Abstract
The authors aimed to investigate the blood pressure (BP)–lowering ability of eplerenone in drug‐resistant hypertensive patients. A total of 57 drug‐resistant hypertensive patients whose home BP was ≥135/85 mm Hg were investigated. The patients were randomized to either an eplerenone group or a control group and followed for 12 weeks. The efficacy was evaluated by clinic, home, and ambulatory BP monitoring. Urinary albumin, pulse wave velocity, and flow‐mediated vasodilation (FMD) were also evaluated. Home morning systolic BP (148±15 vs 140±15 mm Hg) and evening systolic BP (137±16 vs 130±16 mm Hg) were significantly lowered in the eplerenone group (n=35) compared with baseline (both P<.05), while unchanged in the control group (n=22). BP reductions in the eplerenone group were most pronounced for ambulatory awake systolic BP (P=.04), awake diastolic BP (P=.004), and 24‐hour diastolic BP (P=.02). FMD was significantly improved in the eplerenone group. In patients with drug‐resistant hypertension, add‐on use of eplerenone was effective in lowering BP, especially home and ambulatory awake BP.
Resistant hypertension (RH) is defined as failure to attain a target clinic blood pressure (BP) level <140/90 mm Hg despite treatment with at least three antihypertensive classes including at least one diuretic.1, 2 RH is frequently seen in clinical practice and is often difficult to manage. It is important to uncover the factors that could make patients drug‐resistant, including white‐coat RH, substances that elevate BP, insufficient drug regimen, and secondary hypertension such as sleep apnea. After confirming that patients are truly drug‐resistant, clinicians should refer them to hypertension specialists to consider improvement in medical regimens or enhancement of lifestyle modification in order to achieve better BP control.3
Mineralocorticoid receptor antagonists, especially spironolactone, have been established as drug regimens in the management of RH.4, 5 A selective mineralocorticoid receptor antagonist, eplerenone, has also been shown to be effective in patients with RH,6 but it has not been established whether this agent is effective in lowering different types of BP, such as clinic, home, and ambulatory BP. In addition, the effect of eplerenone on the measures of target organ damage (TOD) in patients with RH has not been assessed. In the present study, we sought to test the hypothesis that additional use of eplerenone could lower various measures of BP and improve the measures of TOD in patients with drug‐resistant hypertension who were already treated with at least three antihypertensive drugs, including calcium channel blockers (CCBs), renin‐angiotensin system (RAS) inhibitors, and diuretics.
Methods
The inclusion criteria in this study were: (1) patients with essential hypertension who were classified as having drug‐resistant hypertension treated with at least three antihypertensive drugs (CCBs, RAS inhibitors, and diuretics) for more than 3 months; and (2) home morning BP ≥135/85 mm Hg (either) despite the above treatment plus nonpharmacologic treatments (diet and exercise). The exclusion criteria were: allergy or allergic reaction to study drugs; patients with hyperkalemia or serum potassium level >5.0 mEq/L; hepatic damage such as liver cirrhosis (Child‐Pugh class C); renal dysfunction (estimated glomerular filtration rate [eGFR] <50 mL/min); severe hepatic damage; use of potassium or spironolactone, itraconazole, ritonavir, or nelfinavir; age younger than 20 years, or dementia.
The protocol of this study was registered on the University Hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR) Web site under the trial number UMIN000014186. The study was designed as a prospective, randomized, open‐label, and parallel‐controlled trial. The primary endpoint of this study was the reduction of home BP, especially in the morning, by the add‐on use of eplerenone. As shown in Figure 1, the patients were randomized to either the eplerenone group or control group in a 2:1 fashion. This randomization was carried out at an independent research center. Once‐daily morning dose of eplerenone was started at either 25 mg or 50 mg and adjusted based on the patient's age, BP, and laboratory data. Following the observational period, in the interventional period, eplerenone was added in one arm (eplerenone group) or current treatment was continued in the other arm (control group). The patients were followed for 12 weeks, and the efficacy of treatment was evaluated by clinic, home, and ambulatory BP monitoring (ABPM). The patients were asked to continue their lifestyles such as food intake and exercise, and the antihypertensive medications were unchanged throughout the study period. Informed consent was obtained from all study participants and the study was approved by the institutional ethics committees of Jichi Medical University and each participating institution.
Figure 1.
