribution to morning surge (Table 3).
Table 3.
Linear Regression Results for Morning Surge
| Variables | Univariable | P Value | Multivariable | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | Partial R 2 | Model R 2 | |||
| Age, y | −0.009 | 0.155 | .952 | −0.109 | 0.151 | 0.006 | 0.006 | .567 |
| AHI, events per h | 0.336 | 0.210 | .115 | |||||
| Lowest SaO2, % | −0.796 | 0.376 | .039 | −0.475 | 0.380 | 0.090 | 0.097 | .028 |
| 24‐h SBP SD | 1.596 | 0.466 | .001 | 0.999 | 0.499 | 0.080 | 0.177 | .032 |
| 24‐h DBP SD | 0.986 | 0.433 | .027 | |||||
| Night SBP SD | 0.954 | 0.449 | .038 | |||||
| Night DBP SD | 1.164 | 0.456 | .014 | |||||
| PRA, ng/mL/h | 0.340 | 1.034 | .744 | |||||
| PAC, ng/dL | −0.599 | 0.354 | .096 | |||||
| eGFR, mL/min/1.73 m2 | 0.146 | 0.070 | .043 | 0.101 | 0.071 | 0.033 | 0.209 | .161 |
Abbreviations: AHI, apnea‐hypopnea index; DBP, diastolic blood pressure; PAC, plasma‐aldosterone concentration; eGFR, estimated glomerular filtration rate; PRA, plasma‐renin activity; SaO2, oxygen saturation; SBP, systolic blood pressure; SD, standard deviation; SE, standard error.
SPSS for Windows (version 18.0, SPSS, Inc, Chicago, IL) was used. A probability level of P<.05 was considered statistically significant.
Results
The clinical characteristics of the patients, including ABPM parameters and PWV according to non‐OSA and OSA status, are presented in Table 1. Body mass index (BMI), 24‐hour SBP and DBP, daytime SBP and DBP, nighttime SBP, sleep‐trough morning SBP increase, and preawakening morning SBP increase were higher in the OSA patients than in the non‐OSA patients (Table 1) (BMI: 23.6±3.4 in the non‐OSA patients, 26.2±2.9 in the OSA patients; P=.004) (sleep‐trough morning SBP increase, 19.6±12.8 mm Hg in the non‐OSA patients, 29.7±11.8 mm Hg in the OSA patients; P=.008) (preawakening morning SBP increase: 5.3±11.4 mm Hg in the non‐OSA patients, 11.6±9.7 mm Hg in the OSA patients, P=.028).
Table 1.
Patient Characteristics, BP Parameters, and Arterial Stiffness According to OSA Status
| Non‐OSA (n=23) | OSA (n=35) | P Value | |
|---|---|---|---|
| Age, y | 49.3±12.78 | 53.2±9.8 | .194 |
| Men, No. (%) | 15 (65.2) | 17 (48.5) | .283 |
| Body mass index, kg/m2 | 23.6±3.4 | 26.2±2.9 | .004 |
| Ever‐smoker, No. (%) | 6 (26.1) | 13 (37.1) | .410 |
| Daily drinker, No. (%) | 2 (8.7) | 2 (5.7) | 1.00 |
| Total time sleep, min | 373.3±87.4 | 351.0±85.2 | .338 |
| AHI, events per h | 2.5±1.5 | 12.9±7.9 | <.001 |
| Lowest SaO2, % | 91.3±3.1 | 87.6±4.5 | .001 |
| ODI, events per h | 0.86±0.69 | 7.67±6.47 | <.001 |
| BP measurement | |||
