Abstract
The association between central pulsatile hemodynamic load, arterial stiffness, and orthostatic hypotension (OH) is unclear. The authors recruited 1099 participants from the community. Questionnaire, physical examination, and laboratory tests were performed. To assess the correlation between central pulsatile hemodynamic load, arterial stiffness, and OH, multiple logistic regression analysis was performed, and the discriminatory power was assessed by the area under the receiver operating curve. The prevalence of OH in this population was 5.6%. After adjusting for potential confounders, brachial‐ankle pulse wave velocity (BaPWV) was significantly and positively correlated with OH in both the hypertension and nonhypertension groups (all P<.05), while central systolic blood pressure (CSBP) was only significantly associated with OH in the hypertension subgroup. In addition, BaPWV seemed to have a better discriminatory power than CSBP in both subgroups. BaPWV appears to be a better indicator of OH than CSBP in routine clinical practice.
Orthostatic hypotension (OH) is defined as a fall in systolic blood pressure (SBP) of 20 mm Hg or more or in diastolic blood pressure (DBP) of at least 10 mm Hg measured within 3 minutes of standing.1 This results from a failure of neural and circulatory mechanisms to compensate for the reduction in venous return during the upright posture. Evidence from cross‐sectional and longitudinal epidemiological studies has confirmed OH is an independent risk factor for cardiovascular (CV) morbidity.2, 3 It is a frequent finding in the elderly, with a prevalence rate of 15% to 25% in that population.4 More important, asymptomatic OH is a far more common condition that is often unrecognized. Therefore, identifying underlying risk factors for OH is crucial for its prevention and management.
As we know, the orthostatic regulatory system compensates via the baroreceptors in the carotid artery, aorta, and cardiopulmonary region. Baroreceptors respond to the drop in blood pressure (BP) and induce cardiac changes as part of the sympathetic reflex to preserve a constant level of arterial pressure and maintain cerebral perfusion against the force of gravity.5 Numerous studies have found age‐associated reductions in baroreflex function associated with the occurrence of OH.6 Arterial stiffening is suggested to be a potential mechanism, as the arterial stretch over segments with the baroreceptors is a key determinant in baroreflex activation.6, 7, 8 The reduction in cardiac output is also a concern, especially in the elderly. The aged heart is stiff and noncompliant, resulting in impaired diastolic filling, which reduces stroke volume, particularly when faced with increased cardiac afterload.4 To our knowledge, central BPs represent the true load imposed to the left ventricular (LV) and central large artery walls.9 This implies that direct measures of central pulsatile hemodynamic load (eg, central SBP [CSBP] and augmentation index [AI]) and arterial stiffness (pulse wave velocity [PWV]) may be associated with OH. Because the heart and brain are exposed to central pressure, it seems that measures of central pulsatile hemodynamic load may represent a better indicator of OH compared with PWV.10 However, few studies in the literature have compared the potential value of identifying OH between arterial stiffness and central pulsatile hemodynamic load. Therefore, the goals of the present study were to evaluate the relationship between OH and indexes of arterial stiffness and central pulsatile hemodynamic load. Furthermore, this study aimed to compare the discriminatory power for identifying OH among CSBP, AI, and PWV.
Patients and Methods
Study Population
A cross‐sectional study was conducted in patients from the Jinyang Community Health Center in Chengdu, China. All patients were recruited from consecutive participants attending the health checkup in the community between September 2011 and May 2012. Participants with peripheral vascular diseases, atrial fibrillation, or incomplete demographic or laboratory data were excluded from the analysis. Initially, a total of 1099 participants were included. Informed consent was obtained from each participant. The medical ethics committee of West China Hospital affiliated with Sichuan University approved all of the procedures.
Data Collection
Standardized questionnaire, physical examination, and laboratory tests were obtained in this survey. Specially trained doctors and nurses performed all data collections. A face‐to‐face interview was conducted to collect demographic (eg, age and sex) and clinical data (eg, smoking status, alcohol consumption level, exercise habits, history of diabetes mellitus, history of hypertension, and use of antihypertensive drugs) by self‐reporting and standardized questionnaires. As in our previous study, physical examinations involved assessments of height, weight, and waist circumference (WC).11, 12 Body mass index (BMI) was calculated using the following formula: weight/height2 (kg/m2). Fasting plasma glucose (FPG), fasting serum total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), high‐density lipoprotein cholesterol (HDL‐C), triglycerides (TGs), serum uric acid, and serum creatinine were included. Blood was drawn from the antecubital vein in the morning after 12‐hour fasting and subsequently analyzed using an automatic biochemical analyzer.
Baseline and Orthostatic Tests of BP and HR
BP and heart rate (HR) were measured in the right arm with patients in a sitting position using a calibrated electronic BP monitor (HEM‐7200, Omron Healthcare Co, Ltd, Kyoto, Japan) by a trained nurse or physician. The cuff widths were adapted to fit each participant's mid‐arm circumference. Two readings were recorded in the sitting position at least 1 minute apart after resting for more than 5 minutes. Following the brachial‐ankle PWV (BaPWV) test and an additional 10 minutes of rest, BP and HR measurements were again recorded with each participant in the supine position. Participants subsequently stood with their forearms relaxed and supported at the level of the heart, and BP and HR measurements were repeated at 30 seconds and 2 minutes after standing. OH was defined as a decline in SBP of at least 20 mm Hg and/or a decline in DBP of at least 10 mm Hg after either 0 or 2 minutes from a supine to an upright posture.1, 13, 14 All sitting and supine BP and HR measurements were performed twice, and the average of these parameters was used for analysis.