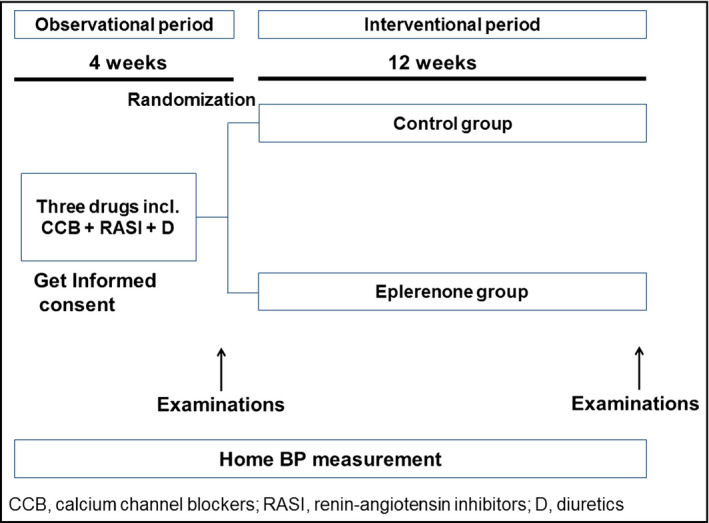
Study protocol.
Measures of TOD
Blood and urine samples were collected in the morning in a fasting state at baseline and at the 12th week of the study. Plasma/serum after separation and urine samples were stored at 4°C in refrigerated containers and sent to a commercial laboratory (SRL Inc., Tokyo, Japan) within 24 hours. The urinary albumin level was measured using a turbidimetric immunoassay and expressed as the urinary albumin/creatinine ratio (UACR, mg/g·cr). The eGFR was calculated using a validated equation based on the modified version of the Modification of Diet in Renal Disease study: eGFR (mL/min/1.73 m2) = 194 × age −0.287 × S‐Cr −1.094 (if female × 0.739).7 Renal dysfunction was defined as eGFR <60 mL/min/1.73 m2. The extent of intravascular fluid volume was assessed by atrial natriuretic peptide (ANP).
Arterial stiffness was assessed by brachial‐ankle pulse wave velocity (baPWV), and arterial wave reflection was assessed by augmentation index. The baPWV was measured using a volume plethysmographic device with four cuffs fitted with oscillometric sensors (form/BP‐203RPE II; Omron Healthcare, Lake Forest, IL). The reproducibility8 and validity9, 10 have been previously confirmed.
Flow‐mediated dilatation (FMD) was measured with the standard technique according to the guidelines for ultrasound assessment of the FMD of the brachial artery.11 Briefly, patients were examined under a fasting condition after a 12‐hour fast. They were not taking any medications, and were instructed to avoid smoking and exercise for at least 4 to 6 hours before the examination.12, 13 Using a 10‐MHz linear array transducer probe, the longitudinal image of the right brachial artery was recorded at baseline and then continuously from 30 seconds before to at least 2 minutes after the cuff deflation that followed suprasystolic compression (50 mm Hg above systolic BP [SBP]) of the right forearm for 5 minutes. The diastolic diameter of the brachial artery was determined semiautomatically using an instrument equipped with software for monitoring the brachial artery diameter (UNEX Co., Ltd., Nagoya, Japan). FMD was estimated as the percent change in the diameter over the baseline value at maximal dilatation during reactive hyperemia. All FMD measurements were obtained by an experienced technician. The reproducibility of FMD measurement was previously confirmed.14 .
Statistical Analysis
Sample size calculations were based on the results of expected efficacy in the eplerenone and control groups in RH. We assumed a difference of 15 mm Hg (−5 mm Hg by nonpharmacologic therapy, and −20 mm Hg by add‐on use of eplerenone) in home SBP between the treatment groups. Assuming a 10% dropout rate with 80% power at the 5% significance level, 29 patients per treatment arm were required.
All statistical analyses were carried out with the SPSS software package, version 19.0 (IBM, Armonk, NY). A two‐tailed paired t test was used to compare the mean values before and after each drug therapy. One‐way analysis of variance was performed to detect differences among groups. The χ2 test was applied to examine differences between the prevalence in the two groups. Pearson's correlation coefficients were used to calculate the correlation between the changes in BP and pulse rate parameters and the changes in the measures of TOD. Data are expressed as mean±standard deviation or prevalence (percentage). Values of P<.05 were considered statistically significant.