| Office SBP, mm Hg | 146.9±11.6 | 154.0±16.1 | .074 |
| Office DBP, mm Hg | 84.8±10.0 | 89.9±11.6 | .096 |
| Office PR, bpm | 78.0±13.1 | 75.0±9.5 | .056 |
| 24‐h SBP, mm Hg | 136.0±12.3 | 145.9±15.5 | .014 |
| 24‐h SBP SD | 15.4±2.4 | 17.1±3.9 | .088 |
| 24‐h DBP, mm Hg | 81.8±17.5 | 92.1±11.2 | .008 |
| 24‐h DBP SD | 11.5±2.6 | 13.4±4.5 | .043 |
| 24‐h PR, bpm | 70.0±9.0 | 70.5±8.8 | .826 |
| 24‐h PR SD | 13.6±15.0 | 10.0±2.8 | .187 |
| Day SBP, mm Hg | 138.7±12.7 | 149.0±15.6 | .011 |
| Day SBP SD | 13.9±2.9 | 15.6±4.1 | .110 |
| Day DBP, mm Hg | 87.3±7.7 | 94.5±11.5 | .007 |
| Day DBP SD | 10.6±3.1 | 12.8±4.5 | .048 |
| Day PR, bpm | 72.5±10.1 | 72.9±9.9 | .884 |
| Night SBP, mm Hg | 124.6±15.7 | 133.3±15.9 | .044 |
| Night SBP SD | 12.1±4.1 | 13.8±3.4 | .099 |
| Night DBP, mm Hg | 77.0±9.8 | 82.3±11.4 | .074 |
| Night DBP SD | 8.3±3.0 | 10.0±4.0 | .075 |
| Night PR, bpm | 58.3±6.5 | 60.4±7.6 | .305 |
| Sleep‐trough morning SBP increase, mm Hg | 19.6±12.8 | 29.7±11.8 | .008 |
| Pre‐awakening morning SBP increase, mm Hg | 5.3±11.4 | 11.6±9.7 | .028 |
| Arterial stiffness | |||
| Pulse wave velocity, both | 1,567.1±325.3 | 1,624.1±248.9 | .454 |
| Ankle‐brachial index, both | 1.13±0.08 | 1.12±0.05 | .608 |
Abbreviations: BP, blood pressures bpm, beats per minute; DBP, diastolic blood pressure; MS, morning surge; ODI, oxygen desaturation index; PR, pulse rate; SBP, systolic blood pressure. Data are expressed as mean±standard deviation. Obstructive sleep apnea (OSA) is defined as an apnea‐hypopnea index (AHI) >5 based on a nonattended portable sleep recorder‐based peripheral arterial tone.
The decreased amplitude of the PAT reflected sympathetic overactivity during sleeping. When a decrease in the PAT signal amplitude occurred in tandem with oxygen desaturation, a relative tachycardia, and an actigraphy signal, sympathetic overactivity caused by OSA was diagnosed. Decreased PAT amplitude was rare in non‐OSA patients, and simultaneous ABPM revealed lower morning SBP and reduced sleep‐trough morning SBP increases in non‐OSA patients compared with OSA patients (Figure 1A). Figure 1B shows the frequent decreased PAT amplitude coupled with desaturation, and ABPM showed elevated morning SBP and sleep‐trough morning SBP increases. Sleep‐trough morning SBP increases were inversely correlated with the lowest oxygen saturation (r=−0.272, P=.039) (Figure 2). AHI was correlated with morning SBP and DBP (r=0.281, P=.032; r=0.319, P=.015). There were no significant differences in PRA or PAC between the non‐OSA and OSA groups (Table 2).
Figure 1.
