Abstract
The aim of this study was to investigate the cognitive state in women and its relation to menopause and hypertension (HTN). The authors included 1034 women aged 47.13±15.71 years. The prevalence of HTN was 47.1%, with 67.8% of patients treated and 48.6% controlled. Cognitive impairment was higher among hypertensive menopausal (mini‐Boston Naming Test: 7.4±3.1 vs 8.5±2.4, P<.001; Clock‐Drawing Test: 5.2±2 vs 5.6±1.6, P<.01). Using logistic regression adjusted by age and education level, statistical differences were found in the results from the mini‐Boston Naming Test between menopausal hypertensive vs menopausal normotensive women (odds ratio, 1.48; 95% confidence interval, 1.06–2.07; P=.021), and no difference between nonmenopausal hypertensive vs menopausal normotensive women (odds ratio, 0.89; 95% confidence interval, 0.51–1.57; P=.697). The P interaction between both groups was significant (P=.038). The possibility of alteration in cortical functions in menopausal hypertensive woman showed a relative increment of 48% (P=.021). The association between HTN and menopause increases the possibility of compromising the semantic memory by 50%.
Historically, women's health has been centered on fertility, and it was widely assumed that both women and men behaved likewise regarding treatment and medical conditions. In the past 2 decades, however, the advances in knowledge, diagnosis, and cardiovascular disease (CVD) treatments have shown that women respond differently.1
The leading causes of death among women are CVD and stroke. CVD in women typically occurs 10 years later than in men, with a significant increased prevalence after menopause.2 This stage is considered critical for women, since risk factors such as hypertension (HTN), dyslipidemia, obesity, and diabetes, among others, increase after menopause.
In the 1970s, the Framingham study showed that menopause was an independent risk factor for CVD.3 This should be considered relevant if we consider that hormonal protection is not present during this period. The Hypertension in Seven Latin American Cities: the Cardiovascular Risk Factor Multiple Evaluation in Latin America (CARMELA) study showed that prevalence in HTN increased with age (>55 years of age) and was higher in women than in men.4
Hypertension affects 25% of adult women worldwide. Moreover, in the United States, more than 75% of women older than 60 years are hypertensive.5, 6 Multiple mechanisms to explain HTN in menopausal women were applied. However, CVD and HTN in women could not only be attributed to hormonal changes.
HTN and other vascular risk factors have been shown to contribute to cognitive impairment and dementia. Therefore, the risk of cognitive disorders is higher among women than among men. HTN in middle‐aged individuals is a predictor of cognitive disorders later in life.7
Recent studies on the relationship between estrogen and Alzheimer's disease have prompted interest in memory experiences around the time of menopause. Seattle Midlife Women's Health Study (SMWHS) reports relevant declines in cognitive performance following menopause.8
The latest evidence shows that estradiol modulates cognitive function in animals and humans. Modulation begins in the uterus, when direct estrogen prompts sexual differentiation over several brain regions that control reproduction and some cognitive functions. With age, circulating estrogen levels decrease and seem to contribute to age‐related decline in learning and memory.9
Cognitive function, put in its most basic context, is the ability to learn, retain, and recall information. In humans, it also represents a complex, multidimensional set of intellectual functions such as judgment and evaluation. Thus, in a broader context, cognition includes all mental abilities and processes related to knowledge, including, but not limited to, attention, memory, reasoning, comprehension, and language production.9
Our objective was to study the cognitive state in menopausal hypertensive women compared with normotensive and premenopausal women participating in the Corazon Sano Program (Healthy Heart Program) in the city of Villa Maria, Córdoba, Argentina.
Methods
Study Population
According to the census conducted by the INDEC,10 Villa Maria is an urban area with a population of 80,006 inhabitants.
A total of 22 of 34 neighborhoods were randomly surveyed between February 2010 and August 2011.