Results
Baseline characteristics are shown in Table 1. There were no significant differences in the clinical characteristics between the groups except for a slight difference in the rate of hyperlipidemia. With regard to the baseline use of medications (Table 2), ACE inhibitors and antidiabetic medications tended to be more prescribed in the control group but were otherwise similar between the groups. Thiazide diuretics were prescribed in all patients in this study. The dose of eplerenone used most frequently was 50 mg (n=24), followed by 25 mg (n=10) and 100 mg (n=1).
Table 1.
Baseline Characteristics of Subjects
| Control Group | Eplerenone Group | P Value | |
|---|---|---|---|
| No. | 22 | 35 | |
| Age, y | 65.9±10.8 | 60.0±13.0 | .08 |
| Male sex, % | 50.0 | 71.4 | .11 |
| Body mass index, kg/m2 | 28.5±6.7 | 27.1±3.3 | .31 |
| Waist circumference, cm | 91.6±14.6 | 89.5±9.1 | .52 |
| History of angina pectoris, % | 22.7 | 14.3 | .42 |
| History of myocardial infarction, % | 4.5 | 0 | .21 |
| History of dissecting aneurysm, % | 4.5 | 2.9 | .74 |
| History of stroke, % | 4.5 | 11.4 | .38 |
| History of heart failure, % | 9.1 | 0 | .08 |
| History of peripheral artery disease, % | 9.1 | 2.9 | .31 |
| History of hypertension, y | 12.5±11.3 | 12.2±10.3 | .92 |
| Treatment of hypertension, y | 8.4±9.3 | 8.7±7.6 | .87 |
| Family history of hypertension, % | 68.2 | 85.7 | .12 |
| Current smoking, % | 27.3 | 11.4 | .13 |
| Hyperlipidemia, % | 45.5 | 20.0 | .04 |
| Renal dysfunction, % | 22.7 | 14.3 | .42 |
| Diabetes mellitus, % | 59.1 | 37.1 | .12 |
| Atrial fibrillation, % | 4.5 | 5.7 | .85 |
| Antihypertensive medications, No. | 3.95±1.05 | 3.69±0.76 | .27 |
| Calcium channel blockers, % | 95.5 | 100.0 | .21 |
| Second calcium channel blockers, % | 9.1 | 8.6 | .95 |
| Angiotensin receptor blockers, % | 90.9 | 94.3 | .63 |
| ACE inhibitors, % | 22.7 | 5.7 | .058 |
| Thiazide diuretics, % | 100 | 100 | – |
| β‐Blockers, % | 40.9 | 31.4 | .47 |
| α‐Blockers, % | 18.2 | 25.7 | .52 |
| Antiplatelets, % | 27.3 | 17.1 | .37 |
| Antidiabetic medications, % | 36.4 | 14.3 | .054 |
| Statins, % | 31.8 | 22.9 | .46 |
| Antihyperuricemic medications, % | 22.7 | 14.3 | .42 |
Abbreviation: ACE, angiotensin‐converting enzyme.
Table 2.
Blood Pressure Parameters at Baseline
| Control Group | Eplerenone Group | P Value | |
|---|---|---|---|
| No. | 22 | 35 | |
| Clinic SBP, mm Hg | 146±9 | 145±17 | .77 |
| Clinic DBP, mm Hg | 77±11 | 80±10 | .33 |
| Clinic PR, bpm | 68±15 | 71±13 | .41 |
| Home morning SBP, mm Hg | 142±10 | 147±14 | .27 |
| Home morning DBP, mm Hg | 76±10 | 81±11 | .11 |
| Home morning PR, bpm | 65±12 | 65±8 | .96 |
| Home evening SBP, mm Hg | 134±13 | 137±16 | .46 |
| Home evening DBP, mm Hg | 70±12 | 73±10 | .32 |
| Home evening PR, bpm | 69±12 | 69±10 | .87 |
| 24‐hour SBP, mm Hg | 136±11 | 134±10 | .50 |
| 24‐hour DBP, mm Hg | 75±8 | 78±7 | .099 |
| 24‐hour PR, bpm | 68±11 | 68±10 | 1.0 |
| Awake SBP, mm Hg | 139±11 | 140±11 | .87 |
| Awake DBP, mm Hg | 77±7 | 82±8 | .019 |
| Awake PR, bpm | 70±11 | 71±11 | .83 |
| Sleep SBP, mm Hg | 128±14 | 120±12 | .052 |
| Sleep DBP, mm Hg | 69±10 | 69±7 | .98 |
| Sleep PR, bpm | 61±10 | 60±10 | .56 |
| Morning SBP, mm Hg | 142±16 | 139±15 | .56 |
| Morning DBP, mm Hg | 80±11 | 82±10 | .38 |
| Morning PR, bpm | 67±12 | 69±10 | .64 |
Abbreviations: bpm, beats per minute; DBP, diastolic blood pressure; PR, pulse rate; SBP, systolic blood pressure.
