Abstract
This retrospective claims database analysis compared two strategies of hypertension treatment in outpatient, emergency, and inpatient departments: a fixed‐dose combination (FDC) of amlodipine/valsartan vs free combinations of angiotensin receptor blockers (ARBs) and calcium channel blockers (CCBs) (ARB+CCB group). After a mean follow‐up of 15.2 months, the FDC group had significantly lower total healthcare costs (US $1844 vs US $2158; P<.001) and hospitalization rates (14.57% vs 18.43%; P<.001), a higher proportion of days covered (80.35% vs 72.57%; P<.001), and better persistence (266 vs 225 days; P<.001) compared with the ARB+CCB group. The FDC group also had a better major adverse cardiovascular event (MACE)–free survival (hazard ratio, 0.83; 95% confidence interval, 0.73–0.94; P=.003) and decreased rates of heart failure (2.12% vs 3.26%; P<.001), malignant dysrhythmia (0.18% vs 0.42%; P=.021), and percutaneous coronary intervention (0.76% vs 1.26%; P=.015). Compared with free combinations of ARB+CCB, an FDC of amlodipine/valsartan improved MACE‐free survival and medication compliance and decreased total healthcare costs and hospitalization rates in hypertensive patients.
Hypertension is recognized as one of the most important risk factors for cardiovascular disease, with a substantial impact on morbidity and mortality.1 The worldwide prevalence of hypertension was reported to be around 26% in 2000, and is expected to increase to 29% by 2025.2 Blood pressure (BP) reduction has been reported to effectively protect against complications such as myocardial infarction, heart failure, stroke, and renal function impairment.3, 4, 5 However, despite enormous advances in antihypertensive drug therapy, the BP control rate remains low.6 In the United States, about 50% of patients with hypertension achieve BP control.6 In the Taiwanese Survey on Hypertension, Hyperglycemia, and Hyperlipidemia conducted between 1993 and 2002, 50.4% of hypertensive patients were treated with antihypertensive drugs, only 24.5% of whom had good BP control.7
In addition to a low treatment rate, nonadherence and lack of persistence are two of the main reasons for inadequate BP control.8, 9 Adherence and persistence rates with antihypertensive drugs are often low, ranging from 24% to 51% and 29% to 58% in the United States, respectively.10 Given that the majority of patients with hypertension require two or more medications to maintain BP control,11, 12 the complexity of treatment regimens has been assumed to be responsible for the low adherence and persistence.13 Among the strategies to improve medication adherence and persistence, fixed‐dose combination (FDC) medications are commonly used. FDC medications combining two active agents in a single pill and therefore simplifying drug regimens have been demonstrated to improve compliance.13, 14 Prior meta‐analyses have reported improved adherence and lower healthcare costs associated with FDC medications compared with free‐drug combinations of the same classes in treating patients with chronic illnesses or hypertension.13, 14, 15 However, data on the impact of FDCs on major adverse cardiovascular events (MACEs) are sparse. The most frequently prescribed FDC of a renin‐angiotensin system inhibitor and a calcium channel blocker (CCB) in Taiwan is amlodipine/valsartan (Exforge; Novartis Pharmaceutical, Basel, Switzerland). In the present study, we aimed to compare the clinical outcomes and heathcare costs of hypertension treatment with an FDC of amlodipine/valsartan vs free‐drug combinations of angiotensin receptor blockers (ARBs) and CCBs.
Methods
Data Sources
We obtained data from the National Health Insurance Research Database (NHIRD) of Taiwan. The National Health Insurance (NHI) program, a state‐operated, universal health insurance program implemented in March 1995, covers approximately 99% of the entire population of Taiwan. The database contains inpatient registries from all medical facilities contracted with the NHI Administration, and provides information regarding all admissions, including new‐onset MACEs in inpatients with one principal and four secondary International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes. All personal identifiers are encrypted by the Bureau of the NHI before release to researchers. Confidentiality assurances were addressed by following the data regulations of the Bureau of the NHI, and institutional review board approval was waived.
Study Cohorts
Two study cohorts of patients with the diagnosis of hypertension (ICD‐9‐CM: 401.x) were generated from the NHIRD. The first included patients receiving an FDC of amlodipine/valsartan, and the second included those receiving ARB+CCB combination therapy.