A closed‐answer questionnaire was used, including questions about sex, age, family background, socioeconomic situation, physical activity, cardiovascular risk factors (such as HTN), dyslipidemia, obesity, diabetes, drug treatments, menopausal status, and hormone replacement therapy. All participants performed a self‐evaluation about their health using the Likert scale, including five items (eg, poor, regular, good, very good, excellent health). According to the level of schooling, the participants were divided into four groups: (1) uneducated, (2) grammar school (finished or unfinished), (3) high school (finished or unfinished) and, (4) higher education (finished or unfinished). All participants were evaluated for anthropometric measures: weight (recorded to the nearest 0.1 kg), height (cm), and waist circumference (WC) (cm) (measuring the midpoint between the lowest rib and the iliac crest in which the participants were standing with nonforced exhalation), body mass index (BMI), and blood pressure (BP) measurements (according to standardized procedures and national and international guidelines).11, 12
All participants had a 12‐hour fasting period before venous blood sampling. The samples were used to determine glucose, C‐reactive protein (quantitative), triglycerides (TGs), total cholesterol (TC), and high‐density lipoprotein (HDL) and low‐density lipoprotein (LDL) cholesterol. All samples were sent to and processed at the core laboratory.
Diagnosis Criteria (Cutoff Values)
BP (diastolic and systolic) considering an average value of the last two of three measurements of a seated patient using a validated semiautomatic digital sphygmomanometer with an adjustable cuff according to the arm circumference. Definitions were: hypertension (systolic BP [SBP] ≥140 mm Hg and/or diastolic BP [DBP] ≥90 mm Hg or antihypertensive medication treatment; controlled hypertension (SBP ≤140 mm Hg and/or DBP ≤90 mm Hg); and normotension (SBP ≤140 mm Hg and/or DBP ≤90 mm Hg) without treatment.11, 12
Dyslipidemia: TC ≥200 mg/dL, and/or HDL ≤50 mg/dL and/or LDL ≥130 mg/dL and/or TGs ≥150 mg/dL.13
Abdominal obesity: WC ≥88 cm.14
Hyperglycemia while fasting or diabetes: glycemia ≥100 mg/dL or antidiabetic medication treatment.15
Menopause was defined according to World Health Organization (WHO) as amenorrhea ≥1 year.
Neuropsychological Assessments
The Minimal Cognitive Examination (MCE)16 was used and included five tests: (1) The Mini‐Mental State Examination (MMSE), 30‐point version, was administered according to the standards of the Neuropsychology Working Group of the Argentinean Society of Neurology.17 It is used to assess global cognition (orientation, attention, memory, language, visual construction). For the five questions asked in the MMSE for orientation assessment, a score is given; (2) Benton Orientation Test to assess orientation as to time; (3) Clock‐Drawing Test (CDT) for executive function assessment (subcortical damage); (4) Alternating Series Test to assess the presence of repetitions, with dichotomic results (yes/no); and (5) the abbreviated form of the Boston Naming Test (BNT),18 Version 12 flashcards, to assess semantic memory (cortical damage). All participants completed an anxiety/depression scale.19 A 14‐point questionnaire, including two subscales (one to detect anxiety and the other depression), which assesses either intensity or frequency of symptoms with the four‐point Likert scale (eg, never, from time to time, very often, always), with a 0 to 21 score for each subscale and a global scale from 0 to 42. Two blinded neuropsychologists studied the results of each participant to confirm internal agreement. Two neuropsychologists blindly conducted the neuropsychological assessments. The cutoff values of the neuropsychological test were: (1) MMSE ≤24 points for cognitive impairment, (2) CDT ≤5 points for executive function impairment, and (3) abbreviated BNT ≤9 points for semantic memory impairment. Anxiety/depression scale were analyzed together and the cutoff point was normal 0 to 7 points, possible 8 to 10 (significant), and probable ≥11 points (very significant).
Participants signed informed consent before participating in the trial. Both the trial protocol and the informed consent were approved by an independent ethics committee (Favaloro Foundation). The trial was conducted pursuant to Good Clinical Practice, local regulations, and the Declaration of Helsinki and its amendments.