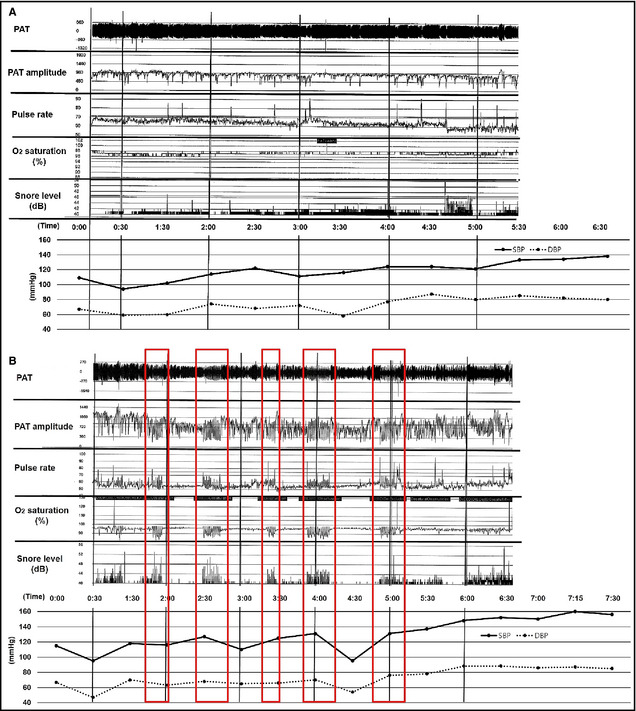
Decreased peripheral arterial tonometry (PAT) amplitude was not frequent in nonobstructive sleep apnea (OSA) patients, and simultaneous ambulatory blood pressure monitoring (ABPM) revealed lower values of morning systolic blood pressure (SBP) and sleep‐trough morning SBP increases in non‐OSA patients compared with OSA patients (A). The frequently decreased PAT amplitude coupled with desaturation is shown (red box), and ABPM showed elevated morning SBP and sleep‐trough morning SBP increases in OSA patients (B).
Figure 2.
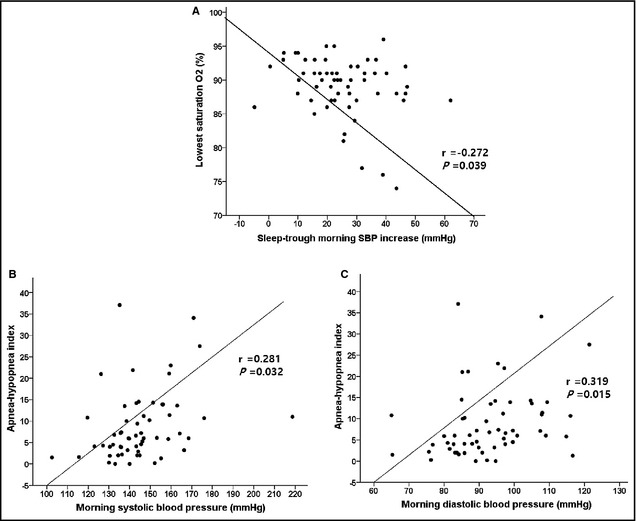
The sleep‐trough morning systolic blood pressure (SBP) increase was inversely correlated with the lowest oxygen (O2) saturation. The apnea‐hypopnea index was significantly correlated with morning SBP and diastolic blood pressure.
Table 2.
Laboratory Data According to Obstructive Sleep Apnea Status
| Non‐OSA (n=23) | OSA (n=35) | P Value | |
|---|---|---|---|
| Fasting glucose, mg/dL | 100.4±17.5 | 110.0±21.5 | .083 |
| HbA1c, % | 5.46±0.49 | 5.86±0.63 | .031 |
| Total cholesterol, mg/dL | 189.8±28.0 | 204.4±46.1 | .187 |
| Triglycerides, mg/dL | 139.4±95.6 | 163.8±85.9 | .326 |
| High‐density lipoprotein, mg/dL | 58.8±12.6 | 49.3±12.8 | .009 |
| Low‐density lipoprotein, mg/dL | 103.1±31.4 | 122.3±41.0 | .067 |
| eGFR, mL/min/1.73 m2 | 92.4±25.4 | 94.9±21.8 | .671 |
| Uric acid, mg/dL | 5.55±1.60 | 6.71±8.43 | .548 |
| Microalbumin creatinine ratio, mg/L | 8.4±11.8 | 14.2±18.0 | .216 |
| hs‐CRP, mg/dL | 2.4±3.7 | 2.3±3.0 | .901 |
| Fibrinogen, mg/dL | 282.2±67.2 | 289.0±89.5 | .825 |
| PRA, ng/mL/h | 2.04±2.24 | 1.48±1.41 | .243 |
| PAC, ng/dL | 10.85±5.83 | 9.88±4.87 | .497 |
| ARR | 12.2±11.9 | 17.3±18.2 | .320 |
Abbreviations: ARR, aldosterone‐renin ratio (PAC/PRA); eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; hs‐CRP, high‐sensitivity C‐reactive protein; PAC, plasma‐aldosterone concentration; PRA, plasma‐renin activity; SaO2, oxygen saturation. Data are expressed as mean±standard deviation.