The patients who received an FDC of amlodipine/valsartan between April 1, 2008, and December 31, 2010, were identified and classified as the FDC cohort. The date of the first prescription of this regimen during this period was identified as the index date, and the 6‐month period prior to the index date was defined as the baseline period. The patients were followed for 12 months after the index date (defined as the follow‐up period), and the patients with hypertension and/or high costs were identified. The inclusion criteria were at least two filled prescriptions for an FDC of amlodipine/valsartan (including the index prescription) during the follow‐up period with a total supply of 90 days or more, continuous enrollment with pharmacy and medical claims in both the baseline and follow‐up periods, and an age of 18 years or older at the index date.
The patients who received free‐dose ARB+CCB combination therapy between April 1, 2008, and December 31, 2010 were identified and classified as the ARB+CCB cohort. The date of the first prescription for ARB+CCB combination therapy during this period was identified as the index date, and the 6‐month period prior to the index date was defined as the baseline period. The patients were followed for 12 months after the index date (defined as the follow‐up period). The inclusion criteria were at least two filled prescriptions for ARB+CCB (including the index prescription) during the follow‐up period with a total supply of 90 days or more, continuous enrollment with pharmacy and medical claims in both the baseline and follow‐up periods, and an age of 18 years or older at the index date.
To evaluate the effectiveness of treatment and patient compliance between the two cohorts, we measured the proportion of days covered (PDC) and discontinuation (persistence) with claims for the medications.16
To identify an appropriate control group, propensity score matching17 was deemed to be a valid method for the real‐world data obtained from NHIRD. A propensity score, which is the probability an individual will be assigned to a group based on conditions that exist at the time of the group assignment, was calculated to correct for potential sample selection bias caused by nonrandom assignment. In this study, the variables used for the matching process in the propensity score model included the baseline variables age, sex, coronary heart disease (ICD‐9‐CM: 410–414), myocardial infarction (ICD‐9‐CM: 410), peripheral vascular disease (ICD‐9‐CM: 443), stroke (ICD‐9‐CM: 434.91), congestive heart failure (ICD‐9‐CM: 428), dyslipidemia (ICD‐9‐CM: 272), diabetes mellitus (ICD‐9‐CM: 250), obesity (ICD‐9‐CM: 278), chronic kidney disease (ICD‐9‐CM: 585), medical costs, and overall pill burden. The overall pill burden was defined as the total number of pills prescribed in the baseline period. The control group was matched at a ratio of 4:1 to the FDC group.
MACEs included any of the following: (1) myocardial infarction (ICD‐9‐CM codes 410–410.9); (2) heart failure (ICD‐9‐CM codes 428.0–428.10); (3) percutaneous coronary intervention (ICD‐9‐CM codes 36.0–36.03 and 36.05–36.09); (4) coronary artery bypass surgery (ICD‐9‐CM codes 36.1–36.99 and V45.81); (5) stroke (ICD‐9‐CM codes 430–437); (6) thrombolysis therapy (ICD‐9‐CM codes 36.0–36.99); (7) malignant dysrhythmia (ICD‐9‐CM codes 426.0, 426.12–426.13, 426.51–426.52, 426.54, 427.1, 427.4, 427.41–427.42, and 427.5); and (8) cardiogenic shock (ICD‐9‐CM code 785.51). Mortality related to a MACE was identified using death certificate data files with any diagnosis code, which also indicated the cause of death related to the cardiovascular event.
The following ICD‐9‐CM codes were not used to define MACEs unless they were accompanied with a diagnosis code of cerebral infarction or cerebral hemorrhage: occlusion or stenosis of extracranial arteries without infarction (ICD‐9‐CM codes 430.00, 431.00, 433.20, 433.30, 433.80, 43.390, 434.90, 434.00, 434.10, and 434.90); basilar, vertebral, and subclavian artery syndrome (ICD‐9‐CM codes 435.0–435.3); hypertensive encephalopathy (ICD‐9‐CM code 437.2); cerebral aneurysm, nonruptured (ICD‐9‐CM code 437.3); cerebral arteritis (ICD‐9‐CM code 437.4); and moyamoya disease (ICD‐9‐CM code 437.5).
Statistics
Continuous variables were compared using Student t test, and categorical variables were compared by chi‐square test. Data are presented as means, standard deviations, medians, or percentages. A linear regression model was used for the variable of cost, logistic regression for binary outcome, and a Cox proportional hazard model for time to event analysis. All analyses were conducted using SAS statistical software, version 9.3 (SAS Institute Inc, Cary, NC) and R statistical software, version 3.0.1 (R Foundation for Statistical Computing). A P value <.05 was considered to be statistically significant.