Statistical Analyses
This is an epidemiological and transversal study. The categoric variables are expressed in percentages and continuous variables as mean±standard deviation. Chi‐square test was used to prove the association between the categoric variables. Student t test was used to verify significant statistical differences in continuous variables according to different groups. Logistic regressions were calculated to study if the differences observed in the t test support themselves when controlling with variables such as age and schooling. Adjusted odds ratios (ORs) were calculated with 95% confidence intervals (CIs). Estimated bootstrap interaction was also calculated. All tests were conducted at the 5% level of significance. The statistical software programs used were SPSS version 15 (SPSS, IBM, Armonk, NY) and STATA version 12 (StataCorp, College Station, TX).
Results
In this study, 1191 women 18 years and older were evaluated, and 157 (7.9%) were excluded for the following reasons: they had sensorial, motor, or neurological disorders that prevented them from answering the survey or neuropsychological tests; they had a diagnosis of dementia; or they were taking medication for dementia or did not complete the study procedures.
We included 1034 women (47.13±15.71 years; age range, 18–87 years), and 48.4% of the sample were menopausal women. Table 1 shows the characteristics of the total sample and the difference between hypertensive and nonhypertensive women (age, schooling, BP, anthropometric measures, risks factors, and biochemical data). There were 501 menopausal women (48.4%) and the prevalence of hypertension was 47.1% (485). The Figure shows the distribution of the prevalence of HTN and the relationship between controlled/treated patients in the different age groups. A total of 67.8% of hypertensive patients were treated, and 48.6% of the treated were controlled. The prevalence of HTN increased with age and controlling BP decreased with age.
Table 1.
Characteristics of the Sample
| Characteristics | Total Sample | Hypertension | Normotension | P Value |
|---|---|---|---|---|
| Sample, No. | 1034 | 485 | 545 | |
| Age, y | 47.1 (15.7) | 54.6 (14.0) | 40.5 (13.9) | <.001 |
| Education level, % | ||||
| Uneducated | 17.4 | 2.47 | 1.1 | |
| Grammar school | 48.1 | 58.3 | 39.4 | |
| Middle/high school | 35.2 | 28.0 | 42.0 | |
| College | 14.7 | 11.1 | 18.1 | <.001 |
| Blood pressure, mm Hg | ||||
| SBP | 132.7 (22.5) | 148.3 (22.0) | 118.8 (10.9) | <.001 |
| DBP | 78.9 (12.3) | 86.2 (12.2) | 72.5 (8.1) | <.001 |
| Anthropometry | ||||
| Weight, kg | 72.9 (17.9) | 78.0 (17.6) | 69.3 (16.6) | <.001 |
| BMI, kg/m2 | 29.0 (7.3) | 31.8 (6.8) | 27.7 (6.3) | <.001 |
| WC, cm | 94.9 (15.3) | 99.8 (14.4) | 90.6 (14.8) | <.001 |
| Biochemical, mg/dL | ||||
| Glycemia | 90 (18) | 96 (34) | 84 (12) | <.001 |
| CRP | 3.53 (5.0) | 3.77 (5.5) | 3.29 (4.5) | .171 |
| Cholesterol | 200 (44) | 203 (41) | 188 (43) | <.001 |
| HDL | 50 (11) | 50 (11) | 51 (12) | .112 |
| LDL | 120 (33) | 129 (34) | 117 (33) | <.001 |
| Triglyceride | 150 (88) | 155 (100) | 116 (62) | <.001 |
| Vascular risk factors, % | ||||
| Dyslipidemia | 32.6 | 46.6 | 20.4 | <.001 |
| Hyperglycemia | 14.4 | 20.7 | 9.0 | <.001 |
| Obesity | 68.9 | 81.2 | 57.9 | <.001 |
Abbreviations: BMI, body mass index; CRP, C‐reactive protein; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure; WC, waist circumference. Values are expressed as mean±standard deviation unless otherwise indicated.
Figure 1.
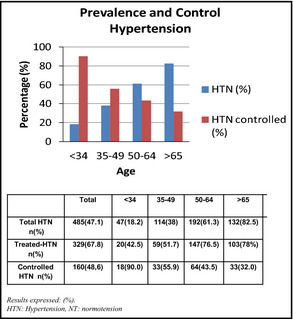
Relationship between prevalence of hypertension and controls according to age group.