The patients in the OSA group had higher levels of gylcated hemoglobin (HbA1c) and lower levels of high‐density lipoprotein (HDL) (HbA1c: 5.46%±0.49% in non‐OSA patients, 5.86%±0.63% in OSA patients; P=.031) (HDL: 58.8±12.6 mg/dL in non‐OSA patients, 49.3±12.8 mg/dL in OSA patients; P=.009) (Table 2). Multivariable linear regression analysis revealed that lowest oxygen saturation during sleep and 24‐hour SBP SD were independent predictors of MS (Table 3).
Discussion
In the present study, the OSA group diagnosed by sleep recorder–based PAT had higher 24‐hour SBP and DBP, day SBP and DBP, night SBP, preawakening morning SBP, and sleep‐trough morning SBP increases compared with the non‐OSA group. Furthermore, the lowest oxygen saturation as one of the parameters of OSA was inversely correlated with the sleep‐trough morning SBP increases and was an independent predictor of MS.
The association between OSA and sympathetic activity has been extensively studied using various autonomic or hemodynamic signals such as muscle sympathetic nerve activity, PAT, BP, and heart rate.1, 5 Recurrent episodes of airway obstruction result in hypoxia and hypercapnia, increasing sympathetic neural tone, which, in turn, causes vasoconstriction and marked increases in BP.22 The PAT signal is acquired by applying a subdiastolic pressure of approximately 40 mm Hg in a finger cuff with two air‐inflatable pressure chambers. The fingers are populated with a high density of α‐receptors, which are needed for the regulation of vascular tone; these receptors represent one branch of sympathetic activity.16, 23 Although the PAT signal could be affected by BP, peripheral vascular resistance, blood volume in the finger, and activation of the autonomic nervous system, the Watch‐PAT software automatic algorithm was used to determine whether such an effect occurred. The automatic algorithm could determine the timing of the PAT signal amplitude decrease combined with a relative tachycardia, desaturation, and actigraphy signal to facilitate PAT arousal scoring.16, 23
Sympathetic nervous system activation has also been proposed as the mechanism underlying MS.11, 13 Narkiewicz and colleagues24 reported in a muscle sympathetic nerve activity study in patients without hypertension that higher sympathetic traffic is associated with an increase in daytime BP and a greater difference in day and night BP. Furthermore, MS in elderly patients with hypertensive cerebrovascular disease is known to be dependent on the α‐adrenergic sympathetic nervous system.25 Therefore, the present study is helpful for understanding the association between MS and OSA through sympathetic activity in relatively young and never‐treated patients with essential hypertension. Our previous study demonstrated that relatively young and never‐treated patients with essential hypertension may be less affected by the renin‐angiotensin‐aldosterone system, which is one of the underlying mechanisms of MS.11
Even mild sleep apnea is known to increase nocturnal BP. Additionally, in the present study, the OSA group had higher night SBP than the non‐OSA group (Table 1). The present study suggests that the association of increased nocturnal BP and extreme MS in patients with OSA may be controversial. In the present study, increased nocturnal BP in OSA is not in agreement with extreme MS in OSA. An increase in sleep‐trough morning SBP was defined as the difference between the morning SBP and the average of three readings centered on the lowest SBP taken while the patients were asleep.9, 11 Therefore, an increase in sleep‐trough morning SBP may not reflect nocturnal BP; rather, it may reflect an abrupt elevation in morning SBP.