Results
Using propensity score matching, 3301 patients taking an FDC of amlodipine/valsartan, and 13,204 patients taking free combinations of ARB+CCB were enrolled between April 1, 2008, and December 31, 2010. The demographic and clinical characteristics of the two groups are shown in Table 1. The mean follow‐up duration was 15.2 months. No significant differences were found between the two groups in terms of age, sex, and comorbid conditions including coronary heart disease, peripheral vascular disease, congestive heart failure, dyslipidemia, diabetes, obesity, and chronic kidney disease. Angiotensin‐converting enzyme inhibitors, ARB, CCB, and diuretics were significantly more frequently used in the ARB+CCB group at baseline. No significant differences were noted in the duration of follow‐up (15.23 vs 15.27 months; P=.63) and overall pill burden (419 vs 427; P=.4) between the two groups.
Table 1.
Patient Demographic Characteristics
| FDC (n=3301) | ARB+CCB (n=13204) | P Value | |
|---|---|---|---|
| Age, mean±SD, y | 60.30±12.53 | 60.37±13.09 | .767 |
| Male, % | 1724 (52.23) | 6861 (51.96) | .785 |
| Duration of follow‐up, mo | 15.23±3.93 | 15.27±4.11 | .627 |
| Baseline comorbid conditions, % | |||
| Coronary heart disease | 590 (17.87) | 2280 (17.27) | .411 |
| Peripheral vascular disease | 38 (1.15) | 132 (1.00) | .441 |
| Congestive heart failure | 24 (0.73) | 81 (0.61) | .463 |
| Dyslipidemia | 1097 (33.23) | 4478 (33.91) | .459 |
| Diabetes | 1101 (33.35) | 4434 (33.58) | .805 |
| Obesity | 24 (0.73) | 104 (0.79) | .723 |
| Chronic kidney disease | 117 (3.54) | 428 (3.24) | .384 |
| Baseline concomitant medications, % | |||
| ACE inhibitor‐mono | 550 (16.66) | 3381 (25.61) | <.001 |
| ACE inhibitor‐combo | 249 (7.54) | 285 (2.16) | <.001 |
| Angiotensin receptor blocker | 605 (18.33) | 4034 (30.55) | <.001 |
| β‐Blockers | 1331 (40.32) | 5209 (39.45) | .360 |
| Calcium channel blocker | 2146 (65.01) | 9307 (70.49) | <.001 |
| Diuretics | 580 (17.57) | 2781 (21.06) | <.001 |
| Other antihypertensive agents | 321 (9.72) | 1213 (9.19) | .341 |
| Antidiabetic agents | 1037 (31.42) | 3985 (30.18) | .168 |
| Overall pill burden | 419.31±478.83 | 427.17±477.35 | .398 |
Abbreviations: ACE inhibitor‐combo, angiotensin‐converting enzyme inhibitor in combination with other antihypertensive agents in a single pill; ACE inhibitor‐mono, angiotensin‐converting enzyme inhibitor only in a pill; ARB+CCB, free combinations of angiotensin receptor blockers and calcium channel blockers; FDC, fixed‐dose combination of amlodipine/valsartan; SD, standard deviation.
We analyzed the healthcare costs, including medical and pharmacy costs, of the two study groups (Figure 1, Tables S1 and S2). At baseline, there was no significant difference in total healthcare costs between the FDC and ARB+CCB groups ($787 vs $750; P=.21), although the pharmacy cost was higher in the FDC group ($311 vs $282; P=.019). The post‐index total healthcare cost was significantly lower in the FDC group compared with the ARB+CCB group ($1844 vs $2158; P<.001). In subgroup analysis, adherence status was divided into two categories: PDC ≥80% and PDC <80%. The patients taking an FDC of amlodipine/valsartan with a PDC ≥80% had insignificantly higher total healthcare costs than those taking ARB+CCB ($1710 vs $1587; P=.07). Of the patients with a PDC ≥80%, the total pharmacy cost was higher in the FDC group ($801 vs $726; P=.03). Of the patients with a PDC <80%, those taking an FDC of amlodipine/valsartan had a significantly lower total healthcare cost ($2109 vs $2791; P<.001).
Figure 1.