Table 2 shows hypertensive and normotensive women according to their menopausal status, the anxiety and depression status, and the average punctuation of the neuropsychological test. There was a statistically significant difference between HTN and cognitive performance, with patients with HTN having lower scores on the cognitive tests. In the multiple linear models, this association was lost after being adjusted for age and level of schooling. Menopause did not change cognitive results, but when it was related to HTN, differences were found in all of the cognitive domains studied: MMSE: menopausal women, 26.7 (3.38) vs nonmenopausal women, 27.7 (2.86) (P=.001); CDT: menopausal women, 5.23 (2.0) vs nonmenopausal women, 5.6 (1.6) (P=.014); BNT: menopausal women, 7.4 (3.1) vs nonmenopausal women, 8.54 (2.4) (P<.0001). Statistically significant differences were not found in regards to HTN, menopause, and anxiety/depression scoring scale (P>.5).
Table 2.
Hypertensive and Normotensive Women According to Menopausal Status: Anxiety, Depression Status, and the Average Punctuation of the Neuropsychological Test
| Variables | Hypertension | Normotension | ||||
|---|---|---|---|---|---|---|
| Menopausal | Nonmenopausal | P Value | Menopausal | Nonmenopausal | P Value | |
| Sample, No. (%) | 341 (70.3) | 141 (29.0) | <.001 | 160 (29.1) | 382 (70.1) | <.0001 |
| Age, y | 61.3 (9.5) | 38.4 (8.8) | <.001 | 56.1 (9.4) | 33.8 (9.5) | <.0001 |
| Blood pressure, mm Hg | ||||||
| SBP | 150.6 (22.9) | 142.8 (19.1) | <.001 | 121.9 (11.2) | 117.6 (10.7) | <.001 |
| DBP | 85.2 (12.0) | 88.6 (12.6) | .007 | 73.5 (7.6) | 72.0 (8.2) | .046 |
| Anxiety and depression status, No. (%) | ||||||
| Anxiety and depression | 122 (35.8) | 73 (51.8) | .786 | 79 (49.3) | 198 (51.8) | .351 |
| Neuropsychological test results | ||||||
| MMSE | 26.7 (3.3) | 27.7 (2.8) | <.001 | 26.4 (2.9) | 27.8 (2.9) | .198 |
| CDT | 5.2 (2.0) | 5.6 (1.6) | .014 | 5.4 (1.6) | 5.7 (1.7) | .050 |
| BNT | 7.4 (3.1) | 8.5 (2.4) | <.001 | 8.1 (2.8) | 8.5 (2.6) | .165 |
Abbreviations: BNT, Boston Naming Test; CDT, Clock‐Drawing Test; DBP, diastolic blood pressure; MMSE, Mini‐Mental State Examination; SBP, systolic blood pressure. Values are expressed as mean±standard deviation unless otherwise indicated.
When we study the cross between menopause and hypertension, MMSE and CDT lost significance. Women with HTN and menopause showed a higher percentage of alteration in their memory (BNT <9) (Table 3).
Table 3.
Prevalence of Cognitive Impairment According to the State of Menopause and Hypertension
| Menopausal | Nonmenopausal | |
|---|---|---|
| Boston Naming Test | ||
| HTN |
55.1% (CI, 49.7–60.6) 188/341 |
42.6% (CI, 34.0–51.1) 60/141 |
| NT |
43.8% (CI, 35.8–51.8) 70/160 |
42.7% (CI, 37.6–47.8) 163/382 |
| Clock‐Drawing Test | ||
| HTN |
29% (CI, 24.3–34.1) 99/341 |
24.3% (CI, 17.9–31.8) 39/160 |
| NT |
22.6% (16.1–30.5) 32/141 |
17.2% (CI, 13.6–21.4) 66/382 |
| Mini‐Mental State Examination | ||
| HTN |
17.8% (CI, 13.9–22.4) 61/341 |
10.6% (CI, 6.3–16.4) 17/160 |
| NT |
9.21% (CI, 5–15.3) 13/141 |
7.32% (CI, 4.9–10.4) 28/382 |
Abbreviations: CI, confidence interval; HTN, hypertension; NT, normotension. Cognitive impairment: Boston Naming Test <9, Clock‐Drawing Test <5, and Mini‐Mental State Examination <24.