Baseline BP and pulse rate were similar between the groups, except for the significantly lower awake diastolic BP (DBP) in the control group than in the eplerenone group (Table 2). Sleep SBP tended to be higher in the control group than the eplerenone group.
Figures 2 and 3 show the comparisons of the changes of SBP and DBP between the groups. Eplerenone significantly reduced awake DBP and 24‐hour DBP compared with the baseline value. Home morning and evening SBP and DBP were significantly reduced in the eplerenone group from baseline, but no such effects were observed in the control group. With regard to intergroup comparisons, the extent of the reduction in 24‐hour DBP and awake SBP/DBP were greater in the eplerenone group than in the control group. Sleep BP and morning BP were not significantly reduced in either group. With regard to diurnal BP rhythm, when the patients were divided into dippers (nocturnal SBP dip ≥10%) vs nondippers (nocturnal SBP dip <10%), the rate of nondippers and the extent of night BP dip did not change significantly from baseline. Comparisons of changes in laboratory data are shown in Table 3. The extent of the changes in laboratory data were similar between the groups except for higher urinary potassium excretion in the eplerenone group and a minor difference in fasting blood glucose.
Figure 2.
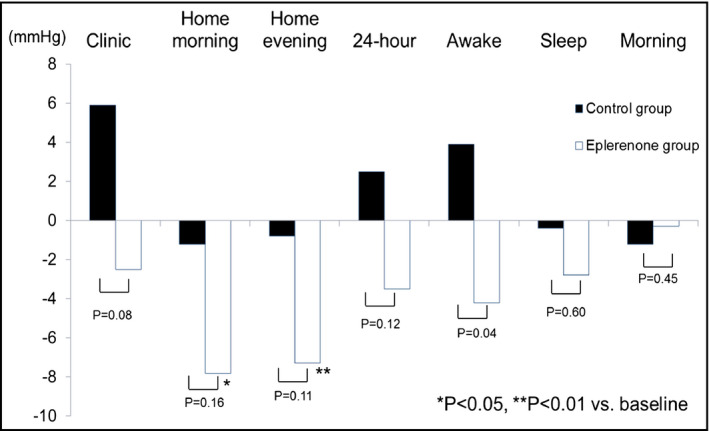
Changes in clinic, home and ambulatory systolic BP from the baseline.
Figure 3.
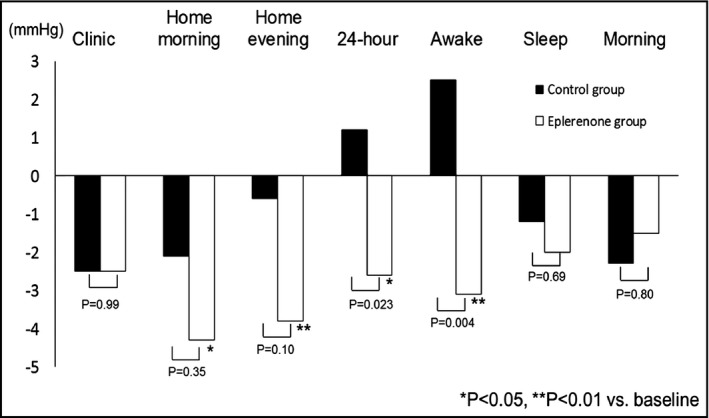
Changes in clinic, home and ambulatory diastolic BP from the baseline.
Table 3.