Arterial Stiffness Measurements
Although carotid‐femoral PWV is one of the most reliable methods for measuring arterial elasticity, BaPWV has been used as a simple, convenient, and automatic method for measuring PWV and is considered useful in screening for arterial stiffness in primary care settings and large populations.15, 16, 17
BaPWV was measured by an automatic device (VP1000, ColinCo, Ltd, Komaki, Japan) with an appropriately sized cuff. For at least 5 minutes before the test, each patient rested in a supine position in a room at 25°C. Participants were instructed to refrain from consuming food, tea, caffeine, or smoking for 3 hours before their measurements and also to refrain from consuming alcohol for 24 hours before their measurements. The device simultaneously recorded PWV, BP, electrocardiogram records, and heart sounds. Detailed information on BaPWV measurement has been reported elsewhere.13
rAI and CSBP Measurement
Radial AI (rAI), a simple and quick surrogate of central AI reflecting the contribution of reflexive waves to pulse pressure (PP), is another index of arterial stiffness.18, 19 For at least 10 minutes of rest, each patient was placed in a sitting position, and the rAI was estimated using an automated device (HEM‐9000AI; Omron Healthcare Co, Ltd, Kyoto, Japan) fixed to the left wrist of each participant. Because rAI is affected by HR, its value was adjusted corresponding to an HR of 75 beats per minute (rAIP75). CSBP was also estimated using the same device. Information on rAI and CSBP measurement has been reported elsewhere.13, 20
Related Definitions
Hypertension was defined as having SBP of at least 140 mm Hg and/or DBP of at least 90 mm Hg and/or currently taking antihypertensive medications. Diabetes mellitus was defined as one of the following at follow‐up assessment: (1) FPG ≥7.0 mmol/L, (2) a positive response to the question, “Has a doctor ever told you that you have diabetes?,” or (3) current use of insulin or oral hypoglycemic agents. Smoking was defined as average cigarette consumption of at least 1 per day. Alcohol intake was defined as average intake of alcohol of at least 50 g/d. Physical activity was defined as exercise three or more times per week for at least 30 minutes each time.
Statistical Analyses
Continuous variables were expressed as mean±standard deviation (SD). Differences of baseline characteristics between participants with and without OH were tested by independent t test for normally distributed variables and by the nonparametric Mann‐Whitney or Wilcoxon test for skewed variables. Categorical variables are expressed as frequencies and percentages. Differences between participants with and without OH were tested by chi‐square test. To determine BaPWV, rAIP75, CSBP, and PP in the sitting position associated with OH, a multiple logistic regression analysis was performed to determine odds ratios (ORs) and 95% confidence intervals (CIs) in different regression models and the discriminatory power of these measurements for OH was assessed by the area under the receiver operating characteristic (ROC) curve. Covariates including age, WC, BMI, HR in the sitting position, FPG, TGs, TC, HDL‐C, LDL‐C, creatinine, uric acid, BaPWV, rAIP75, CSBP, and PP in the sitting position were used as continuous variables. Sex, alcohol intake, smoking, regular physical exercise, diabetes, hypertension, and antihypertensive drug use were used as categorical variables. BaPWV, rAIP75, CSBP, and PP in the sitting position were not simultaneously included in regression analysis to avoid any colinearity that these independent variables may have. The point representing the largest sum of sensitivity and specificity on the ROC curve was calculated. The difference between area ROC curves was assessed using the algorithm developed by the DeLong nonparametric approach. BaPWV was split into four quartiles (Q1: BaPWV ≤14.47 m/s; Q2: 14.47 <BaPWV≤16.65 m/s; Q3: 16.65 <BaPWV≤19.40 m/s; and Q4: BaPWV ≥19.40 m/s). A comparison of orthostatic HR changes at 30 seconds and 2 minutes were conducted across the four BaPWV quartiles. The mean of both orthostatic HR changes was chosen for data analysis. The least significant difference test for pairwise comparisons was used when needed. SPSS 19.0 (IBM, Armonk, NY) and MedCalc 11.0 (MedCalc Software, Ostend, Belgium) software were used. Statistical significance was defined as P<.05.
Results
Basic Characteristics of Patients
Overall, our sample had a mean age of 64.8±7.7 years (n=1099): 41.9% of the participants were male (460 patients), 39.6% had hypertension (438 patients), and 13.0% had diabetes mellitus (143 patients). Of the 1099 participants enrolled in this study, OH was detected in 61 (5.6%). Table 1 shows characteristics of participants at baseline. Compared with those without OH, patients with OH had a higher age, higher SBP and PP values in the sitting position, and higher BP in the supine position, BaPWV, and CSBP (all P<.05, Table 1).
Table 1.