Education level showed an influence on cognitive status. Uneducated women showed lower scores on BNT (OR, 5.18; 95% CI, 3.31–8.13; P<.001) and women with a high education level (college/university) had higher scores on BNT (OR, 0.40; 95% CI, 0.27–0.59; P<.001). The variation of BNT score/age did not show differences in this analysis.
When we used a logistic regression model estimated by the bootstrap method and adjusted by age and education level, we found statistical differences between menopausal HTN vs menopausal normotension (OR, 1.48; 95% CI, 1.06–2.07; P=.021), and no differences between nonmenopausal HTN vs nonmenopausal normotension (OR, 0.89; 95% CI, 0.51–1.57; P=.697). The P interaction between both groups was significant (P=.038). The possibility of alteration in cortical functions (measured by BNT) in hypertensive menopausal woman vs normotensive menopausal showed a relative increment of 48% (P=.021) (Table 4).
Table 4.
Risk of Cognitive Impairment According to the State of Menopause and Hypertension
| OR | 95% CI | P Value | P Interaction | |
|---|---|---|---|---|
| BNT | ||||
| Nonmenopausal/menopausal | 1.06 | 0.59–1.89 | .852 | |
| Menopausal HTN vs NT | 1.48 | 1.06–2.07 | .021 | .038 |
| Nonmenopausal HTN vs NT | 0.89 | 0.51–1.57 | .697 | |
| CDT | ||||
| Nonmenopausal/menopausal | 1.07 | 0.60–1.932 | .852 | |
| Menopausal HTN vs NT | 1.29 | 0.79–2.11 | .303 | .621 |
| Nonmenopausal HTN vs NT | 1.09 | 0.70–1.71 | .687 | |
| MMSE | ||||
| Nonmenopausal/menopausal | 0.72 | 0.32–1.63 | .433 | .648 |
| Menopausal HTN vs NT | 1.19 | 0.57–2.41 | .654 | |
| Nonmenopausal HTN vs NT | 1.46 | 0.79–2.69 | .221 | |
| Education level | ||||
| Uneducated | 5.18 | 3.31–8.13 | .001 | |
| Grammar school | 1.70 | 1.07–2.70 | .024 | |
| Middle/high school and college | 0.40 | 0.27–0.59 | .001 | |
Abbreviations: BNT, Boston Naming Test; CDT, Clock‐Drawing Test; CI, confidence interval; HTN, hypertension; NT, normotension; MMSE, Mini‐Mental State Examination; OR, odds ratio. Cognitive impairment: Boston Naming Test <9, Clock‐Drawing Test <5, and Mini‐Mental State Examination <24. Logistic regression model estimated by Bootstrap method, corrected by educational level.
Health perception was self‐evaluated using Likert scale and the results were poor in 9.6%, regular in 40.2%, good in 38.4%, very good in 9.7%, and excellent in 2.1% of patients. There was an inverse association between HTN and health perception. There were more hypertensive women than expected in the groups with a poor health perception (χ2=48.691; P<.0001).
Discussion
The Corazón Sano Program conducted in Villa Maria, Córdoba, is the first recording of health and CVD data of women in Argentina. Prevalence of HTN is directly related to age, and our study was no exception; 47.1% of the population presented with HTN, and we found that the prevalence of HTN increased with age. Distribution was variable according to age: from 18.2% in women younger than 34 years to 82% in women older than 65 years. In treated/controlled participants, we found an inverse relationship with age (90% in <34 years vs 32% in >65 years). These results can likely be explained by the chronic nature of the condition as well as how it affects pathological mechanisms.
This should be contrasted with aging and vascular damage in older women, when control is more difficult to achieve, even if treatment compliance is acceptable. These data are consistent with those provided by the Women's Health Initiative (WHI),20 in which 64% of patients were treated and 36% were controlled. In our study, 67.8% (n=329) of the women with HTN were taking medication and only 48.6% of the treated patients were controlled. Once again, the noncontrolled group included elderly women (56.6±12.0 vs 39.8±14.5 years).