Comparisons of Changes in Laboratory Data
| Control group | a P Value | Eplerenone group | a P Value | b P Value | |||
|---|---|---|---|---|---|---|---|
| 0W | 12W | 0W | 12W | ||||
| Hemoglobin, g/dL | 13.7±1.5 | 13.8±1.7 | .43 | 14.0±1.4 | 14.0±1.6 | 1.00 | .86 |
| Hematocrit, % | 40.9±4.4 | 40.9±4.5 | .90 | 40.8±4.2 | 40.9±4.3 | .78 | .98 |
| Total cholesterol, mg/dL | 191±48 | 192±22 | .61 | 196±29 | 199±33 | .19 | 1 |
| Triglycerides, mg/dL | 197±190 | 174±92 | .32 | 169±146 | 157±103 | .54 | .92 |
| HDL cholesterol, mg/dL | 47±11 | 47±10 | .36 | 56±18 | 56±17 | .86 | .75 |
| LDL cholesterol, mg/dL | 114±22 | 111±24 | .64 | 108±26 | 114±32 | .05 | .12 |
| AST, U/L | 31±21 | 32±22 | .96 | 27±10 | 26±11 | .95 | .87 |
| ALT, U/L | 37±37 | 41±36 | .62 | 30±15 | 32±21 | .48 | .71 |
| Uric acid, mg/dL | 6.0±1.4 | 6.1±1.9 | .55 | 6.1±1.4 | 6.2±1.6 | .28 | .822 |
| BUN, mg/dL | 16.7±4.3 | 16.6±5.7 | .92 | 16.1±4.9 | 17.1±4.6 | .14 | .311 |
| Creatinine, mg/dL | 0.91±0.38 | 0.88±0.36 | .35 | 0.77±0.18 | 0.80±0.19 | .08 | .067 |
| Na, mmol/L | 141±2 | 141±2 | .36 | 140±2 | 140±2 | .84 | .81 |
| K, mmol/L | 4.3±0.4 | 4.3±0.5 | .24 | 4.2±0.4 | 4.3±0.4 | .12 | .059 |
| Fasting blood glucose, mg/dL | 147±48 | 127±36 | .01 | 131±42 | 127±33 | .45 | .049 |
| Fasting Insulin, μIU/mL | 34.4±66.2 | 18.6±23.6 | .16 | 19.9±20.1 | 15.0±12.0 | .07 | .22 |
| logHOMA‐IR | 0.58±0.51 | 0.51±0.27 | .51 | 0.59±0.49 | 0.51±0.43 | .23 | .93 |
| Glycated hemoglobin, % | 6.4±1.2 | 6.2±1.0 | .06 | 6.0±1.1 | 6.0±1.0 | .59 | .16 |
| logANP, pg/mL | 1.55±0.37 | 1.60±0.34 | .17 | 1.46±0.31 | 1.48±0.30 | .59 | .91 |
| log UACR | 1.87±0.83 | 1.64±0.92 | .16 | 1.43±0.7 | 1.42±0.74 | .91 | .15 |
| Urinary Na, mEq/L | 120±42 | 130±49 | .72 | 136±53 | 144±59 | .56 | .97 |
| Urinary K, mEq/L | 45±18 | 32±9 | <.001 | 49±21 | 58±30 | .01 | <.001 |
Abbreviations: ALT, alanine aminotransferase; ANP, atrial natriuretic peptide; AST, aspartate aminotransferase; BUN, serum urea nitrogen; HOMA‐IR, homeostatic model assessment of insulin resistance; Na, sodium; K, potassium; UACR, urinary albumin/creatinine ratio.
P value indicates the comparison between week 0 (0W) and week 12 (12W).
P value indicates the comparison of the intergroup difference.
Table 4 shows the changes in the measures of TOD in the control and eplerenone groups. UACR and PWV did not change between baseline and the 12th week. However, FMD was significantly increased after eplerenone therapy, but was not changed in the control group.
Table 4.
Changes in Measures of Target Organ Damage
| Control Group | P Value | Eplerenone Group | P Value | |||
|---|---|---|---|---|---|---|
| 0W | 12W | 0W | 12W | |||
| Log UACRa | 1.83±0.85 | 1.68±0.93 | .16 | 1.41±0.69 | 1.42±0.74 | .91 |
| FMD, %b | 5.5±1.9 | 5.8±1.9 | .21 | 4.9±1.5 | 5.5±1.7 | .044 |
| Average baPWV, cm/sc | 1590±305 | 1577±209 | .80 | 1529±303 | 1481±336 | .23 |
Abbreviations: 0W, week 0; 12W, week 12; baPWV, brachial‐ankle pulse wave velocity; FMD, flow‐mediated vasodilation; UACR, urinary albumin/creatinine ratio. Data are shown as mean±standard deviation. an=19 for the control group, n=33 for the eplerenone group. bn=19 for the control group, n=31 for the eplerenone group. cn=20 for the control group, n=32 for the eplerenone group.
The correlations between the changes in BP parameters and the changes in the measures of TOD are shown in Table S1. In the eplerenone group, the changes in home and ambulatory BP were associated with the change in logANP; the changes in clinic and home BP were significantly associated with the change in log UACR. The changes in home BP were associated with the change in FMD in the control group. However, there were no significant associations with PWV.
