I recently proposed a novel disease entity, systemic hemodynamic atherothrombotic syndrome (SHATS), which is characterized by a synergistic risk of exaggerated hemodynamic stress (exaggerated variability of blood pressure [BP] and blood flow) and vascular disease, not only for advancing organ damage but also for triggering cardiovascular events.1, 2, 3 This entity serves to separate physical hemodynamic stress from other material risk factors such as glucose, lipids, and inflammatory reactions.
Hypertension is defined by the average of BP levels, of which its clinical implication is well established. The latest international and domestic guidelines for the management of hypertension were published recently,4, 5, 6 and the management of hypertension in all of these guidelines is based on the average of clinic, home, or ambulatory BP values. However, average‐based BP management may miss the risk for BP.
There is growing evidence that excess variability in BP presents a risk for organ damage and cardiovascular events.7 The various types of BP variability include shortest beat‐by‐beat BP variability, diurnal BP variability, day‐by‐day variability in home BP, visit‐to‐visit variability in clinic BP, seasonal BP variability, and long‐term age‐related BP variability for several years. Various neurohumoral networks are involved in the regulation of BP, and the disruption of these networks and changes in the vascular properties of small and large arteries are potential underlying causes of excessive BP variability and its related organ damage.
Here, I present a typical case of SHATS in which the patient developed ischemic stroke despite well‐controlled classical risk factors, and I discuss this new disease entity in relation to her case.
A 72‐year‐old Japanese woman who had been registered and was being followed in the Japan Morning Surge Home Blood Pressure (J‐HOP)8 study experienced an acute stroke just after rising in the morning to take a walk (Figure 1). Her neurological deficits were dizziness and conjugate deviation. The responsible lesion (shown in diffusion magnetic resonance imaging) was located in the small artery, in the paramedian branch (the perforating artery supplying the posterior circulation) of the posterior cerebral artery (Figure 1). The patient had a history of hypertension from age 36, hyperlipidemia from age 57, and acute myocardial infarction from age 58. Before the onset of stroke, she had achieved good control of clinic BP (<130/80 mm Hg), home BP self‐measured in the sitting position (125/75 mm Hg), average 24‐hour BP (<120/75 mm Hg) (Figure 2), and other metabolic risk factors with a regimen of aspirin, candesartan, hydrochlorothiazide, bisoprolol, and a statin.
Figure 1.
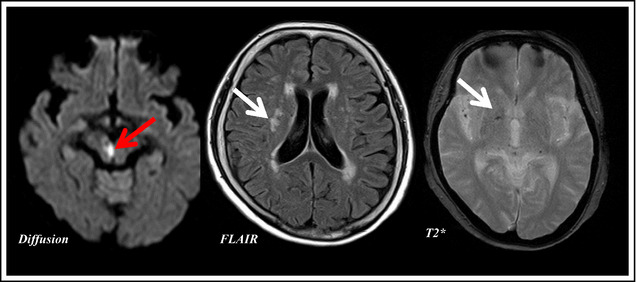
Imaging of a 72‐year‐old woman who developed a morning‐onset stroke. The paramedian branch of the posterior cerebral artery exhibited signs of branch atheromatous disease, and this anatomical location corresponded to the site of high intensity of diffusion magnetic resonance imaging and her neurological deficit. The red arrow indicates the acute infarction in diffusion magnetic resonance imaging and the white arrows show old deep white matter infarcts (fluid‐attenuated inversion recovery) and microbleed (T2*).
Figure 2.