Prevalence of HTN in menopausal women was higher. An Italian study (the Study on Hypertension Prevalence in Menopause in the Italian Population [SIMONA])21 showed a higher prevalence in postmenopausal women (64.1%) as compared with perimenopausal and premenopausal women, with this increase independent of age and BMI. In our sample, the relationship between HTN in menopausal vs nonmenopausal women (70.3% vs 29.0%) was conflicting as compared with normotensive women (menopausal vs nonmenopausal women: 29.1% vs 70.1%).
The level of knowledge about HTN is essential for treatment compliance and, therefore, control of the condition. Data from the National Health and Nutrition Examination Survey (NHANES)22 conducted in 2011 to 2012 reported that the awareness of hypertensive status in the US population was 82.7%. In our study, 72.9% of the women who were asked “Has any physician or healthcare professional ever told you that you have HTN, also known as high blood pressure?” (the same question asked by NHANES) answered in an affirmative manner. The number of treated patients in our study were 84.1% hypertensive patients, and 43.5% were controlled patients. NHANES showed that 75.7% were treated HTN patients and 51.9% were controlled. Compared with our data, NHANES showed a higher treatment rate (10%) and lower control rate (10%).
Psychosocial factors (anxiety and depression) are well‐known vascular risk factors and are closely related to the incidence and progression of HTN. They are common in women and even more common in menopausal women. The INTERHEART23 study showed that psychosocial factors doubled HTN and the risk of having CVD. The Women's Health Initiative‐Observational Study (WHI‐OS)20 proves that subclinical depression increases HTN. Finally, a subanalysis conducted in the Atherosclerosis Risk in Communities (ARIC) study24 showed that the high anxiety scores came from switching from pre‐HTN to HTN and therefore an increased risk of CVD. Therefore, HTN increases the risk of having a mental disorder (and vice versa), and it is vital to recognize the early association between the conditions. In our study, 22.4% of the hypertensive women had symptoms compatible with anxiety, while 14.8% had symptoms compatible with depression. The presence of menopause did not modify the result (hypertensive menopausal women: anxiety 21.4%, depression 14.3%). These results were not enough to establish statistically significant differences between the groups; however, they are still significant in the clinical aspect. Finally, for the past 20 years, HTN has been associated with cognitive impairment and dementia. The magnitude of brain artery damage (expressed in white matter lesions, lacunar infarction, and microhemorrhages) is a well‐known risk factor for stroke as well as cognitive impairment.25, 26
In our study, a statistically significant relationship was found between HTN and cognitive performance in all domains under study (executive function and semantic memory) as well as a relationship between menopause and cognition. However, this association was lost when the multiple linear regressions model was used in which age and schooling were the variables responsible for such an association. When the hypertensive menopausal group was analyzed, cognitive impairment was higher than in the nonmenopausal hypertensive group.
Typically, low performance on the MMSE and CDT proves cognitive decline in general; hopefully, this was related to lower level of education in women. However, differences were found in the test that evaluates cortical functions (BNT). Such difference persisted even when uneducated women were excluded. The possibility of alteration in cortical functions (measured by BNT) in hypertensive menopausal women was 48%.
Vascular brain injury as a result of HTN causes executive dysfunction (subcortical damage). It could also worsen the neurodegenerative component (cortical) expressed clinically as amnestic cognitive impairment or Alzheimer‐type dementia.27
An important aspect of the programming of sex differences during development is that these differences may contribute to the development and patterns of adult cognitive loss seen in some neurodegenerative and psychiatric diseases. Women have a higher rate of Alzheimer's disease than men, and with more negative symptoms.28
A recent study reported no changes in immediate or working memory, delayed recall, verbal learning, or verbal fluency across the menstrual cycle.29 Nevertheless, postmenopausal women (with presumably low estradiol levels) performed significantly worse than premenopausal and perimenopausal women on delayed verbal memory tasks and significantly worse than perimenopausal women on phonemic verbal fluency tasks.30
Future research is needed on estrogen treatments for women with impaired memory and other symptoms caused by either premature menopause (oophorectomy) menopause or aging.
