Abstract
Arterial stiffness is a well‐established risk factor for cardiovascular disease and mortality. Carotid to femoral pulse wave velocity (cfPWV) as a measure of arterial stiffness was obtained in 155 (47 women; 67.2±9.1 years, range 44–87 years) patients with detected coronary artery disease (CAD) scheduled for coronary artery bypass surgery. The authors set out to analyze how cfPWV in CAD patients correlates with reference values for healthy, normotensive volunteers and whether cfPWV values reflect the extent of CAD. cfPWV was measured with an oscillometric device. Mean cfPWV value of CAD patients was 9.3±1.9 m/s vs 7.7±1.1 m/s in healthy volunteers (P<.0001). In a multiple regression model, age (P<.0001), sex (P=.006), systolic arterial pressure (P=.04), mean arterial pressure (P=.04), and severity of CAD (P<.001) emerged as independent predictive markers for cfPWV in CAD patients. This study established reference values for cfPWV in CAD patients measured with an oscillometric device and confirmed the strong association between arterial stiffness and severity of CAD.
Cardiovascular diseases are the leading cause of morbidity and mortality in industrial countries. Aging of the vascular system is characterized by intimal thickening, calcium deposition, endothelial dysfunction, and accumulation of interstitial collagen.1, 2 As a result, vessel wall elasticity and compliance decrease, whereas arterial stiffness and pulse wave velocity increase. Therefore, assessment of carotid to femoral pulse wave velocity (cfPWV) has been recommended by the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) since 2007. The measurement of cfPWV is the “gold standard” to estimate central arterial changes and a good predictor of increased cardiovascular risk.3, 4 Normally, the pressure wave reflected from the periphery reaches the heart during diastole. However, with increasing arterial stiffness caused by aging or diseases, the velocity of the wave increases and the reflected pressure wave returns to the heart earlier. The pressure wave then reaches the heart during systole, resulting in elevated systolic and decreased diastolic blood pressure. As a result, the cardiac left ventricular workload increases, which subsequently leads to left ventricular hypertrophy. Moreover, the decrease in diastolic pressure compromises the coronary blood flow and increases the risk for myocardial ischemia.5
In the past, cfPWV has been mostly assessed using applanation tonometry,6 which is time‐consuming and requires considerable operator training as well as the exposure and palpation of the femoral artery.7 Since the introduction of an oscillometric device with good intraobserver and interobserver viability and good agreement with applanation tonometry, the measurement of cfPWV was performed in a number of studies with this easy‐to‐use and well‐tolerated device.8, 9, 10, 11, 12, 13, 14, 15 However, a wider implementation of cfPWV into clinical practice is hampered by the fact that larger cohort studies with established reference values for a standardized, convenient, and viable cfPWV assessment method are lacking.16 The aim of this study was to assess reference values for CAD patients with the oscillometric device and to evaluate whether an increase in cfPWV values is related to the severity of CAD.
Patients and Methods
Study Patients
In the present study we assessed 155 patients (108 men, 47 women) with diagnosed CAD scheduled for coronary artery bypass surgery. For all patients, height and weight were measured and body mass index was determined as kg/m2. Smoking status was classified as nonsmoker or current smoker (smoking within the past 2 months). Diabetes was defined as previously diagnosed diabetes. CAD was defined as 1‐vessel, 2‐vessel, 3‐vessel, and multivessel disease. At the preoperative visit, all study parameters including cfPWV, blood for laboratory analysis, and use of medications were determined. According to the guidelines of the JNC8 (2014)17 and the ESH‐ESC (2013)18 and orientated on the study from Pucci and colleagues,13 patients were categorized as hypertensive if their systolic blood pressure (SBP) was >140 mm Hg or diastolic blood pressure (DBP) >90 mm Hg, or if they were receiving blood pressure (BP)–lowering drug treatment. BP was measured automatically with an oscillometric device and mean arterial pressure (MAP) was calculated from SBP and DBP as MAP=DBP+0.33×(SBP−DBP). The study was approved by the local medical ethics committee and was carried out in accordance with the Declaration of Helsinki guidelines. Written informed consent was obtained from all study participants.
