Abstract
Recently, endocan—formerly known as endothelial cell‐specific molecule 1 (ESM‐1)—was found to be associated with entities such as cancer, hypertension, renal transplant rejection, and chronic renal failure. Endothelial cells of many organs secrete endocan, but the exact functions of this relatively new molecule have not been elucidated completely. Emerging evidence suggests, however, that endocan plays an important role in inflammation, upregulation of cell adhesion molecules, lymphocyte functions, and endothelial cytoskeleton rearrangement. As suggested above, endocan has a prognostic impact in hypertension, transplant rejection, and chronic renal failure. In the current review, the evidence regarding endocan, hypertension, and chronic renal failure are summarized.
In the past few years, the vascular endothelium has been shown to play a crucial role in inflammation, coagulation, angiogenesis, and tumor invasion, primarily through regulation of receptor/ligand interactions and secretion of different mediators. One of the molecules secreted by endothelial cells is endothelial cell‐specific molecule 1 (ESM‐1).1 The carbohydrate moiety of ESM‐1 is a single chain of the dermatan sulfate proteoglycan comprising a mature polypeptide of 165 amino acids. Because this proteoglycan is specifically secreted by endothelial cells, the name proposed was “endocan.”2
Endocan is secreted by vascular endothelial cells in the skin, kidney, gastrointestinal tract, lungs, liver, brain, lymph nodes, heart, and thyroid gland.3 Normally, endocan is proposed to have a major role in the regulation of cell adhesion, inflammatory disorders, and tumor progression. Endocan has been shown to have prognostic value in various pathologic entities, such as cancer, sepsis, inflammatory disorders, hypertension (HTN), transplant rejection, and chronic kidney disease.4, 5, 6, 7, 8, 9, 10, 11, 12, 13
In the current review, we summarize the recent findings regarding endocan and disease states, specifically focusing on HTN, chronic kidney disease, renal transplantation, underlying potential mechanisms, and future perspectives.
Endocan and HTN
Since endothelial dysfunction, atherosclerosis, and HTN are related with each other and since endocan is linked with endothelial dysfunction and inflammation, it was suggested that endocan might also be related to HTN.11
Indeed, in one pilot study, the relationship between endocan and HTN was investigated. Balta and colleagues showed that endocan levels were higher in newly diagnosed hypertensive patients compared with normotensive patients. Furthermore, endocan levels correlated with carotid intima–media thickness and high‐sensitivity C‐reactive protein (hsCRP).11 However, this study had many drawbacks including low patient numbers, not using ambulatory blood pressure, and lack of data regarding echocardiographic parameters.
In another study, the effect of calcium channel blocker (amlodipine) treatment on serum endocan levels and endothelial cell adhesion molecules were investigated in patients with essential HTN. Patients were treated with amlodipine 5 mg/d to 10 mg/d for 8 weeks and blood samples were taken at the beginning and after an 8‐week period. Following treatment, intracellular cell adhesion molecule 1 (ICAM‐1) and vascular cell adhesion molecule 1 (VCAM‐1) levels decreased while E‐selectin increased. Endocan levels tended to decrease (P=.063). On the other hand, the CD11a/lymphocyte function‐associated antigen 1 (LFA‐1) ICAM‐1 and endocan ligand was significantly increased in lymphocytes, monocytes, and granulocytes. Thus, the authors concluded that the increased expression of ICAM/VACM ligands, together with a decrease of soluble cell adhesion molecules and endocan, suggest a de‐activation of endothelium consecutive to a reduction in blood pressure by amlodipine in hypertensive patients.14
Endocan and Renal Transplantation
First reports came from Li and colleagues investigating the prognostic utility of ESM‐1—a precursor of endocan—in acute rejection after renal transplantation. This study has demonstrated that in patients with acute rejection, ESM‐1 mRNA and protein expression increased significantly. The authors concluded that ESM‐1 appeared to reflect the degree of endothelial cell injury in renal allografts and might have the potential to serve as a highly sensitive and specific marker for acute rejection after renal transplantation.15
Soon after this initial report, another study examined the relationship between endocan levels and chronic renal allograft injury in renal transplant patients. Su and colleagues recruited 97 renal transplant patients with a mean age of 43.6±13.2 years and with a mean transplant duration of 7.0±5.7 years. They demonstrated that higher endocan levels were found in more advanced chronic kidney disease (CKD) stages in a dose‐dependent manner and that endocan levels were inversely correlated with estimated glomerular filtration rate. In addition, renal function decline was significantly greater in the group with higher serum endocan levels. Moreover, tumor necrosis factor α (TNF‐α) correlated with endocan and TNF‐α–activated human umbilical vein endothelial cells secrete high levels of endocan and transforming growth factor β1. All these findings suggest a relationship between endocan and endothelial dysfunction.12