In the eplerenone group, minor transient dizziness was reported in four cases, but these patients completed the study protocol.
Discussion
In the present study, eplerenone was effective in further reducing ambulatory BP and home BP in patients with drug‐resistant hypertension. Moreover, endothelial function was improved by eplerenone. This study was among the first to examine various measures of out‐of‐clinic BP and measures of TOD in patients with drug‐resistant hypertension.
Eplerenone and Drug‐Resistant Hypertension
In the present study, the add‐on use of eplerenone was effective in lowering awake ambulatory BP in patients with drug‐resistant hypertension. Although there were no significant differences in home BP levels between the eplerenone and control groups, the extent of the reduction of BP was significant only in the eplerenone group. The results of this study are in agreement with a previous report in which eplerenone was effective in lowering clinic and ambulatory BP levels in 52 RH patients in a single‐arm study.6 In another study, an aldosterone synthase inhibitor, LCI699, effectively reduced BP levels compared with placebo.15 In contrast to the studies of spironolactone in RH,16 studies of eplerenone in drug‐resistant hypertension are surprisingly scarce. Although the extent of the clinic BP reduction was small, both ambulatory and home BP were reduced in the present study, suggesting that eplerenone is an important treatment strategy in RH.
The Supplemental Figure shows the BP‐lowering effects based on baseline BP levels in each BP profile. Although baseline clinic BP was not associated with the extent of clinic BP‐lowering effects, the higher the baseline ambulatory and home BPs, the greater the extent of corresponding BP reductions in both the control and eplerenone groups. These results indicate that the higher BP tended to be lowered, but eplerenone could lower BP independent of baseline BP levels.
Eplerenone and Ambulatory and Home BP
In the present study, ambulatory awake BP was significantly reduced from baseline, but sleep BP was not. In previous studies of RH, spironolactone effectively reduced ambulatory BP levels to a similar extent both in the daytime and at night.17, 18 This discrepancy was attributable to the difference in baseline awake BP level (140±11/82±8 mm Hg) and sleep BP (120±12/69±7 mm Hg) in our study vs awake (151±15/88±12 mm Hg) and sleep BP levels (139±18/79±12 mm Hg) in the previous study.17 There were no significant changes in the patterns of diurnal BP variation. Nonsignificant reductions in nondippers with eplerenone therapy could be attributable to the relatively small number of nondippers in this study.
With regard to home BP, eplerenone effectively lowered morning and evening home BP levels compared with baseline in the present study. There have been no studies that have examined the effect of eplerenone on home BP levels in RH, and thus this is the first study to clarify the effectiveness of eplerenone on home BP in RH. Williams and colleagues19 recently reported in the Optimum Treatment for Drug‐Resistant Hypertension (PATHWAY‐2) study that spironolactone was effective in lowering home BP in 314 patients with RH. There have been only two studies that examined the effect of eplerenone on home BP (but not in RH): one was a retrospective study of 83 hypertensive patients20 with 8 weeks of eplerenone treatment wherein home SBP/DBP decreased by −7.1±10.1/−2.6±5.0 mm Hg (P <.0001), but this was a single‐arm study. The other study compared the efficacy of add‐on eplerenone and add‐on indapamide on home BP and showed that eplerenone (−10±10/−4±6 mm Hg) and indapamide (−15±10/−7±6 mm Hg) were equally effective in lowering home BP.21 On the other hand, low‐dose spironolactone was shown to be effective in lowering home BP.22 Because ambulatory BP assesses only 1‐day BP, the additional confirmation of BP‐lowering effects by home BP monitoring could provide a more robust assessment of BP lowering.
Eplerenone and TOD
In the present study, UACR was not reduced in the eplerenone or the control group. In contradiction to our data, in a recent study on nondiabetic hypertensive patients treated with eplerenone, UACR was significantly reduced in the eplerenone group but not in the placebo group.23 However, unlike in this previous study, 37% of patients in our study had diabetes, history of cardiovascular disease was included, and all of the patients had drug‐resistant hypertension despite the use of diuretics. Although PWV was not changed, FMD was significantly improved in the eplerenone group. Improvement of FMD by mineralocorticoid receptor blockade has been reported,24, 25 but PWV was unchanged by mineralocorticoid receptor blockade.26 Our findings are in agreement with these reports. Reasons for this finding in the present study may have been the relatively short observation period or the use of multiple antihypertensive treatments, meaning that further improvement of arterial stiffness was not observed. We calculated the correlation coefficients of the relationship between changes in BP and vascular parameters. As may be seen in supplemental Table 2, changes in home and some of the ambulatory BP parameters in the control group were significantly associated with changes in PWV and FMD. On the other hand, these relations were not observed in the eplerenone group except for changes in home evening DBP and PWV. These results indicate that pharmacologic effects of eplerenone, but not BP‐lowering effects, could have influenced the improvement in FMD.