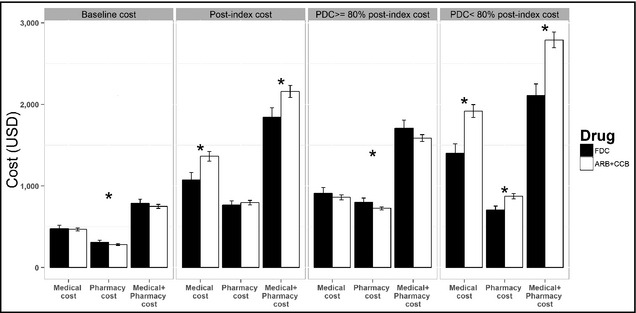
Comparison of total healthcare costs. The patients taking a fixed‐dose combination of amlodipine/valsartan (FDC) had significantly lower post‐index total healthcare costs (medical plus pharmacy costs). ARB+CCB indicates free combinations of angiotensin receptor blockers and calcium channel blockers; PDC, proportion of days covered. *P<.05.
[Correction added after initial online publication on December 5, 2014: Figure 1 has been revised.]
The factors of total healthcare and pharmacy costs were analyzed using multiple linear regression analysis (Figure 2, Table S3). A PDC ≥80%, compared with a PDC <80%, was associated with both lower total healthcare (coefficient, −25,152; 95% confidence interval [CI], −28,522 to −21,783; P<.001) and pharmacy (coefficient, −1434; 95% CI, −2759 to −110; P=.034) costs. The FDC group had a significantly lower total healthcare cost (coefficient, −9063; 95% CI, −13,316 to −4811; P<.001), and a borderline significant reduction in pharmacy costs (coefficient, −1597; 95% CI, −3269 to 75; P=.06). Increased age and baseline pill burden were significantly associated with higher total healthcare and pharmacy costs.
Figure 2.
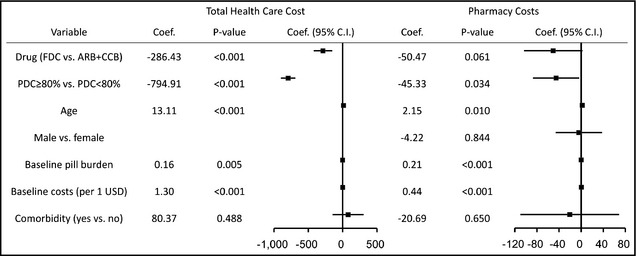
Multivariate analysis of total healthcare and pharmacy costs. Proportion of days covered (PDC) ≥80% predicted both lower total healthcare and pharmacy costs. A fixed‐dose combination of amlodipine/valsartan (FDC) significantly reduced lower total healthcare costs but not pharmacy costs. Age and baseline pill burden were significantly associated with higher total healthcare and pharmacy costs. ARB+CCB indicates free combinations of angiotensin receptor blockers and calcium channel blockers.
Table 2 demonstrates medication adherence, persistence, and utilization of healthcare resources in the two groups. The patients in the FDC group had a significantly lower hospitalization rate (14.57% vs 18.43%; P<.001). In the patients with a PDC ≥80%, no significant difference in hospitalization rate was detected between the two groups. However, in the patients with a PDC <80%, an FDC of amlodipine/valsartan resulted in a significant reduction in hospitalization rate compared with the ARB+CCB regimen (19.5% vs 25.0%; P<.001). The FDC group also had a higher PDC (80.35% vs 72.57%; P<.001) and better medication persistence (266 vs 225 days, P<.001) than the ARB+CCB group. The improvement in medication compliance remained significant even in the patients with a PDC ≥80% (PDC: 93.42% vs 92.98%, P=.002; persistence: 341 vs 335 days, P=.001).
Table 2.