Endocan and CKD
Very recently, we investigated the relationship between plasma endocan levels and inflammation, endothelial dysfunction, cardiovascular events, and survival in CKD. A total of 251 patients with CKD (stage 1–5) not undergoing dialysis were recruited. Plasma endocan concentrations were negatively correlated with estimated glomerular filtration rate and positively correlated with inflammatory markers such as pentraxin 3 (PTX3) and hsCRP. In addition, endocan was associated with endothelial dysfunction as evaluated by flow‐mediated vasodilatation (FMD) and carotid intima–media thickness (CIMT). More importantly, after adjusting for multiple confounders, endocan levels were independently associated with cardiovascular events (hazard ratio, 3.54; 95% confidence interval [CI], 2.36–5.28) and all‐cause mortality (hazard ratio, 2.38; 95% CI, 1.17–4.82). In addition, the authors demonstrated some important and interesting findings. Using receiver operating curve analysis, endocan had an area under the receiver operating curve for survival of 0.774 (95% CI, 0.717–0.824;P<.001), which was greater than that of estimated glomerular filtration rate, proteinuria, FMD, CIMT,hsCRP, and PTX3. The discriminative power for predicting cardiovascular events was also significantly higher for endocan (area under the corresponding receiver operating characteristic curve, 0.851; 95% CI, 0.800–0.892; P<.001) than for all of the already mentioned variables.13
Possible Mechanisms of Action
Why does endocan appear to be related to worse outcomes and what are its deleterious actions in vivo?These are relevant questions but unfortunately the answers are not completely known, although some insights emerged from previous studies. These include:
Upregulation of cell adhesion monecules, such as ICAM‐1, LFA‐1, VCAM‐1, and E‐selectin, helping leukocyte extravasation.12, 16, 17, 18
Inhibition of the interaction between ICAM‐1 and LFA‐1 on leukocytes.
Endothelial cell activation by induction of molecules such as resistin.19
Close relationship with systemic inflammatory markers such as CRP and PTX3.13
Endothelial cytoskeleton rearrangement and increased cytoskeleton‐associated protein secretion such as zonula occludens leading to cellular contraction.17
How Does Endocan Affect Prognosis in CKD and HTN?
All above‐mentioned mechanisms regarding endocan may play a role in different stages; however, growing experimental evidence suggests that inflammation plays a major role in HTN. Along these lines, it was demonstrated that lymphocyte infiltration occurs in experimental models of HTN.20 At the same time, perivascular lymphocyte infiltration impairs vasodilatation and increases contractility.21 Besides, in angiotensin II‐dependent HTN, lymphocyte deficiency facilitates natriuresis likely via stimulation of endothelial nitric oxide synthase‐ and cyclooxygenase‐2–dependent pathways.22
Thus, endocan may affect these pathways and lead to HTN and chronic kidney disease. The hypothetical effects of endocan on the development of HTN, CKD, and cardiovascular disease are shown in the Figure.
Figure 1.
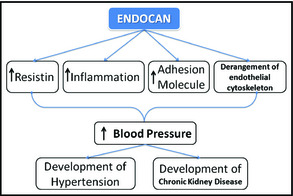
The hypothetical effects of endocan on the development of hypertension, chronic kidney disease, and cardiovascular disease.
Future Perspectives
The specific relationship between endocan and end organ damage such as cardiac hypertrophy, renal damage, and stroke must be determined. In addition, the role of endocan in different diseases such as in dialysis patients and in patients with diabetes must be studied. The specific relationship between endocan and heart disease should be more intensively studied. For example, a recent study showed that endocan may be related to severity of coronary artery disease.23 Kinetics of endocan should be studied in different disease stages such as sepsis and diabetes, as opposed to single‐measurement studies. The role of endocan in the renin angiotensin system, sympathetic system, and oxidative stress and the treatment with anti‐endocan antibodies to possibly improve HTN, endothelial dysfunction, and transplant rejection must be determined. For example, experimental evidence suggests that treatment with anti‐endocan antibody improved survival in patients with sepsis.18
Conclusions
Endocan—an emerging molecule secreted by endothelial cells—seems to have many roles in pathologic processes. It has a prognostic implication in various pathologic conditions such as cancer, sepsis, CKD, renal transplant rejection, and HTN. Various pathophysiologic processes that result in endothelial dysfunction attributable to endocan likely play a role in these outcomes. Further studies are needed to highlight whether endocan is only a marker of a negative prognosis in these conditions or whether it has an active role.
Disclosures
The authors declare that they have no conflicts of interest.
Financial Disclosure
None.
J Clin Hypertens (Greenwich). 2014;16:914–916. DOI: 10.1111/jch.12440. © 2014 Wiley Periodicals, Inc.