Measurement of cfPWV
cfPWV and oscillometric BP were measured after the patients had rested in a supine position (~30°) for 10 minutes. An oscillometric device with Food and Drug Administration approval in 2007 (Vicorder; SMT Medical, Würzburg, Germany) was used for simultaneous recording of carotid and femoral pulse waves according to the manufacturer's instruction as previously described.9, 19 To measure cfPWV, a carotid pressure cuff was applied over the right common carotid artery and a femoral pressure cuff was placed around the right upper thigh, as proximal as possible. Then the distance between the suprasternal notch and the top of the femoral cuff was measured with a tape and this value was entered into the computer. Both cuffs were inflated simultaneously up to 65 mm Hg and the corresponding oscillometric signal from each cuff was digitally analyzed to extract in real time the pulse time delay. After acquiring 10 to 15 steady pulses, the investigator saved the recording and the pulse transit time in milliseconds was reported. The cfPWV was calculated from the device software by dividing traveled pulse wave distance by pulse transit time. All measurements were made in triplicate and averaged for the analyses.
Data Analyses
Categorical variables were expressed as frequencies and percentages. Metric variables were expressed as mean±standard deviation. Statistical analysis was performed using MedCalc 13.02 program (Windows, Redmond, WA). Differences between two groups were analyzed using a t test, and when normality was not met, the Mann‐Whitney U test was used. Differences between more than two groups regarding normally distributed variables were analyzed by analysis of variance. P values <.05 were considered significant.
Results
In the present study, we assessed 155 patients (47 women; mean age, 67.2±9.1 years, range 44–87 years) with diagnosed CAD scheduled for coronary artery bypass surgery. As expected, only 30% of the patients were women. A total of 92.9% of the participants had treated arterial hypertension, 80.6% had treated hyperlipidemia, 21.9% smoked within the past 2 months, and 36.1% had diagnosed type 2 diabetes. Regarding the degree of CAD, 5.8% (n=9) of patients had single‐vessel, 26.5% (n=41) had two‐vessel, 60.6% (n=94) had three‐vessel, and 7.1% (n=11) had multivessel disease. Further demographic and clinical data of the study individuals are shown according to age groups in Table 1.
Table 1.
Demographic and Clinical Data of 155 Patients According to Age Groups
| Study Group (N=155) | 40–49 y (n=8) | 50–59 y (n=21) | 60–69 y (n=52) | 70–79 y (n=65) | 80–89 y (n=9) | |
|---|---|---|---|---|---|---|
| Age, y | 67.2±9.1 | 46.8±2.0 | 54.8±2.9 | 64.7±3.1 | 73.7±2.6 | 82.0±2.2 |
| Men/women | 108/47 | 7/1 | 19/2 | 36/16 | 40/25 | 6/3 |
| BMI, kg/m2 | 28.5±4.5 | 30.9±5.2 | 29.8±5.4 | 28.3±4.4 | 28.1±4.1 | 28.3±4.3 |
| SBP, mm Hg | 138.5±19.8 | 140.4±24.9 | 135.1±18.3 | 138.1±19.2 | 139.9±20.6 | 139.3±16.6 |
| DBP, mm Hg | 72.4±10.3 | 83.6±13.6 | 74.7±10.9 | 73.4±9.6 | 69.9±9.7 | 69.1±7.4 |
| MAP, mm Hg | 94.2±12.0 | 102.3±16.9 | 94.6±12.5 | 94.7±11.1 | 93.0±11.9 | 92.3±9.6 |
| cfPWV, m/s | 9.3±1.9 | 7.9±1.5 | 8.3±1.5 | 9.0±1.7 | 10.0±1.9 | 9.7±1.4 |
Abbreviations: BMI, body mass index; cfPWV, carotid to femoral pulse wave velocity; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.
When comparing cfPWV values of CAD patients and reference values of volunteers (Table 2) from data published by Müller and colleagues,12 we noticed a significant increase in cfPWV values in the age group from 60 to 75 years as shown in Table 3 and Figure 1, which is also in accordance with data by McEniery and colleagues.16
Table 2.
Demographic and Clinical Data of 318 Volunteers12 According to Age Groups
| Volunteers12 (N=318) | <30 y (n=196) | 30–39 y (n=32) | 40–49 y (n=36) | 50–59 y (n=36) | ≥60 y (n=18) | |
|---|---|---|---|---|---|---|
| Age, y | 28.7±17.6 | 46.8±2.0 | 34.6±3.0 | 44.9±2.8 | 54.6±2.8 | 66.8±7.4 |
| Men/women | 153/165 | 97/99 | 13/19 | 20/16 | 12/24 | 11/7 |
| BMI, kg/m2 | 22.5±4.7 | 21.4±4.0 | 23.4±3.6 | 24.4±3.2 | 25.4±4.4 | 26.9±3.8 |
| SBP, mm Hg | 122.1±9.6 | 120.8±8.7 | 123.4±10.4 | 124.3±7.7 | 124.9±11.2 | 127.9±10.5 |
| DBP, mm Hg | 65.6±9.3 | 62.4±7.1 | 68.3±7.6 | 73.6±8.2 | 71.5±7.9 | 69.6±7.4 |
| MAP, mm Hg | 84.4±8.4 | 81.8±6.7 | 86.4±7.7 | 90.5±7.6 | 89.3±8.1 | 89.1±6.8 |
| cfPWV, m/s | 6.1±1.4 | 5.3±0.6 | 6.5±0.6 | 7.1±0.9 | 7.9±1.1 | 8.2±1.2 |
Abbreviations: BMI, body mass index; cfPWV, carotid to femoral pulse wave velocity; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.