Medication Adherence, Persistence, and Utilization of Healthcare Resources
| FDC (n=3301) | ARB+CCB (n=13,204) | P Value | |
|---|---|---|---|
| Baseline | |||
| Length of stay, mean±SD | 6.38±6.25 | 6.72±7.11 | .415 |
| Visits | |||
| Patients with ≥1 ED visits, % | 506 (15.33) | 2154 (16.31) | .169 |
| Patients with ≥1 outpatient visits, % | 3248 (98.39) | 12,993 (98.40) | .975 |
| Patients with ≥1 inpatient visits, % | 300 (9.09) | 1091 (8.26) | .127 |
| Post‐index, all patient | |||
| Length of stay, mean±SD | 7.04±10.08 | 8.40±13.89 | .012 |
| Visits | |||
| Patients with ≥1 ED visits, % | 788 (23.87) | 3297 (24.97) | .191 |
| Patients with ≥1 outpatient visits, % | 3301 (100) | 13,204 (100) | – |
| Patients with ≥1 inpatient visits, % | 481 (14.57) | 2433 (18.43) | <.001 |
| Adherence‐PDC, mean±SD, % | 80.35±21.90 | 72.57±25.95 | <.001 |
| Persistence‐days, mean±SD | 265.75±130.89 | 224.67±142.60 | <.001 |
| Post‐index, patients with PDC ≥80% | |||
| Length of stay, mean±SD | 6.39±11.72 | 7.54±18.45 | .231 |
| Visits | |||
| Patients with ≥1 ED visits, % | 460 (20.98) | 1337 (19.27) | .080 |
| Patients with ≥1 outpatient visits, % | 2193 (100) | 6939 (100) | – |
| Patients with ≥1 inpatient visits, % | 265 (12.08) | 869 (12.52) | .586 |
| Adherence‐PDC, mean±SD, % | 93.42±5.62 | 92.98±5.77 | .002 |
| Persistence‐days, mean±SD | 340.5±69.04 | 334.88±78.07 | .001 |
| Post‐index, patients with PDC <80% | |||
| Length of stay, mean±SD | 7.83±7.56 | 8.87±10.52 | .073 |
| Visits | |||
| Patients with ≥1 ED visits, % | 328 (29.60) | 1960 (31.29) | .265 |
| Patients with ≥1 outpatient visits, % | 1108 (100) | 6265 (100) | – |
| Patients with ≥1 inpatient visits, % | 216 (19.50) | 1564 (24.96) | <.001 |
| Adherence‐PDC, mean±SD, % | 54.47±18.93 | 49.96±20.24 | <.001 |
| Persistence‐days, mean±SD | 117.81±93.05 | 102.57±88.02 | <.001 |
Abbreviations: ARB+CCB, free combinations of angiotensin receptor blockers and calcium channel blockers; ED, emergency department; FDC, fixed‐dose combination of amlodipine/valsartan; PDC, proportion of days covered; SD, standard deviation.
The results of multivariate analysis using Cox regression for medication persistence and PDC are presented in Figure 3 and Table S4. An FDC of amlodipine/valsartan and presence of comorbidities were significantly associated with better persistence (hazard ratio [HR], 0.69; 95% CI, 0.66–0.73; P<.001) and PDC ≥80% (odds ratio [OR], 1.82; 1.67–1.98; P<.001). Baseline pill burden and healthcare costs were negative preditors of persistence and PDC ≥80%.
Figure 3.
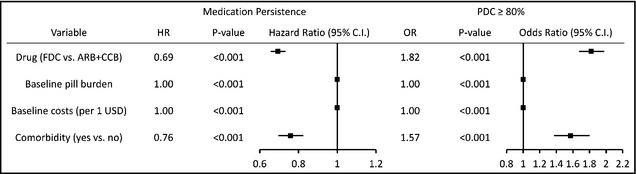
Multivariate analysis of persistence and proportion of days covered. A fixed‐dose combination of amlodipine/valsartan (FDC) and the presence of comorbidities were significantly associated with better persistence and proportion of days covered (PDC) ≥80%. Baseline pill burden and healthcare costs were negative predictors of persistence and PDC ≥80%. ARB+CCB indicates free combinations of angiotensin receptor blockers and calcium channel blockers; HR: hazard ratio; OR: odds ratio.
Figure 4 illustrates Kaplan‐Meier curves of MACE‐free survival. The patient taking an FDC of amlodipine/valsartan had better MACE‐free survival than those taking free combinations of ARB+CCB (HR, 0.83; 95% CI, 0.73–0.94; P=.003). Compared with the ARB+CCB group, the FDC group had significantly decreased rates of heart failure (2.12% vs 3.26%; P<.001), malignant dysrhythmia (0.18% vs 0.42%; P=.021), and percutaneous coronary intervention (0.76% vs 1.26%; P=.015), and a borderline significant decrease in myocardial infarction (0.58% vs 0.92%; P=.052) (Table S5).
Figure 4.