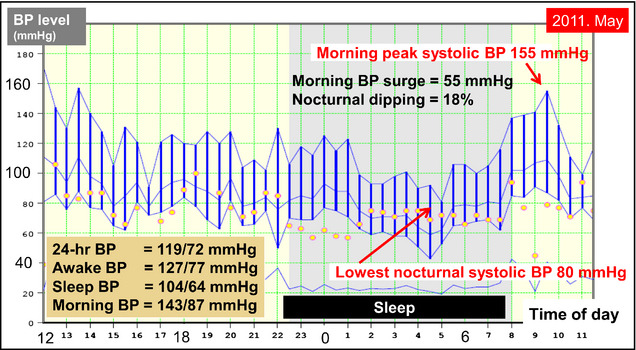
Ambulatory blood pressure (BP) monitoring (ABPM) before the onset of the patient's stroke. ABPM performed 13 months before the stroke demonstrated an exaggerated morning BP surge with marked daytime BP variability, even when the average of the 24‐hour BP level was well controlled to <130/80 mm Hg.
Vascular evaluation tests performed before the onset of the stroke disclosed advanced systemic vascular disease (flow‐mediated dilatation of the brachial artery: 2.4% [normal range: ≥5%]; intima‐media thickness of the common carotid artery: 2.2 mm (right), 2.2 mm (left) [normal range: <1.0 mm]; brachial‐ankle pulse wave velocity (1935 cm/s, corresponding to 93‐year‐old healthy reference patients); carotid augmentation index: 26%). However, the patient had risk factors for SHATS, including exaggerated morning blood pressure surge (MBPS) (systolic BP ≥55 mm Hg) (Figure 2) and orthostatic hypertension detected by orthostatic home BP monitoring before stroke (Figure 3).
Figure 3.
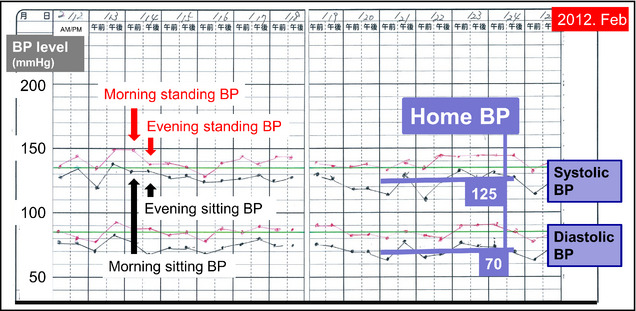
The patient's self‐measured home active standing blood pressure (BP) monitoring 4 months before the onset of stroke. This home active standing BP monitoring performed in the morning and evening (twice per day) for 14 days clearly demonstrated persistent orthostatic hypertension (orthostatic increase in systolic BP >15 mm Hg) at home. The black dots and lines represent the second reading of the sitting BP measurements and the red dots and lines represent the standing BPs consecutively measured just after standing.
Although the patient's conventional risk factors including ambulatory and home BP values were well‐controlled below 130 mm Hg, she experienced a stroke event one morning. Current Japanese and international guidelines for the management of hypertension stress the importance out‐of‐office BP, and the BP control status is assessed by the average of clinic, home, or 24‐hour BP values.4, 5, 6 However, even if the average value of one of these BP measures is well below the guideline‐recommended threshold for the target BP level, there is still a blind spot in disease management, ie, exaggerated BP variability still poses a risk for advancing organ damage and for triggering cardiovascular events.
There are several phenotypes of BP variability in SHATS, such as morning BP surge, orthostatic hypertension, sleep BP surge, and extreme‐dipping of nocturnal BP, that have been reported to confer cardiovascular risk.1, 2, 3, 9, 10, 11, 12, 13 Some of these conditions overlap, indicating a partly shared common pathophysiology involving vascular damage such as impaired baroreceptor sensitivity caused by increased sympathetic activity or increased large artery stiffness and small artery remodeling. Exaggerated BP variability not only promotes vascular damage, thereby perpetuating a vicious cycle, but may also trigger cardiovascular events at the time of BP peak. Patients who have a history of cardiovascular events and/or increased large artery stiffness may have an augmented risk of BP variability, because the power of the pulse wave transmits to peripheral sites of the vascular tree without attenuation, resulting in cardiovascular events of large artery disease (atherothrombotic disease) and small artery disease (branch atheromatous disease, lacunar infarct).1, 2, 3
It is therefore imperative that clinicians recognize the synergistic relationship between the risk of exaggerated BP variability and vascular damage (Figure 4). The diagnosis of SHATS is based on both exaggerated BP variability and advanced vascular disease. Even when patients are well controlled for conventional risk factors and their average BP values are controlled, hypertensive patients may be at risk for advanced organ damage and cardiovascular events—especially high‐risk patients with advanced vascular disease. The detection of phenotypes of BP variability to assess SHATS and an integrated strategy for cardiovascular protection targeting BP variability and vascular damage may be effective.