Table 3.
Association of cfPWV Values of CAD Patients and Volunteers12
| Age Group, y | CAD Patients cfPWV, m/s | Volunteers12 cfPWV, m/s | P Value |
|---|---|---|---|
| 40–49 | 7.9±1.5 (n=8) | 7.1±0.9 (n=36) | .05 |
| 50–59 | 8.3±1.5 (n=21) | 7.9±1.1 (n=36) | .2 |
| 60–75 | 9.6±1.9 (n=100) | 8.2±1.2 (n=18) | .003 |
| 40–87 | 9.3±1.9 (n=155) | 7.7±1.1 (n=90) | <.0001 |
Abbreviations: CAD, coronary artery disease; cfPWV, carotid to femoral pulse wave velocity.
Figure 1.
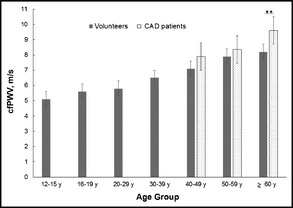
Carotid to femoral pulse wave velocity (cfPWV) of healthy volunteers and patients with coronary artery disease (CAD). **P<.01.
Risk factors such as type 2 diabetes (r=0.13, P=.11), treated hypertension (r=0.01, P=.9), hyperlipidemia (r=0.08, P=.3), and smoking (r=0.09, P=.3) did not show any association with cfPWV values in univariate analyses. Body mass index (r=0.17, P=.03) was associated with cfPWV in univariate correlation, but not in multivariate analyses.
In the multivariate regression model, including all significant demographic variables, age, sex, severity of CAD, SBP, and MAP emerged as independent predictive markers for cfPWV in CAD patients (Table 4). The regression parameter ß was 1.4 for the two‐vessel CAD group, which means that this group had a mean 1.4 higher cfPWV value compared with the reference group with one‐vessel CAD. The three‐vessel CAD group had a 2.98 and the multivessel CAD group a 5.75 higher mean cfPWV value compared with the reference group. As shown in Figure 2, cfPWV was strongly associated with the severity of the patient's CAD in one‐way analysis of variance (ANOVA).
Table 4.
Multivariate Regression Analyses of cfPWV Including All Significant Demographic Variables
| Variable | Regression Coefficient ß | Standard Error | P Value |
|---|---|---|---|
| Age, y | 0.06 | 0.01 | <.0001 |
| Male sex (vs female) | 0.58 | 0.21 | .006 |
| 1‐vessel CAD | Reference group | ||
| 2‐vessel CAD | 1.4 | 0.4 | .0007 |
| 3‐vessel CAD | 2.98 | 0.4 | <.0001 |
| Multivessel CAD | 5.75 | 0.5 | <.0001 |
| SBP, mm Hg | 0.01 | 0.006 | .04 |
| MAP, mm Hg | 0.037 | 0.02 | .04 |
Abbreviations: CAD, coronary artery disease; cfPWV, carotid to femoral pulse wave velocity; MAP, mean arterial pressure; SBP, systolic blood pressure.
Figure 2.
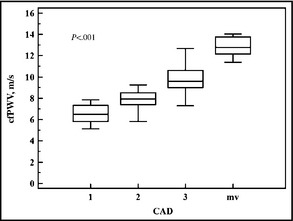
Carotid to femoral pulse wave velocity (cfPWV) in association with severity of coronary artery disease (CAD). mv indicates multivessel. P value from one‐way analysis of variance.