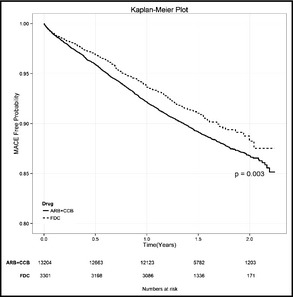
Kaplan‐Meier survival curves of major adverse cardiovascular events. The patients taking a fixed‐dose combination of amlodipine/valsartan (FDC) had better major adverse cardiovascular event (MACE)–free survival than those taking free‐combination regimens of angiotensin receptor blockers and calcium channel blockers (ARB+CCB).
Discussion
This retrospective claims database analysis compared two strategies for hypertension treatment: an FDC of amlodipine/valsartan vs free combinations of ARBs and CCBs. After a mean follow‐up of 15.2 months, the FDC group had significantly lower total healthcare costs and hospitalization rates, a higher PDC, and better persistence compared with the ARB+CCB group. The FDC group also had better MACE‐free survival than the ARB+CCB group.
In this study, total healthcare costs were significantly lower in the patients taking an FDC of amlodipine/valsartan than those in the patients taking ARB+CCB, mainly due to significant cost reduction in the patients with a PDC <80%. This reduction in healthcare costs is consistent with the results of prior reports. In a meta‐analysis comparing annual healthcare costs of FDC and free‐combination regimens for hypertension treatment, Sherrill and colleagues15 estimated a $2039 reduction (95% CI, $1030–$3047) in all‐cause total costs and a $709 reduction (95% CI, $117–$1302) in hypertension and cardiovascular‐related costs in the FDC group. In their analysis of pharmacy costs, patients taking an FDC had an average $605 reduction (95% CI, $376–$835) in annual pharmacy costs compared with those taking free drug combinations. In contrast to their results, we found no significant difference in total pharmacy costs between the two groups (P=.36), which may be the result of relatively lower pharmacy costs and reimbursements from the NHI program in Taiwan. Because of the limitations of data retrieval from the NHIRD, we could not specifically estimate pharmacy costs related to hypertension or cardiovascular disease, and inconsistent results have been reported in previous analyses.18, 19
By reducing overall pill burden and simplifying medication regimens, FDCs have been shown to improve medication compliance and persistence in numerous studies. We used a PDC ≥80% to define the threshold of adherence. The patients taking an FDC of amlodipine/valsartan had better medication adherence and persistence, regardless of their adherence status. In a meta‐analysis of chronic diseases including diabetes mellitus, hypertension, and human immunodeficiency virus, Bangalore and associates13 reported a 26% reduction in the risk of noncompliance in those taking an FDC compared with those taking a free‐drug combination (relative risk, 0.74, 95% CI, 0.69–0.8; P<.0001). In another meta‐analysis of hypertension treatment with the use of an FDC, Gupta and colleagues14 found that an FDC was associated with a 29% increase in compliance and persistence (OR, 1.29; 95% CI, 1.11–1.50).
In the current study, the patients taking an FDC of amlodipine/valsartan had a lower hospitalization rate than those taking ARB+CCB, which was mainly the result of a reduction in hospitalization rates in the patients with a PDC <80%. Moreover, we also observed an improvement in MACE‐free survival in the FDC group. It has been well documented that medication compliance is associated with decreased use of medical care services in various clinical diseases20, 21, 22, 23, 24 and improvement in cardiovascular outcomes.25, 26, 27, 28 However, to the best of our knowledge, no meta‐analyses or randomized prospective trials have demonstrated the superiority of an FDC over free‐combination regimens in MACEs. An observational, multicenter study of 1605 patients in Spain in 2006 reported a decreased cumulative incidence of cerebrovascular events in patients taking single‐pill combinations for hypertension control (2.4% in single‐pill combinations vs 4.6% in free combinations; P=.041) after 2 years of follow‐up.29 In the meta‐analysis conducted by Gupta and colleagues,14 there was a beneficial trend in the use of an FDC, with a reduction of 4.1 mm Hg in systolic BP (95% CI, −9.8 to 1.5; P=.15) and a 3.1‐mm Hg reduction in diastolic BP (95% CI, −7.1 to 0.9; P=.13). The recently published Use of a Multidrug Pill in Reducing Cardiovascular Events (UMPIRE trial) was an open‐label, randomized, blinded end‐point trial comparing an FDC of aspirin, statin, and two antihypertensive agents with usual care in patients with established cardiovascular disease or at risk for cardiovascular disease.30 After a median follow‐up of 15 months, the FDC group showed a significant improvement in adherence and small but statistically significant reductions in systolic BP (2.6 mm Hg; 95% CI, −4.0 to −1.1 mm Hg; P<.001) and low‐density lipoprotein cholesterol (4.2 mg/dL; 95% CI, −6.6 to −1.9 mg/dL; P<.001). However, no significant differences were found between the groups in terms of serious adverse events or cardiovascular events. In contrast to the results of the UMPIRE trial, we found that patients taking an FDC of amlodipine/valsartan had better MACE‐free survival compared with those taking free combinations of ARB+CCB. Given the better compliance in the FDC group in this study, we hypothesized that it was the result of improved compliance with the use of an FDC regimen, not necessarily the pharmacologic effect of it, that may lead to better efficacy in BP control and improvement in clinical outcomes as well.