Baseline Characteristics Between Patients With and Without OH
| Variables | Patients Without OH (n=1038) | Patients With OH (n=61) | P Value |
|---|---|---|---|
| Age, y | 64.4±7.8 | 68.4±7.5 | <.001 |
| Male, No. (%) | 431 (41.8) | 29 (47.5) | .354 |
| Smoking, No. (%) | 82 (7.9) | 8 (13.1) | .149 |
| Drinking, No. (%) | 121 (11.7) | 6 (9.8) | .665 |
| Physically active, No. (%) | 651 (62.7) | 39 (63.9) | .848 |
| Hypertension, No. (%) | 411 (39.6) | 27 (44.3) | .469 |
| Diabetes mellitus, No. (%) | 134 (12.9) | 9 (14.8) | .677 |
| Sitting | |||
| SBP, mm Hg | 132.6±18.0 | 149.5±18.6 | .004 |
| DBP, mm Hg | 71.7±10.1 | 71.2±9.9 | .748 |
| PP, mm Hg | 61.0±14.4 | 68.3±18.0 | <.001 |
| HR, beats per min | 75.2±10.3 | 74.5±12.0 | .598 |
| Supine | |||
| SBP, mm Hg | 129.9±16.8 | 142.6±19.5 | <.001 |
| DBP, mm Hg | 71.0±9.6 | 76.6±10.7 | <.001 |
| HR, beats per min | 71.7±9.8 | 72.4±12.6 | .644 |
| Standing | |||
| SBP, mm Hg | 135.0±18.4 | 132.6±22.4 | .417 |
| DBP, mm Hg | 77.1±10.0 | 72.5±11.1 | <.001 |
| HR, beats per min | 78.0±10.6 | 79.5±12.2 | .278 |
| Blood pressure and HR changes after 30‐s postural changes | |||
| SBP, mm Hg | 2.7±9.9 | −14.1±15.0 | <.001 |
| DBP, mm Hg | 4.7±5.8 | −7.4±7.9 | <.001 |
| HR, beats per min | 9.2±6.5 | 8.4±5.8 | .344 |
| Blood pressure and HR changes after 2‐min postural changes | |||
| SBP, mm Hg | 5.1±9.3 | −10.3±13.6 | <.001 |
| DBP, mm Hg | 6.1±5.6 | −3.9±9.0 | <.001 |
| HR, beats per min | 6.4±6.2 | 7.4±5.5 | .204 |
| Drugs | |||
| Antihypertensive drugs used, No. (%) | 282 (27.2) | 15 (24.6) | .66 |
| CCB, No. | 155 | 7 | .459 |
| β‐Blocker, No. | 47 | 3 | .887 |
| α‐Blocker, No. | 1 | 0 | 1 |
| RAS blocker, No. | 69 | 4 | .978 |
| Diuretics, No. | 36 | 4 | .211 |
| Other drugs, No. | 44 | 3 | .799 |
| WC, cm | 83.8±8.8 | 83.2±8.1 | .643 |
| BMI, kg/m2 | 23.93±2.93 | 23.23±2.73 | .07 |
| FPG, mmol/L | 6.0±1.6 | 6.3±1.6 | .134 |
| Creatinine, μmol/L | 92.6±33.0 | 95.8±13.8 | .519 |
| Uric acid, μmol/L | 356.5±96.7 | 358.1±84.0 | .913 |
| TG, mmol/L | 1.62±0.91 | 1.75±1.02 | .368 |
| TC, mmol/L | 5.18±3.62 | 5.25±1.58 | .904 |
| HDL‐C, mmol/L | 1.54±0.37 | 1.42±0.30 | .04 |
| LDL‐C, mmol/L | 2.50±0.70 | 2.63±0.97 | .235 |
| Central hemodynamic indexes | |||
| CSBP, mm Hg | 135.9±18.9 | 143.6±21.3 | .002 |
| BaPWV, m/s | 17.10±3.69 | 20.20±4.90 | <.001 |
| rAIP75, % | 84.09±10.93 | 85.48±9.94 | .37 |
Abbreviations: BaPWV, brachial‐ankle pulse wave velocity; BMI, body mass index; CCB, calcium channel blocker; CSBP, central systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; HR, heart rate; LDL‐C, low‐density lipoprotein cholesterol; PP, pulse pressure; rAIP75, radial augmentation index normalized to a heart rate of 75 beats per minute; RAS, renin‐angiotensin system; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
Multiple Logistic Regression Analysis in Different Models
The univariate logistic regression analysis presented that CSBP (OR, 1.020; 95% CI, 1.007–1.033; P=.003), BaPWV (OR, 1.180; 95% CI, 1.116–1.248; P<.001), and PP in the sitting position (OR, 1.031; 95% CI, 1.014–1.047; P<.001) were associated with OH except rAIP75 (OR, 1.012; 95% CI, 0.986–1.038; P=.37). In the multivariate logistic regression models, CSBP, BaPWV, and PP in the sitting position were still significantly associated with OH after adjustment for potential risk factors including age, WC, BMI, HR in the sitting position, sex, alcohol intake, smoking, regular physical exercise, diabetes, hypertension, and antihypertensive drug use. After further adjustment for FPG, TGs, TC, HDL‐C, LDL‐C, creatinine, and uric acid, the associations remained significant (Table 2). While the sitting brachial SBP was higher in patients with OH than in those without OH, the frequency of antihypertensive drug treatment was nearly the same between patients with OH and those without OH, which meant that hypertension was better controlled in non‐OH than in OH patients. In order to reduce the effect of higher BP on the results, the population was divided into two subgroups: hypertension group and nonhypertension group. The results revealed that CSBP was insignificantly associated with OH after adjustment for potential risk factors in the nonhypertension group; however, BaPWV was still significantly associated with OH in both groups (Table 3).