The chronic intermittent hypoxia in OSA patients is known to increase the levels of various inflammatory markers, oxidative stress, and procoagulant and thrombotic activity.26, 27 These factors may contribute to the development of endothelial dysfunction, metabolic syndrome, atherosclerosis, and cardiovascular disease associated with OSA.28 Our study also demonstrated the presence of higher levels of HbA1c and lower levels of HDL compared with those in the non‐OSA group. However, the heart rate and BP variability that is generally accepted to be affected by the sympathetic nervous system did not show differences between the groups. These results could be caused by the relatively small sample size.
Study Limitations
This study has several limitations. First, the enrolled patients in the present study had OSA diagnosed with the WP200. The WP200 is able to detect large respiratory events related to sympathetic activations in OSA based on PAT recordings.16 However, the PAT amplitude does not provide absolute values; thus, only within‐subject changes in the pulse wave analysis during a limited time interval could be evaluated.22 The WP200 could not distinguish between obstructive sleep apnea and central sleep apnea without activation of the respiratory muscles because it does not use electroencephalography to determine the sleep stage. The WP200 detects arousal based on the timing of the decrease in the PAT signal amplitude combined with relative tachycardia and actigraphy signals. In addition, several studies have demonstrated the accuracy of the WP200 in diagnosing OSA.16, 17, 18, 19, 22, 23, 29 Second, the sleep‐trough morning SBP increase in this study did not meet the criteria of extreme morning surges employed in other studies because the enrolled patients were relatively young and were never‐treated early hypertensive patients.10 MS is significantly associated with older hypertensive patients, especially those with established target organ damage and a long duration of hypertension.9, 30
Conclusions
Our study showed that patients with OSA had higher daytime, nighttime, and 24‐hour SBPs and DBPs and an increase in sleep‐trough morning SBP. Furthermore, lowest oxygen saturation during sleep and 24‐hour SBP SD were independent predictors of MS in this study. The association of OSA and MS may be caused by sympathetic activity. These results suggest that never‐treated hypertensive patients with MS detected by ABPM should be evaluated for OSA because the management of OSA, such as continuous positive airway pressure, may relieve MS.
Disclosure
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Acknowledgments
The authors wish to acknowledge the financial support of the Catholic Medical Center Research Foundation.
J Clin Hypertens (Greenwich). 2015:675–681. DOI: 10.1111/jch.12581. © 2015. Wiley Periodicals, Inc.
References
- 1. Okada H, Iwase S, Mano T, et al. Changes in muscle sympathetic nerve activity during sleep in humans. Neurology. 1991;41:1961–1966. [DOI] [PubMed] [Google Scholar]
- 2. Bar A, Pillar G, Dvir I, et al. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003;123:695–703. [DOI] [PubMed] [Google Scholar]
- 3. Parish JM. Metabolic syndrome, obstructive sleep apnea, and risk of cardiovascular disease. Sleep Breath. 2012;16:595–597. [DOI] [PubMed] [Google Scholar]
- 4. Shimizu M, Ishikawa J, Eguchi K, et al. Association of an abnormal blood glucose level and morning blood pressure surge in elderly subjects with hypertension. Am J Hypertens. 2009;22:611–616. [DOI] [PubMed] [Google Scholar]
- 5. Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10:253–264. [DOI] [PubMed] [Google Scholar]
- 6. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467–472. [PMC free article] [PubMed] [Google Scholar]
- 7. Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79:1036–1046. [DOI] [PubMed] [Google Scholar]