Discussion
That higher aortic stiffness assessed by pulse wave velocity is associated with an increased risk for cardiovascular events is widely accepted since the publication of the Framingham Heart Study.20 Laurent and colleagues21 highlighted that cfPWV is strongly associated with cerebral vessel disease and cardiovascular events and therefore suggested the use of aortic stiffness as a tissue biomarker for the prediction of future cardiovascular events in specific risk groups. Addressing the importance of cfPWV measurement, reference values for healthy people and patients with cardiovascular risk factors were published by the Arterial Stiffness' Collaboration Group.22 For the collection of cfPWV data, the majority of the studies used the applanation tonometry method. However, this method is highly operator‐dependent, time‐consuming, and intrusive for the patient because, for the palpation of the femoral pulse, the groin has to be exposed. Therefore, there is a demand for devices that allow a quicker, less operator‐dependent, and accurate measurement of cfPWV. The device used in this study estimates aortic stiffness by an oscillometric technique. In a number of studies, cfPWV was assessed with this method.6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Hickson and colleagues,6 Kracht and colleagues,9 and McGreevy and colleagues10 have found a good inter‐observer and intraobserver viability and high reproducibility for this oscillometric device in a clinical setting with little operator training. Müller and colleagues12 as well as other investigators noted the importance of a correct measurement of the path length and suggested the direct measurement from the suprasternal notch to the top of the tight cuff. Using this technique, our study results are in good agreement with cfPWV values of other investigators. In accordance with the data of McEniery and colleagues,16 cfPWV values of patients with CAD were dependent on age and BP. Traditional risk factors such as diabetes, hyperlipidemia, and smoking had no effect on cfPWV in multivariate analysis. Interestingly, in the multivariate analysis, we found a strong association between cfPWV values and the degree of CAD. To our knowledge, this connection was expected but never shown before in a clinical study. Studies have shown a close relationship between increased arterial stiffness and microvascular damage in the heart, brain, retina, and kidney.20, 21 It seems that this is the result of a vicious cycle of mechanisms. First, the local control of blood flow in organs such as the heart, brain, and kidney is mediated in part by the myogenic tone in the resistance vessels. When perfusion pressure increases, the vessels constrict to maintain flow at a relatively constant level. Under these conditions over time, microvascular remodeling occurs, leading to an increase in resistance that compromises resting flow and flow reactivity. Second, reduced wave reflection in the larger muscular arteries that is observed particularly after the age of 60 leads to increased pulsatile energy penetration into small arteries and the microcirculation where excessive dissipation of this energy causes a pulsatile barotrauma, leading to endothelial and vascular damage, atherosclerotic plaques, and remodeling. Third, excessive pressure pulsatility has been shown to impair endothelial function, leading to endothelial dysfunction in large and small arteries associated with elevated levels of circulating inflammatory markers and atherosclerotic vascular changes. Most age‐related disorders such as coronary disease, cognitive impairment, and kidney dysfunction have microvascular changes as pathophysiology. Elevated cfPWV values are a risk factor for these disorders, suggesting a close relationship between large artery stiffness, reflected by cfPWV, and microvascular changes. In a recent publication by Ikonomidis and colleagues,23 arterial stiffness, quantified by pulse wave velocity, was identified as a valid marker of atherosclerosis and was associated with impaired coronary flow reserve and CAD.
Figure 3.
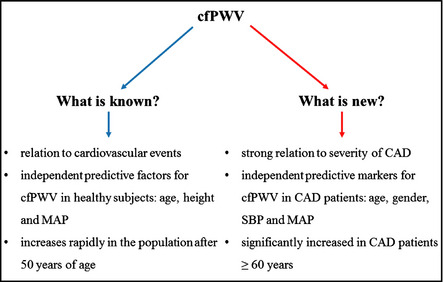
Scheme of knowledge and news about carotid to femoral pulse wave velocity (cfPWV).
Study Limitations
Nevertheless, there are some clear limitations of our study. Although we found a sex dependency of cfPWV values in CAD patients, it should be noted that the female patient group was underrepresented and further research on this is recommended. Moreover, our study population was small and as easy‐to‐use devices for cfPWV measurement are available, larger studies should be conducted to establish normal cfPWV values with this device for all age and sex groups as well as cutoff values for patients at higher risk for cardiovascular disease and cerebrovascular events.
Conclusions
In our study, cfPWV is a strong noninvasive predictor of severity of CAD and is associated with age, sex, and BP values. Therefore, cfPWV is, in our view, a valuable biomarker that should be added to standard risk assessments to improve cardiovascular risk prediction in the community.
Funding
Financial support was given from the Roux Funding Program of the Martin‐Luther‐University Halle‐Wittenberg.
Disclosure
None.
Acknowledgments
We thank our students Beatrice Mühlberg and Silvia Paasch for their contribution to the study.
J Clin Hypertens (Greenwich). 2014;16:629–633. © 2014 Wiley Periodicals, Inc.