Table 2.
Univariate and Multivariate Logistic Regression Models for Prediction of OH in Different Models
| Variable | Univariate Regression | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| CSBP, mm Hg | 1.020 (1.007–1.033) | .003 | 1.023 (1.006–1.040) | .007 | 1.039 (1.016–1.062) | .001 |
| BaPWV, m/s | 1.180 (1.116–1.248) | <.001 | 1.203 (1.110–1.304) | <.001 | 1.259 (1.130–1.402) | <.001 |
| rAIP75, % | 1.012 (0.986–1.038) | .37 | 1.006 (0.975–1.038) | .695 | 1.020 (0.797–1.063) | .347 |
| PP, mm Hg | 1.031 (1.014–1.047) | <.001 | 1.025 (1.004–1.047) | .017 | 1.033 (1.007–1.060) | .013 |
Abbreviations: BaPWV, brachial‐ankle pulse wave velocity; CI, confidence interval; CSBP, central systolic blood pressure; OH, orthostatic hypotension; OR, odds ratio; PP, pulse pressure in the sitting position; rAIP75, radial augmentation index normalized to a heart rate of 75 beats per minute. Model 1: adjusted for age, waist circumference, body mass index, heart rate in the sitting position, sex, alcohol intake, smoking, regular physical exercise, diabetes, hypertension, and antihypertensive drug use. Model 2: Model 1 + fasting plasma glucose, triglycerides, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, creatinine, and uric acid.
Table 3.
Univariate and Multivariate Logistic Regression Models for Prediction of OH in Different Models in Two Subgroups
| Variable | Univariate Regression | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Nonhypertensive group (n=661; 34 patients with OH) | ||||||
| CSBP, mm Hg | 1.019 (0.999–1.038) | .061 | 1.015 (0.992–1.039) | .205 | 1.017 (0.985–1.050) | .310 |
| BaPWV, m/s | 1.211 (1.111–1.320) | <.001 | 1.1881 (1.056–1.336) | .004 | 1.218 (1.0348–1.434) | .018 |
| rAIP75, % | 1.010 (0.978–1.043) | .543 | 1.001 (0.962–1.0462 | .951 | 1.024 (0.972–1.080) | .372 |
| PP, mm Hg | 1.040 (1.009–1.058) | .006 | 1.029 (1.001–1.059) | .043 | 1.027 (0.989–1.065) | .165 |
| Hypertensive group (n=438; 27 patients with OH) | ||||||
| CSBP, mm Hg | 1.025 (1.004–1.047) | .019 | 1.030 (1.003–1.057) | .028 | 1.083 (1.028–1.142) | .003 |
| BaPWV, m/s | 1.179 (1.087–1.278) | <.001 | 1.232 (1.083–1.400) | .001 | 1.455 (1.166–1.816) | .001 |
| rAIP75, % | 1.015 (0.973–1.059) | .493 | 1.015 (0.955–1.079) | .632 | 1.044 (0.945–1.153) | .395 |
| PP, mm Hg | 1.029 (1.005–1.055) | .019 | 1.014 (0.981–1.047) | .142 | 1.040 (0.992–1.091) | .107 |
Abbreviations: BaPWV, brachial‐ankle pulse wave velocity; CI, confidence interval; CSBP, central systolic blood pressure; OH, orthostatic hypotension; OR, odds ratio; PP, pulse pressure in sitting position; rAIP75, radial augmentation index normalized to a heart rate of 75 beats per minute. Model 1: adjusted for age, waist circumference, body mass index, heart rate in the sitting position, sex, alcohol intake, smoking, regular physical exercise, diabetes, hypertension, and antihypertensive drug use. Model 2: Model 1 + fasting plasma glucose, triglycerides, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, creatinine, and uric acid.
ROC Curve Analyses
The area under the ROC curves in the nonhypertens‐ion group were 0.625 (95% CI, 0.532–0.719; P=.018) for CSBP, 0.752 (95% CI, 0.668–0.836; P<.001) for BaPWV, 0.536 (95% CI, 0.431–0.641; P=.496) for rAIP75, and 0.671 (95% CI, 0.578–0.764; P=.001) for PP in the sitting position, respectively (Table 4). The area under the ROC curves for these four measurements in the hypertension group were displayed in Table 4. BaPWV seemed to be a better measurement in discriminating OH in both subgroups (Figure 1).
Table 4.