References
- 1. Lassalle P, Molet S, Janin A, et al. ESM‐1 is a novel human endothelial cell‐specific molecule expressed in lung and regulated by cytokines. J Biol Chem. 1996;271:20458–20464. [DOI] [PubMed] [Google Scholar]
- 2. Béchard D, Gentina T, Delehedde M, et al. Endocan is a novel chondroitin sulfate/dermatan sulfate proteoglycan that promotes hepatocyte growth factor/scatter factor mitogenic activity. J Biol Chem. 2001;276:48341–48349. [DOI] [PubMed] [Google Scholar]
- 3. Zhang SM, Zuo L, Zhou Q, et al. Expression and distribution of endocan in human tissues. Biotech Histochem. 2012;87:172–178. [DOI] [PubMed] [Google Scholar]
- 4. Delehedde M, Devenyns L, Maurage CA, Vivès RR. Endocan in cancers: a lesson from a circulating dermatan sulfate proteoglycan. IntJCell Biol. 2013;2013:705027 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balta I, Balta S, Koryurek OM, et al. Serum endocan levels as a marker of disease activity in patients with Behçet disease. J Am Acad Dermatol. 2014;70:291–296. [DOI] [PubMed] [Google Scholar]
- 6. Balta I, Balta S, Demirkol S, et al. Elevated serum levels of endocan in patients with psoriasis vulgaris: correlations with cardiovascular risk and activity of disease. Br J Dermatol. 2013;169:1066–1070. [DOI] [PubMed] [Google Scholar]
- 7. Scherpereel A1, Depontieu F, Grigoriu B, et al. Endocan, a new endothelial marker in human sepsis. Crit Care Med. 2006;34:532–537. [DOI] [PubMed] [Google Scholar]
- 8. Grigoriu BD Depontieu F Scherpereel A, et al. Endocan expression and relationship with survival in human non–small cell lung cancer. Clin Cancer Res. 2006;12:4575–4582. [DOI] [PubMed] [Google Scholar]
- 9. Maurage CA Adam E Minéo JF, et al. Endocan expression and localization in human glioblastomas. J Neuropathol Exp Neurol. 2009;68:633–641. [DOI] [PubMed] [Google Scholar]
- 10. Kim JH, Park MY, Kim CN, et al. Expression of endothelial cell‐specific molecule‐1 regulated by hypoxia inducible factor‐1α in human colon carcinoma: impact of ESM‐1 on prognosis and its correlation with clinicopathological features. Oncol Rep. 2012;28:1701–1708. [DOI] [PubMed] [Google Scholar]
- 11. Balta S, Mikhailidis DP, Demirkol S, et al. Endocan—a novel inflammatory indicator in newly diagnosed patients with hypertension: a pilot study. Angiology. 2014;65:773–777. [DOI] [PubMed] [Google Scholar]
- 12. Su YH, Shu KH, Hu CP, et al. Serum endocan correlated with stage of chronic kidney disease and deterioration in renal transplant recipients. Transplant Proc. 2014;46:323–327. [DOI] [PubMed] [Google Scholar]
- 13. Yilmaz MI, Siriopol D, Saglam M, et al. Plasma endocan levels associate with inflammation, vascular abnormalities, cardiovascular events, and survival in chronic kidney disease. Kidney Int. 2014. July 2 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Tadzic R, Mihalj M, Vcev A, et al. The effects of arterial blood pressure reduction on endocan and soluble endothelial cell adhesion molecules (CAMs) and CAMs ligands expression in hypertensive patients on Ca‐channel blocker therapy. Kidney Blood Press Res. 2013;37:103–115. [DOI] [PubMed] [Google Scholar]
- 15. Li S, Wang L, Wang C, et al. Detection on dynamic changes of endothelial cell specific molecule–1 in acute rejection after renal transplantation. Urology. 2012;80:738‐e1. [DOI] [PubMed] [Google Scholar]
- 16. Béchard D, Scherpereel A, Hammad H, et al. Human endothelial‐cell specific molecule‐1 binds directly to the integrin CD11a/CD18 (LFA‐1) and blocks binding to intercellular adhesion molecule‐1. J Immunol. 2001;167:3099–3106. [DOI] [PubMed] [Google Scholar]
- 17. Abid MR, Yi X, Yano K, Shih SC, Aird WC. Vascular endocan is preferentially expressed in tumor endothelium. Microvasc Res. 2006;72:136–145. [DOI] [PubMed] [Google Scholar]
- 18. Lee W, Ku SK, Kim SW, Bae JS. Endocan elicits severe vascular inflammatory responses in vitro and in vivo. J Cell Physiol. 2014;229:620–630. [DOI] [PubMed] [Google Scholar]
- 19. Oltean S, Pullerits R, Flodén A, Olausson M, Oltean M. Increased resistin in brain dead organ donors is associated with delayed graft function after kidney transplantation. J Transl Med. 2013;11:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi P, Diez‐Freire C, Jun JY, et al. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madhur MS, Lob HE, McCann LA, et al. Interleukin 17 promotes angiotensin II–induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II‐dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1089–R1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kose M, Emet S, Akpinar TS, et al. Serum endocan level and the severity of coronary artery disease: a pilot study. Angiology. Aug 28, 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