Clinical and experimental studies suggest that endogenous estrogens prevent high BP possibly because of reduction in nitric oxide and prostacyclin I–mediated vasodilatations.31, 32 Thus, lack of hormones, genetics, oxidative stress, activation of the renin‐angiotensin system, weight gain, and sympathetic activation may be considered the main causes of HTN in postmenopausal women.28, 33, 34
As defined in previous studies,35, 36 “perception is the knowledge or internal idea resulting from a material impression of our senses.” The perception of the patient about their own health is essential when diagnosing, having the knowledge that through their senses they have an impression “which is different from the surrounding reality.” Our hypertensive patients showed this perception regarding their own health. The perception of health in hypertensive patients and/or patients with vascular risk factors was negative (poor health) compared with nonhypertensive patients whose perception of their health was good (P<.0001). Data showed the disassociation of HTN control and its treatment, the importance of education to create awareness regarding this health issue, and the impact of the disease on cognitive functions, using in this case measures leading to the early diagnosis.
Study Limitations and Strengths
The weak point of our study was that, in this sample, cognitive disorders were detected using the tests mentioned. MCE were not confirmed or checked together with diagnostic neuropsychological studies.
On the other hand, the strengths of our study were that it was an epidemiological and prospective study, and therefore the sample was significant and representative of the population studied and the tools used were validated in our country (in situ). It is important to note that the participants were directly assessed.
Conclusions
In our sample, HTN was shown to be highly prevalent in menopausal women. This association of HTN and menopause seems to have a negative impact on cortical functions (semantic memory). Further prospective studies about the relationship between HTN, menopause, and cognitive impairment are needed.
Disclosures
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
Acknowledgments
The authors of this paper thank the Cardiovascular Prevention Program (Heart Healthy) and the Health Council of the city of Villa Maria, Córdoba, Argentina, for their support.
J Clin Hypertens (Greenwich). 2015;17:970–976. DOI: 10.1111/jch.12643. © 2015 Wiley Periodicals, Inc.
References
- 1. Shaw LJ, Bugiardini R, Merz CNB. Women and Ischemic Heart Disease. J Am Coll Cardiol. 2009. Oct;54:1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension; analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 3. Hjortland MC, McNamara PM, Kannel WB. Some atherogenic concomitants of menopause: the Framingham Study. Am J Epidemiol. 1976;103:304–311. [DOI] [PubMed] [Google Scholar]
- 4. Hernández‐Hernández R, Silva H, Velasco M, et al; CARMELA Study Investigators . Hypertension in seven Latin American cities: the Cardiovascular Risk Factor Multiple Evaluation in Latin America (CARMELA) study. J Hypertens. 2010;28:24–34. [DOI] [PubMed] [Google Scholar]
- 5. Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep. 2012;14:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Engberding N, Wenger NK. Management of hypertension in women. Hypertens Res. 2012;35:251–260. [DOI] [PubMed] [Google Scholar]
- 7. Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchell SE, Woods FN. Midlife women's attributions about perceived memory changes: observations from the Seattle Midlife Women's Health Study. J Womens Health Gend Based Med. 2001;10:351–362. [DOI] [PubMed] [Google Scholar]
- 9. Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66:602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Instituto Nacional de Estadisticas y Censos de la Republica Argentina (INDEC). Censo Nacional de poblaciones, hogares y viviendas 2010. http://www.indec.gov.ar. Accessed October 27, 2010.