Area Under the ROC Curve for Various Measurements Used to Predict OH in Two Subgroups
| Variable | AUC (95% CI) | P Value | Cutoff | Sensitivity | Specificity | Δ AUC (95% CI)a | P Valuea |
|---|---|---|---|---|---|---|---|
| Nonhypertension group | |||||||
| BaPWV, m/s | 0.752 (0.668–0.836)b , c | <.001 | 18.58 | 0.559 | 0.797 | – | – |
| CSBP, mm Hg | 0.625 (0.532–0.719) | .018 | 121 | 0.853 | 0.351 | 0.127 (0.020–0.233) | .02 |
| PP, mm Hg | 0.671 (0.578–0.764) | .001 | 61 | 0.606 | 0.666 | 0.081 (−0.042 to 0.203) | .196 |
| rAIP75, % | 0.536 (0.431–0.641) | .496 | 88 | 0.452 | 0.682 | 0.216 (0.111–0.320) | <.001 |
| Hypertension group | |||||||
| BaPWV, m/s | 0.740 (0.644–0.837)c | <.001 | 18.83 | 0.778 | 0.591 | – | – |
| CSBP, mm Hg | 0.622 (0.490–0.754) | .059 | 154 | 0.556 | 0.737 | 0.118 (−0.030 to 0.267) | .117 |
| PP, mm Hg | 0.629 (0.498–0.760) | .046 | 77.5 | 0.482 | 0.771 | 0.112 (−0.030 to 0.253) | .123 |
| rAIP75, % | 0.539 (0.420–0.657) | .55 | 74 | 0.952 | 0.152 | 0.202 (0.061–0.343) | .005 |
Abbreviations: Δ AUC, difference between area under the curve values; CI, confidence interval; OH, orthostatic hypotension; PP, pulse pressure; ROC, receiver operating characteristic. aCompared with brachial‐ankle pulse wave velocity (BaPWV). bCompared with central systolic blood pressure (CSBP) (P<.05). cCompared with radial augmentation index normalized to a heart rate of 75 beats per minute (rAIP75) (P<.05).
Figure 1.
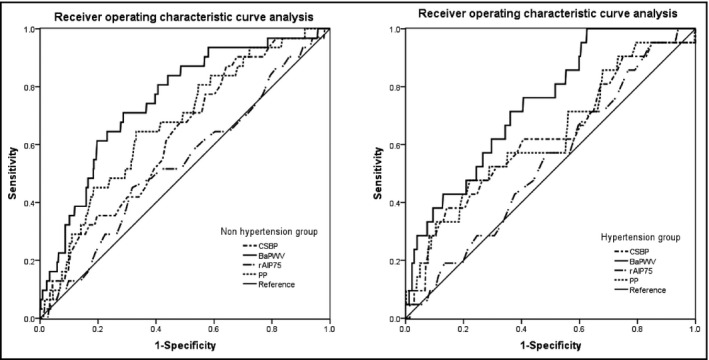
Receiver operating characteristic curve analysis of central systolic blood pressure (CSBP), brachial‐ankle pulse wave velocity (BaPWV), and pulse pressure (PP) in the sitting position and radial augmentation index normalized to a heart rate of 75 beats per minute (rAIP75) for orthostatic hypotension occurrence.
BaPWV and Orthostatic HR Changes at 30 Seconds and 2 Minutes
Figure 2 shows the results of 277 participants whose BaPWV values were in Q1. This result corresponded to a mean orthostatic HR change of 10.05 (95% CI, 9.23–10.87) at 30 seconds. Moreover, 276 and 272 patients who shared a BaPWV value within Q2 and Q3 demonstrated corresponding HR changes of 9.36 (95% CI, 8.58–10.15) and 8.72 (95% CI, 7.94–9.50) after 30 seconds, respectively. A total of 274 participants with CSBP in Q4 demonstrated an HR change of 8.46 (95% CI, 7.72–9.20). Orthostatic HR changes at 2 minutes were 7.95 (7.14–8.76) for Q1, 6.79 (6.02–7.56) for Q2, 5.45 (4.80–6.10) for Q3, and 5.59 (4.93–6.25) for Q4.
Figure 2.
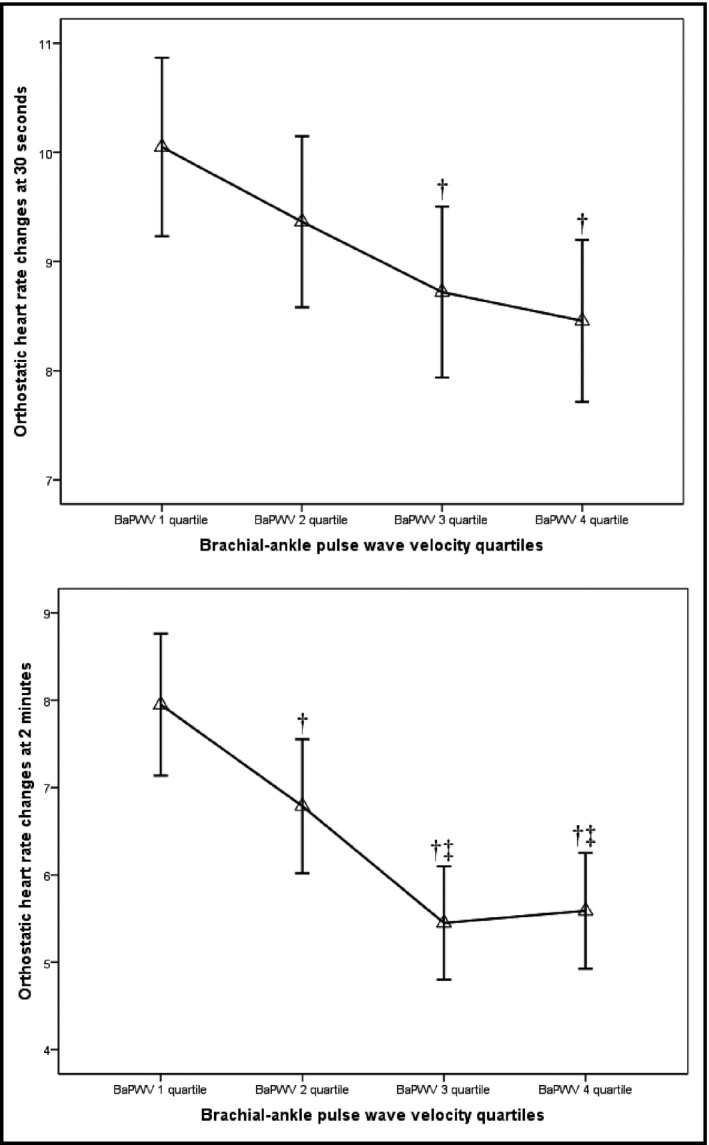
Mean orthostatic heart rate change according to brachial‐ankle pulse wave velocity (BaPWV) quartiles. The triangles represent the mean levels of orthostatic heart rate change and the lines represent the corresponding 95% confidence intervals. †P<.05 vs quartile 1. ‡P<.05 vs quartile 2.