- 8. Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56:765–773. [DOI] [PubMed] [Google Scholar]
- 9. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 10. Li Y, Thijs L, Hansen TW, et al. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension. 2010;55:1040–1048. [DOI] [PubMed] [Google Scholar]
- 11. Cho JS, Ihm SH, Jang SW, et al. Negative association between plasma aldosterone concentration/plasma renin activity and morning blood pressure surge in never‐treated hypertensive patients. Clin Exp Hypertens. 2014;36:195–199. [DOI] [PubMed] [Google Scholar]
- 12. Kuo TB, Hong CH, Hsieh IT, et al. Effects of cold exposure on autonomic changes during the last rapid eye movement sleep transition and morning blood pressure surge in humans. Sleep Med. 2014;15:986–997. [DOI] [PubMed] [Google Scholar]
- 13. Lambert EA, Chatzivlastou K, Schlaich M, et al. Morning surge in blood pressure is associated with reactivity of the sympathetic nervous system. Am J Hypertens. 2014;27:783–792. [DOI] [PubMed] [Google Scholar]
- 14. Amin R, Somers VK, McConnell K, et al. Activity‐adjusted 24‐hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension. 2008;51:84–91. [DOI] [PubMed] [Google Scholar]
- 15. Hoffstein V, Mateika J. Evening‐to‐morning blood pressure variations in snoring patients with and without obstructive sleep apnea. Chest. 1992;101:379–384. [DOI] [PubMed] [Google Scholar]
- 16. Penzel T, Kesper K, Pinnow I, et al. Peripheral arterial tonometry, oximetry and actigraphy for ambulatory recording of sleep apnea. Physiol Meas. 2004;25:1025–1036. [DOI] [PubMed] [Google Scholar]
- 17. Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Onder NS, Akpinar ME, Yigit O, et al. Watch peripheral arterial tonometry in the diagnosis of obstructive sleep apnea: influence of aging. Laryngoscope. 2012;122:1409–1414. [DOI] [PubMed] [Google Scholar]
- 19. Choi JH, Kim EJ, Kim YS, et al. Validation study of portable device for the diagnosis of obstructive sleep apnea according to the new AASM scoring criteria: watch‐PAT 100. Acta Otolaryngol. 2010;130:838–843. [DOI] [PubMed] [Google Scholar]
- 20. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. [DOI] [PubMed] [Google Scholar]
- 22. Pepin JL, Tamisier R, Borel JC, et al. A critical review of peripheral arterial tone and pulse transit time as indirect diagnostic methods for detecting sleep disordered breathing and characterizing sleep structure. Curr Opin Pulm Med. 2009;15:550–558. [DOI] [PubMed] [Google Scholar]
- 23. Schnall RP, Shlitner A, Sheffy J, et al. Periodic, profound peripheral vasoconstriction–a new marker of obstructive sleep apnea. Sleep. 1999;22:939–946. [PubMed] [Google Scholar]
- 24. Narkiewicz K, Winnicki M, Schroeder K, et al. Relationship between muscle sympathetic nerve activity and diurnal blood pressure profile. Hypertension. 2002;39:168–172. [DOI] [PubMed] [Google Scholar]
- 25. Kario K, Pickering TG, Hoshide S, et al. Morning blood pressure surge and hypertensive cerebrovascular disease: role of the alpha adrenergic sympathetic nervous system. Am J Hypertens. 2004;17:668–675. [DOI] [PubMed] [Google Scholar]
- 26. Lurie A. Endothelial dysfunction in adults with obstructive sleep apnea. Adv Cardiol. 2011;46:139–170. [DOI] [PubMed] [Google Scholar]
- 27. Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kallianos A, Trakada G, Papaioannou T, et al. Glucose and arterial blood pressure variability in obstructive sleep apnea syndrome. Eur Rev Med PharmacolSci. 2013;17:1932–1937. [PubMed] [Google Scholar]
- 29. Aronson D, Nakhleh M, Zeidan‐Shwiri T, et al. Clinical implications of sleep disordered breathing in acute myocardial infarction. PLoS One. 2014;9:e88878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee DH, Ihm SH, Youn HJ, et al. Age is an independent risk factor for the early morning blood pressure surge in patients never‐treated for hypertension. Korean Circ J. 2009;39:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]