- 11. Mancia G, De Backer G, Dominiczak A, et al; Authors/Task Force Members . 2007 ESH‐ESC practice guidelines for the management of arterial hypertension: ESH‐ESC Task force on the management of arterial hypertension. J Hypertens. 2007;25:1751–1762. [DOI] [PubMed] [Google Scholar]
- 12. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure , National Heart, Lung, and Blood Institute , National High Blood Pressure Education Program Coordinating Committee . Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 13. Mosca L, Benjamín EJ, Berra K, et al. Effectiveness‐based guidelines for the prevention of cardiovascular disease in women—2011 update a guideline from the American Heart Association. Circulation. 2011;123:1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grundy SM, Brewer HB Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–438. [DOI] [PubMed] [Google Scholar]
- 15. Alberti KG, Simmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 16. Vicario A, Cerezo GH, Zilberman J, et al. Guía para la Evaluación, de Trastornos Cognitivos en pacientes con Enfermedad Vascular. Segunda Edición. Actualización 2013. Rev Arg Fed Cardiol 2013; 42: 1–96. [Google Scholar]
- 17. Allegri RF, Ollari JA, Mangone CA, et al. El “Mini Mental Statement Examination” en la Argentina: instrucciones para su administración. Rev Neurol Arg. 1999;24:31–35. [Google Scholar]
- 18. Serrano CM, Allegri RF, Drake M, et al. A shortened form of the Spanish Boston naming test: a useful tool for the diagnosis of Alzheimer's disease. Rev Neurol. 2001;33:624–627. [PubMed] [Google Scholar]
- 19. Wilkinson MJB, Barczak P. Psychiatric screening in general practice: comparison of the general health questionnaire and the hospital anxiety depression scale. J R Coll Gen Prac. 1988;38:311–313. [PMC free article] [PubMed] [Google Scholar]
- 20. Wassertheil‐Smoller S, Shumaker S, Ockene J, et al. Depression and cardiovascular sequelae in postmenopausal women. The Women's Health Initiative (WHI). Arch Intern Med. 2004;164:289–298. [DOI] [PubMed] [Google Scholar]
- 21. Zanchetti A, Facchetti R, Cesana GC, et al; SIMONA participants . Menopause‐related blood pressure increase and its relationship to age and body mass index: the SIMONA epidemiological study. J Hypertens. 2005;23:2269–2276. [DOI] [PubMed] [Google Scholar]
- 22. Nwankwo T, Yoon SS, Burt V, et al. Hypertension among adults in the United States: national health and nutrition examination survey, 2011‐2012. NCHS Data Brief. 2013;133:1–8. [PubMed] [Google Scholar]
- 23. Yusuf S, Hawken S, Ounpuu S, et al; INTERHEART Study Investigators . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (THE INTERHEART STUDY): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 24. The ARIC Investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 25. Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559–2566. [DOI] [PubMed] [Google Scholar]
- 26. Duron E, Hanon O. Vascular Risk factors, cognitive decline, and dementia. Vasc Health Risk Manag. 2008;4:363–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Carli C. Cerebrovascular disease: assessing the brain as an end‐organ of vascular disease. Nat Rev Cardiol. 2012;9:435–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gillies GE, McArthue S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex‐specific medicines. Pharmacol Rev. 2010;62:155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mihalj M, Drenjančević I, Včev A, et al. Basic cognitive functions across the menstrual cycle in a controlled female cohort. Med Glas (Zenica). 2014;11:177–185. [PubMed] [Google Scholar]
- 30. Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: a systematic review and meta‐analysis. J Steroid Biochem Mol Biol. 2014;142:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11–18. [DOI] [PubMed] [Google Scholar]
- 32. McBride SM, Flynn FW, Ren J. Cardiovascular alteration and treatment of hypertension. Do men and women differ? Endocrine. 2005;28:199–207. [DOI] [PubMed] [Google Scholar]
- 33. Reckelhoff JF. Basic research into the mechanisms responsible for postmenopausal hypertension. Int J Clin Pract Suppl. 2004;139:13–19. [PubMed] [Google Scholar]
- 34. Marañon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci (Lond). 2013;125:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cerezo GH, Vicario A, Vainstein N. Características del síndrome metabólico en la consulta cardiológica. Grupo de estudio Carisma. Caracterización y Análisis del Riesgo en Individuos con Síndrome Metabólico en la Argentina). Rev Insuf Cardíaca. 2008;3:11–15. [Google Scholar]
- 36. Martell‐Claros N, Aranda P, González‐Albarrán O, et al. Perception of health and understanding of cardiovascular risk among patients with recently diagnosed diabetes and/or metabolic syndrome. Eur J Prev Cardiol. 2013;20:21–28. [DOI] [PubMed] [Google Scholar]