Discussion
The goal of the present study was to assess the relationship between OH and indexes of central pulsatile hemodynamic load and arterial stiffness. In addition, it aimed to compare the potential value for identifying OH among CSBP, rAI, PP in the sitting position, and BaPWV. After adjusting for potential confounders, our findings show that BaPWV is significantly and positively correlated with the probability of OH in a community population. Moreover, increases in BaPWV predict a decreased degree of elevation in orthostatic HR. In addition, BaPWV seems to have a better discriminatory power than the other three measurements.
Central BPs are pathophysiologically more relevant than peripheral pressures to the pathogenesis of CV disease, since they represent the true load imposed on the LV and central large artery walls.21 Our results show that CSBP increased the risk of OH in community residents. However, when we divided the population into two subgroups based on residents with hypertension in order to reduce the effect of higher BP on the results, CSBP was only significantly associated with OH in the hypertension group (OR, 1.083; 95% CI, 1.028–1.142; P=.003). Community residents with hypertension had higher SBP values in the sitting position, age, and BaPWV (Table S1). It is known that these factors increase CSBP. Because the heart is coupled with the vasculature, the age‐associated increase in arterial stiffness has critically important effects on cardiac structure and function in the elderly.22 The increased stiffening increases PWV, which results in earlier return of reflected waves from the periphery to the proximal aorta. These returning waves summate with anterograde waves to produce peak systolic pressure (central BP).23 The increased CSBP creates an additional load against which the older heart must increase LV wall thickness and prolonged contractile activation to normalize stroke volume.24, 25, 26 However, this progress reduces endocardial flow and results in diastolic dysfunction and further reduced ventricular filling.27 Although our patient did not have indices of diastolic dysfunction, some studies may provide evidence. First, patients with OH have a higher risk of heart failure with preserved ejection fraction compared with patients without OH (HR, 1.32; 95% CI, 1.07–1.48; P=.033).28 Second, Cwynar and colleagues29 found that hypertension patients with diastolic LV dysfunction have near‐significantly higher values of CSBP than patients without diastolic LV dysfunction (135.1±20.3 mm Hg vs 127.3±19.7 mm Hg, P=.049). Third, Borlaug and colleagues30 showed that LV diastolic and systolic tissue velocities vary inversely with arterial afterload, with late systolic load having the greatest influence on early diastolic velocity. Therefore, orthostatic hypotension has been shown to be associated with increased CSBP in older hypertension adults, which is likely the result of shared pathophysiology.31
We also found that CSBP had a lower discriminatory power than BaPWV in both subgroups. Similarly, a recent meta‐analysis showed that the additively predictive value of central BP compared with brachial BP was not statistically significant in most studies.32 The Framingham Heart Study reported that aortic stiffness emerged as the predominant hemodynamic predictor of major CV events and significantly improved risk discrimination and reclassification, rather than central BP.33 It may be due to errors caused by the method of central BP estimations.34 In addition, determinants of BaPWV and CSBP are different. CSBP is dependent on many factors such as the speed of wave travel, the reflectance point, and the duration and pattern of ventricular ejection. Whereas PWV, which is the speed of wave travel, represents intrinsically arterial stiffness. Antihypertensive drugs may change CSBP without changing PWV over the short term.34, 35 This might show that BaPWV is more stable than CSBP in discriminatory power. More importantly, the results support the hypothesis that impaired baroreflex sensitivity is the mechanism that links arterial stiffness to OH.36 Rather than detecting BP directly, baroreceptors actually determine it from the tension and relaxation of the arterial wall, which are caused by pressure alterations. When the barosensitive region stiffens, the compliance of this vessel segment decreased, leading to restrictions in both stretch and relaxation.37, 38 Therefore, baroreceptor sensitivity gradually decreases. A recent study revealed that older women have greater reliance on vascular conductance to modulate mean arterial pressure via carotid baroreflex during hypotensive stimuli.39 This mechanism also explains why a decreased degree of elevation in orthostatic HR correlates with raised BaPWV. Meanwhile, AI, influenced by many factors, is only an indirect, surrogate measure of arterial stiffness.34, 40 This might not be sufficient to establish an independent association between AI and OH.