The correlations between the change in BP levels and the changes in the measures of TOD in this study have important pathophysiological implications. The significant changes in both home and ambulatory BP, and the change in logANP in the eplerenone group, show that the BP‐lowering effect in the eplerenone group could be dependent on the plasma volume reduction. The significant association between the changes in clinic and home BP and the change in log UACR show that in the eplerenone group, BP reduction can lead to a reduction in UACR, as shown by Ando and colleagues,23 but in some patients, both the BP and the UACR increased. On the other hand, the change in FMD seen in the eplerenone group was independent of BP change.
Study Strengths and Limitations
There were several strengths in this study. Namely, it was a randomized study using three measures of BP in patients with RH and various measures of TOD as surrogate markers of successful BP reduction. There were few adverse effects in the eplerenone treatment group.
This study also had some limitations. First, the number of patients was relatively small. Second, the extent of BP reduction was not large. Third, the 12‐week study period was relatively short for evaluating BP lowering and TOD. However, in our previous investigation of the effects of antihypertensive agents, 12 weeks was sufficient to lower BP, especially in the case of ABPM.27, 28 We observed significant effects on TOD measures. As the control group continued the medications at baseline, we considered that a 12‐week period would be appropriate for this study. Finally, the control group did not change or take any additional medication to lower BP, which might be perceived as somewhat weakening the findings of this study. However, because the main purpose of the study was to evaluate the effect of add‐on use of eplerenone, the design was appropriate in comparing the experimental group with a control group without other added medications. From a safety aspect, baseline BP levels (Table 2) were not very high, and thus the 12‐week study period was suitable because the study ended before natural BP elevation occurred in the control group.
Conclusions
In patients with drug‐resistant hypertension, the add‐on use of eplerenone was effective in lowering BP, especially home and ambulatory awake BP, even when the effects on clinic BP were minimal. Furthermore, endothelial function was improved by eplerenone without causing significant adverse effects. Based on the results of this study, eplerenone could be one of the essential strategies for the treatment of RH in clinical practice.
Supporting information
Figure S1. Correlations between the baseline BP and the changes in BP parameters
Table S1. Correlations between changes in BP parameters and TOD measures
Table S2. Relationship between changes in BP and vascular parameters
Table S3. Rate of nondippers (%)
Conflict of Interest
The authors have no conflicts of interest to report.
Funding Source
This study was funded by Pfizer Japan Inc. Pfizer Japan Inc. was not involved in any significant processes of this study such as design, conduct, monitor, supervise, data analysis, and publication of the study.
J Clin Hypertens (Greenwich). 2016;18:1250–1257. DOI: 10.1111/jch.12860. © 2016 Wiley Periodicals, Inc.