Figure 4.
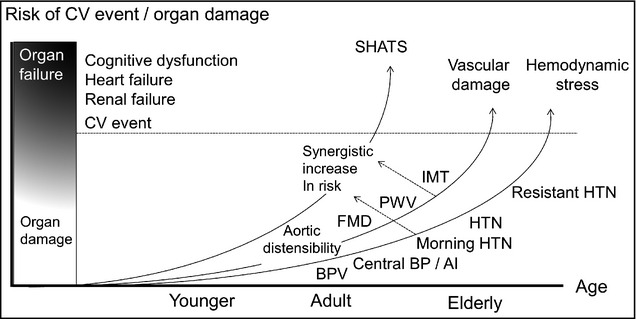
Synergistic risk of systemic hemodynamic atherothrombotic syndrome (SHATS) on cardiovascular event and organ damage worsened by a vicious cycle of hemodynamic stress and vascular damage. FMD indicates flow‐mediated dilatation of the brachial artery; PWV, pulse wave velocity; IMT, intima‐media thickness of the carotid artery; HTN, hypertension; BPV, blood pressure variability; AI, augmentation index.
Recent advances in information technology have enabled the collection of large amounts of BP data from individual patients and easier assessments of BP variability. Further data on antihypertensive treatment considering BP variability should be extensively collected to use in clinical practice in the near future.
Disclosures
The author states that there are no conflicts of interest to report.
References
- 1. Kario K. Proposal of a new strategy for ambulatory blood pressure profile‐based management of resistant hypertension in the era of renal denervation. Hypertens Res. 2013;36:478–484. [DOI] [PubMed] [Google Scholar]
- 2. Kario K. Orthostatic hypertension—a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol. 2013;9:726–738. [DOI] [PubMed] [Google Scholar]
- 3. Kario K. Morning surge in blood pressure—a phenotype of systemic hemodynamic atherothrombotic syndrome. Am J Hypertens. 2015;28:7–9. [DOI] [PubMed] [Google Scholar]
- 4. Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood‐pressure variability. Nat Rev Cardiol. 2013;10:143–155. [DOI] [PubMed] [Google Scholar]
- 5. Kario K, Hoshide S, Haimoto H, et al; on behalf of the J‐HOP study group . Sleep blood pressure self‐measured at home as a novel determinant of organ damage: Japan Morning Surge Home Blood Pressure (J‐HOP) Study. J Clin Hypertens (Greenwich). 2015;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mancia G, Fagard R, Narkiewicz K, et al; Task Force Members . 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 7. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimamoto K, Ando K, Fujita T, et al; Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension . The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37:253–387. [DOI] [PubMed] [Google Scholar]
- 9. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 10. Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56:765–773. [DOI] [PubMed] [Google Scholar]
- 11. Sheppard JP, Hodgkinson J, Riley R, et al. The prognostic significance of the morning blood pressure surge in clinical practice: a systematic review. Am J Hypertens. 2015;28:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Metoki H, Ohkubo T, Kikuya M, et al. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension. 2006;47:149–154. [DOI] [PubMed] [Google Scholar]
- 13. Kario K, Pickering TG, Matsuo T, et al. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. [DOI] [PubMed] [Google Scholar]