Because PP, as an indirect indicator of arterial stiffness, could not accurately and timely reflect development in stiffening of large arteries, PP in the sitting position is not better than BaPWV as an indicator of orthostatic hypotension. This is similar to our previous studies.12 We believe the relatively small sample size of OH is another reason the PP was insignificantly correlated with the probability of OH after the division into two subgroups.
Study Limitations
Limitations of this study should be acknowledged. First, although orthostatic hypotension has been shown to be associated with vascular stiffness in older adults, this cross‐sectional observation was likely the result of shared pathophysiology and may not imply causal associations. Second, this study had a relatively small sample size of patients with OH. This limited the study to analyzing the association by sex. However, the prevalence in our investigation was close to the Irish Longitudinal Study in the same age group (6.4% in 60–69 years).41 This increased the credibility of our results. Third, during the investigation, taking the feasibility into account, we used automatic devices to measure orthostatic BP, but these devices all met the Association for the Advancement of Medical Instrumentation standards.42 Therefore, we believe that the results are reliable. Fourth, the definition for OH used for this project was a drop in SBP/DBP after 2 minutes of standing instead of 3 minutes of standing. Lastly, because the average age of our sample was 64.8±7.7 years, we cannot extend our findings to the general population.
Conclusions
BaPWV appeared to be a better indicator of OH than CSBP in routine clinical practice. Future studies may be needed to assess whether this phenomenon exists in a larger population.
Supporting information
Table S1. baseline characteristics between patients with and without hypertension.
Acknowledgments
The authors thank Dr Xiao's team at Jinyang Community Health Center in Chengdu, China, for their contributions to this study.
Funding
This study was supported by a project from the Science & Technology Pillar Program in Sichuan Province, China (grant number 2012SZ0131).
Conflicts of Interest
The authors have no conflicts of interest to declare.
J Clin Hypertens (Greenwich). 2016;18:655–662. DOI: 10.1111/jch.12726 © 2015 Wiley Periodicals, Inc.
Kai Liu and Si Wang contributed equally to this article.
References
- 1. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. [DOI] [PubMed] [Google Scholar]
- 2. Fedorowski A, Stavenow L, Hedblad B, et al. Orthostatic hypotension predicts all‐cause mortality and coronary events in middle‐aged individuals (The Malmo Preventive Project). Eur Heart J. 2010;31:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benvenuto LJ, Krakoff LR. Morbidity and mortality of orthostatic hypotension: implications for management of cardiovascular disease. Am J Hypertens. 2011;24:135–144. [DOI] [PubMed] [Google Scholar]
- 4. Arnold AC, Shibao C. Current concepts in orthostatic hypotension management. CurrHypertens Rep. 2013;15:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng YC, Vyas A, Hymen E, Perlmuter LC. Gender differences in orthostatic hypotension. Am J Med Sci. 2011;342:221–225. [DOI] [PubMed] [Google Scholar]
- 6. Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R3–R12. [DOI] [PubMed] [Google Scholar]
- 7. Angell James JE. The effects of changes of extramural, ‘intrathoracic,’ pressure on aortic arch baroreceptors. J Physiol. 1971;214:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avolio AP, Deng FQ, Li WQ, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71:202–210. [DOI] [PubMed] [Google Scholar]
- 9. Cohen DL, Townsend RR. Central blood pressure and chronic kidney disease progression. Int J Nephrol. 2011;2011:407801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agabiti‐Rosei E, Mancia G, Rourke MF, et al. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension. 2007;50:154–160. [DOI] [PubMed] [Google Scholar]
- 11. Meng Q, Wang S, Wang Y, et al. Arterial stiffness is a potential mechanism and promising indicator of orthostatic hypotension in the general population. Vasa. 2014;43:423–432. [DOI] [PubMed] [Google Scholar]
- 12. Liu K, Wang Y, He J, et al. Is pulse pressure a predictor of diabetes in Chinese Han nationality population? 15‐year prospective study in Chengdu community. Int J Cardiol. 2014;176:529–532. [DOI] [PubMed] [Google Scholar]
- 13. Luo F, Wang Y, Wang X, et al. A functional variant of NEDD4L is associated with hypertension, antihypertensive response, and orthostatic hypotension. Hypertension. 2009;54:796–801. [DOI] [PubMed] [Google Scholar]
- 14. Gao Y, Lin Y, Sun K, et al. Orthostatic blood pressure dysregulation and polymorphisms of β‐adrenergic receptor genes in hypertensive patients. J Clin Hypertens (Greenwich). 2014;16:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tillin T, Chambers J, Malik I, et al. Measurement of pulse wave velocity: site matters. J Hypertens. 2007;25:383–389. [DOI] [PubMed] [Google Scholar]