References
- 1. Moser M, Setaro JF. Clinical practice. Resistant or difficult‐to‐control hypertension. N Engl J Med. 2006;355:385–392. [DOI] [PubMed] [Google Scholar]
- 2. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 3. Nakano M, Eguchi K, Sato T, et al. Effect of intensive salt‐restriction education on clinic, home, and ambulatory blood pressure levels in treated hypertensive patients during a 3‐month education period. J Clin Hypertens (Greenwich). 2016;18:385–392. doi: 10.1111/jch.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low‐dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–930. [DOI] [PubMed] [Google Scholar]
- 5. Chapman N, Dobson J, Wilson S, et al., On behalf of the Anglo‐Scandinavian Cardiac Outcomes Trial Investigators . Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–845. [DOI] [PubMed] [Google Scholar]
- 6. Calhoun DA, White WB. Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension. J Am Soc Hypertens. 2008;2:462–468. [DOI] [PubMed] [Google Scholar]
- 7. Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. [DOI] [PubMed] [Google Scholar]
- 8. Matsui Y, Kario K, Ishikawa J, et al. Reproducibility of arterial stiffness indices (pulse wave velocity and augmentation index) simultaneously assessed by automated pulse wave analysis and their associated risk factors in essential hypertensive patients. Hypertens Res. 2004;27:851–857. [DOI] [PubMed] [Google Scholar]
- 9. Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial‐ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka H, Munakata M, Kawano Y, et al. Comparison between carotid‐femoral and brachial‐ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–2027. [DOI] [PubMed] [Google Scholar]
- 11. Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 12. Tomiyama H, Matsumoto C, Yamada J, et al. The relationships of cardiovascular disease risk factors to flow‐mediated dilatation in Japanese subjects free of cardiovascular disease. Hypertens Res. 2008;31:2019–2025. [DOI] [PubMed] [Google Scholar]
- 13. Kabutoya T, Hoshide S, Ogata Y, et al. The time course of flow‐mediated vasodilation and endothelial dysfunction in patients with a cardiovascular risk factor. J Am Soc Hypertens. 2012;6:109–116. [DOI] [PubMed] [Google Scholar]
- 14. Eguchi K, Hoshide S, Ishikawa S, et al. Aggressive blood pressure–lowering therapy guided by home blood pressure monitoring improves target organ damage in hypertensive patients with type 2 diabetes/prediabetes. J Clin Hypertens (Greenwich). 2012;14:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karns AD, Bral JM, Hartman D, et al. Study of aldosterone synthase inhibition as an add‐on therapy in resistant hypertension. J Clin Hypertens (Greenwich). 2013;15:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosa J, Widimský P, Toušek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true‐resistant hypertension: six‐month results from the Prague‐15 study. Hypertension. 2015;65:407–413. [DOI] [PubMed] [Google Scholar]
- 17. de Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55:147–152. [DOI] [PubMed] [Google Scholar]
- 18. Oxlund CS, Henriksen JE, Tarnow L, et al. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus: a double blind randomized clinical trial. J Hypertens. 2013;31:2094–2102. [DOI] [PubMed] [Google Scholar]
- 19. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug‐resistant hypertension (PATHWAY‐2): a randomised, double‐blind, crossover trial. Lancet. 2015;386:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elnagar N, Satoh M, Hosaka M, et al. The velocity of home blood pressure reduction in response to low‐dose eplerenone combined with other antihypertensive drugs determined by exponential decay function analysis. Clin Exp Hypertens. 2014;36:83–91. [DOI] [PubMed] [Google Scholar]
- 21. Ohta Y, Ishizuka A, Hayashi S, et al. Effects of a selective aldosterone blocker and thiazide‐type diuretic on blood pressure and organ damage in hypertensive patients. Clin Exp Hypertens. 2015;37:569–573. [DOI] [PubMed] [Google Scholar]
- 22. Hanazawa T, Obara T, Ogasawara K, et al. Low‐dose and very low‐dose spironolactone in combination therapy for essential hypertension: evaluation by self‐measurement of blood pressure at home. Clin Exp Hypertens. 2011;33:427–436. [DOI] [PubMed] [Google Scholar]
- 23. Ando K, Ohtsu H, Uchida S, et al. Anti‐albuminuric effect of the aldosterone blocker eplerenone in non‐diabetic hypertensive patients with albuminuria: a double‐blind, randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2014;2:944–953. [DOI] [PubMed] [Google Scholar]
- 24. Hwang M‐H, Yoo J‐K, Luttrell M, et al. Mineralocorticoid receptors modulate vascular endothelial function in human obesity. Clin Sci. 2013;125:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fujimura N, Noma K, Hata T, et al. ROCK Study Group . Mineralocorticoid receptor blocker eplerenone improves endothelial function and inhibits Rho‐associated kinase activity in patients with hypertension. Clin Pharmacol Ther. 2012;91:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hwang M‐H, Yoo J‐K, Luttrell M, et al. Role of mineralocorticoid receptors in arterial stiffness in human aging. Exp Gerontol. 2013;48:701–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eguchi K, Tomizawa H, Ishikawa J, et al. Effects of new calcium channel blocker, azelnidipine, and amlodipine on baroreflex sensitivity and ambulatory blood pressure. J Cardiovasc Pharmacol. 2007;49:394–400. [DOI] [PubMed] [Google Scholar]
- 28. Eguchi K, Hoshide S, Kario K. Effects of celiprolol and bisoprolol on blood pressure, vascular stiffness, and baroreflex sensitivity. Am J Hypertens. 2015;28:858–867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Correlations between the baseline BP and the changes in BP parameters
Table S1. Correlations between changes in BP parameters and TOD measures
Table S2. Relationship between changes in BP and vascular parameters
Table S3. Rate of nondippers (%)