References
- 1. Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. [DOI] [PubMed] [Google Scholar]
- 2. Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. [DOI] [PubMed] [Google Scholar]
- 3. Mortensen K, Weber T, Baulmann J. Arterielle Gefäßsteifigkeit ‐ Biomarker des kardiovaskulären Risikos und ihr Zusammenhang zu kardiovaskulären Erkrankungen. J Für Hypertonie. 2010;14:31–35. [Google Scholar]
- 4. Nuernberger J, Kribben A, Philipp T, Erbel R. [Arterial compliance (stiffness) as a marker of subclinical atherosclerosis]. Herz. 2007;32:379–386. [DOI] [PubMed] [Google Scholar]
- 5. Sutton‐Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well‐functioning older adults. Circulation. 2005;111:3384–3390. [DOI] [PubMed] [Google Scholar]
- 6. Hickson SS, Butlin M, Broad J, et al. Validity and repeatability of the Vicorder apparatus: a comparison with the SphygmoCor device. Hypertens Res. 2009;32:1079–1085. [DOI] [PubMed] [Google Scholar]
- 7. Butlin M, Qasem A, Battista F, et al. Carotid‐femoral pulse wave velocity assessment using novel cuff‐based techniques: comparison with tonometric measurement. J Hypertens. 2013;31:2237–2243; discussion 2243. [DOI] [PubMed] [Google Scholar]
- 8. Fischer DC, Schreiver C, Heimhalt M, et al. Pediatric reference values of carotid‐femoral pulse wave velocity determined with an oscillometric device. J Hypertens. 2012;30:2159–2167. [DOI] [PubMed] [Google Scholar]
- 9. Kracht D, Shroff R, Baig S, et al. Validating a new oscillometric device for aortic pulse wave velocity measurements in children and adolescents. Am J Hypertens. 2011;24:1294–1299. [DOI] [PubMed] [Google Scholar]
- 10. McGreevy C, Barry M, Bennett K, Williams D. Repeatability of the measurement of aortic pulse wave velocity (aPWV) in the clinical assessment of arterial stiffness in community‐dwelling older patients using the Vicorder device. Scand J Clin Lab Invest. 2013 Apr 2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11. McIntyre NJ, Fluck RJ, McIntyre CW, et al. Determinants of arterial stiffness in chronic kidney disease stage 3. PLoS ONE. 2013;8:e55444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Müller J, Oberhoffer R, Barta C, et al. Oscillometric carotid to femoral pulse wave velocity estimated with the Vicorder device. J Clin Hypertens (Greenwich). 2013;15:176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pucci G, Cheriyan J, Hubsch A, et al. Evaluation of the Vicorder, a novel cuff‐based device for the noninvasive estimation of central blood pressure. J Hypertens. 2013;31:77–85. [DOI] [PubMed] [Google Scholar]
- 14. Stone IS, John L, Petersen SE, Barnes NC. Reproducibility of arterial stiffness and wave reflections in chronic obstructive pulmonary disease: the contribution of lung hyperinflation and a comparison of techniques. Respir Med. 2013;107:1700–1708. [DOI] [PubMed] [Google Scholar]
- 15. Shahin Y, Barakat H, Barnes R, Chetter I. The Vicorder device compared with SphygmoCor in the assessment of carotid‐femoral pulse wave velocity in patients with peripheral arterial disease. Hypertens Res. 2013;36:208–212. [DOI] [PubMed] [Google Scholar]
- 16. McEniery CM, Yasmin, Maki‐Petaja KM, et al. The impact of cardiovascular risk factors on aortic stiffness and wave reflections depends on age: the Anglo‐Cardiff Collaborative Trial (ACCT III). Hypertension. 2010;56:591–597. [DOI] [PubMed] [Google Scholar]
- 17. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 18. ESH/ESC Task Force for the Management of Arterial Hypertension . 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31:1925–1938. [DOI] [PubMed] [Google Scholar]
- 19. Hofmann B, Adam AC, Jacobs K, et al. Advanced glycation end product associated skin autofluorescence: a mirror of vascular function? Exp Gerontol. 2013;48:38–44. [DOI] [PubMed] [Google Scholar]
- 20. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laurent S, Alivon M, Beaussier H, Boutouyrie P. Aortic stiffness as a tissue biomarker for predicting future cardiovascular events in asymptomatic hypertensive subjects. Ann Med. 2012;44(suppl 1):S93–S97. [DOI] [PubMed] [Google Scholar]
- 22. Reference Values for Arterial Stiffness' Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values.’ Eur Heart J. 2010;31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ikonomidis I, Kadoglou NN, Tritakis V, et al. Association of Lp‐PLA2 with digital reactive hyperemia, coronary flow reserve, carotid atherosclerosis and arterial stiffness in coronary artery disease. Atherosclerosis. 2014;234:34–41. [DOI] [PubMed] [Google Scholar]