- 16. Yamashina A, Tomiyama H, Arai T, et al. Brachial‐ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26:615–622. [DOI] [PubMed] [Google Scholar]
- 17. Tsuchikura S, Shoji T, Kimoto E, et al. Brachial‐ankle pulse wave velocity as an index of central arterial stiffness. J Atheroscler Thromb. 2010;17:658–665. [DOI] [PubMed] [Google Scholar]
- 18. Sugawara J, Komine H, Hayashi K, et al. Relationship between augmentation index obtained from carotid and radial artery pressure waveforms. J Hypertens. 2007;25:375–381. [DOI] [PubMed] [Google Scholar]
- 19. Kohara K, Tabara Y, Oshiumi A, et al. Radial augmentation index: a useful and easily obtainable parameter for vascular aging. Am J Hypertens. 2005;18:11S–14S. [DOI] [PubMed] [Google Scholar]
- 20. Wohlfahrt P, Krajcoviechová A, Seidlerová J, et al. Comparison of noninvasive assessments of central blood pressure using general transfer function and late systolic shoulder of the radial pressure wave. Am J Hypertens. 2014;27:162–168. [DOI] [PubMed] [Google Scholar]
- 21. ESH, ESC Task Force for the Management of Arterial Hypertension . Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31:1925–1938. [DOI] [PubMed] [Google Scholar]
- 22. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly. J Am Coll Cardiol. 2011;57:2037–2114. [DOI] [PubMed] [Google Scholar]
- 23. Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. [DOI] [PubMed] [Google Scholar]
- 24. Ahmed S, Shapiro EP, O'Connor FE, Fleg JL. Effect of normative aging on mid wall left ventricular systolic performance. Am J Cardiol. 2001;88:1330–1334. [DOI] [PubMed] [Google Scholar]
- 25. Bryg RJ, Williams GA, Labovitz AJ. Effect of aging on left ventricular diastolic filling in normal subjects. Am J Cardiol. 1987;59:971–974. [DOI] [PubMed] [Google Scholar]
- 26. Benjamin EJ, Levy D, Anderson KM, et al. Determinants of Dopplerindexes of left ventricular diastolic function in normal subjects (the Framingham Heart Study). Am J Cardiol. 1992;70:508–515. [DOI] [PubMed] [Google Scholar]
- 27. Pepine CJ, Nichols WW. The pathophysiology of chronic ischemic heart disease. Clin Cardiol. 2007;30:I4–I9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alagiakrishnan K, Patel K, Desai RV, et al. Orthostatic hypotension and incident heart failure in community‐dwelling older adults. J Gerontol A BiolSci Med Sci. 2014;69:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cwynar M, Gasowski J, Stompór T, et al. Blood pressure and arterial stiffness in patients with high sodium intake in relation to sodium handling and left ventricular diastolic dysfunction status. J Hum Hypertens 2015;29:583–591. 10.1038/jhh.2015.1 [DOI] [PubMed] [Google Scholar]
- 30. Borlaug BA, Melenovsky V, Redfield MM, et al. Impact of arterial load and loading sequence on left ventricular tissuevelocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. [DOI] [PubMed] [Google Scholar]
- 31. Gottdiener JS, Yanez D, Rautaharju P, et al. Orthostatic hypotension in the elderly: contributions of impaired LV filling and altered sympathovagal balance. Am J Geriatr Cardiol. 2000;9:273–280. [DOI] [PubMed] [Google Scholar]
- 32. Vlachopoulos C, Aznaouridis K, O'Rourke MF, et al. Prediction of cardiovascular events and all‐cause mortality with central haemodynamics: a systematic review and meta‐analysis. Eur Heart J. 2010;31:1865–1871. [DOI] [PubMed] [Google Scholar]
- 33. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 35. Trudeau L. Central blood pressure as an index of antihypertensive control: determinants and potential value. Can J Cardiol. 2014;30(5 Suppl):S23–S28. [DOI] [PubMed] [Google Scholar]
- 36. Feldstein C, Weder AB. Orthostatic hypotension: a common, serious and underrecognized problem in hospitalized patients. J Am Soc Hypertens. 2012;6:27–39. [DOI] [PubMed] [Google Scholar]
- 37. Lenard Z, Studinger P, Kovats Z, et al. Comparison of aortic arch and carotid sinus distensibility in humans‐relation to baroreflex sensitivity. Auton Neurosci. 2001;92:92–99. [DOI] [PubMed] [Google Scholar]
- 38. Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age‐ and habitual exercise‐related differences in cardiovagal baroreflex sensitivity. Circulation. 2001;104:1627–1632. [DOI] [PubMed] [Google Scholar]
- 39. Credeur DP, Holwerda SW, Boyle LJ, et al. Effect of aging on carotid baroreflex control of blood pressure and leg vascular conductance in women. Am J Physiol Heart Circ Physiol. 2014;306:H1417–H1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tabara Y, Nakura J, Kondo I, et al. Orthostatic systolic hypotension and the reflection pressure wave. Hypertens Res. 2005;28:537–543. [DOI] [PubMed] [Google Scholar]
- 41. Finucane C, O'Connell MDL, Fan CW, et al. Age‐related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation. 2014;130:1780–1789. [DOI] [PubMed] [Google Scholar]
- 42. Omron Healthcare Corporation official website . Information from the list of HEM‐7200 electronic sphygmomanometer. www.omronhealthcare.com.cn/Home-info-id-66.html#cptd. Accessed May 2, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. baseline characteristics between patients with and without hypertension.