Study Limitations
There are some limitations to this study. This retrospective cohort analysis was based on a claims database and therefore has inherent limitations. The claims data are collected based on prescription information but not for study purposes. A claim for a refill prescription does not indicate that the medication is actually taken, so the assessment of PDC or persistence may have been overestimated or underestimated. Incorrect coding is also possible in daily clinical practice. Instead of specifically focusing on hypertension or cardiovascular disease, we evaluated healthcare costs and medical service utilization of all causes, which could potentially lead to bias. In addition, the claims database does not contain clinical data such as BP records at baseline or at follow‐up visits. Therefore, the efficacy of BP control, an important link between medication compliance and clinical outcomes, could not be evaluated. Prospective randomized control trials are required to evaluate the impact of FDC regimens on major outcomes in treating hypertensive patients.
Conclusions
This retrospective study presents real‐world results of FDC vs free‐combination regimens in the treatment of hypertension. We found that the use of an FDC of amlodipine/valsartan improved MACE‐free survival and medication adherence and persistence and decreased all‐cause healthcare costs and hospitalization rates compared with free combinations of ARBs and CCBs. The reductions in costs and hospitalization rates were more substantial in the nonadherent subgroup. The use of an FDC provides an important opportunity to improve the quality of hypertension treatment.
Supporting information
Table S1. Patient baseline costs and resource use.
Table S2. Patient post‐index costs.
Table S3. Multivariate analysis of total health care and pharmacy costs.
Table S4. Multivariate analysis of medication persistence and proportion of days covered.
Table S5. Number of patients with major adverse cardiovascular events.
Acknowledgments
Dr Chu P‐H is supported by the Ministry of Science and Technology (99‐2314‐B‐182A‐106‐MY3 and 102‐2314‐B‐182A‐060‐MY2). We thank Michael Wu's critical reading of the current paper.
Disclosure
None declared.
J Clin Hypertens (Greenwich). 2015;17:51–58. DOI: 10.1111/jch.12449. © 2014 Wiley Periodicals, Inc.
References
- 1. Ezzati M, Lopez AD, Rodgers A, et al. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. [DOI] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 3. Trialists' Collaboration BPLT . Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet. 2003;362:1527–1535. [DOI] [PubMed] [Google Scholar]
- 4. Trialists' Collaboration BPLT . Effects of different blood pressure‐lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165:1410. [DOI] [PubMed] [Google Scholar]
- 5. Staessen JA, Li Y, Thijs L, Wang J‐G. Blood pressure reduction and cardiovascular prevention: an update including the 2003–2004 secondary prevention trials. Hypertens Res. 2005;28:385–407. [DOI] [PubMed] [Google Scholar]
- 6. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988‐2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 7. Su T‐C, Bai C‐H, Chang H‐Y, et al. Evidence for improved control of hypertension in Taiwan: 1993‐2002. J Hypertens. 2008;26:600–606. [DOI] [PubMed] [Google Scholar]
- 8. Sanson‐Fisher R, Clover K. Compliance in the treatment of hypertension: a need for action. Am J Hypertens. 1995;8:82S–88S. [DOI] [PubMed] [Google Scholar]
- 9. Elliott WJ. Improving outcomes in hypertensive patients: focus on adherence and persistence with antihypertensive therapy. J Clin Hypertens (Greenwich). 2009;11:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerth WC. Compliance and persistence with newer antihypertensive agents. Curr Hypertens Rep. 2002;4:424–433. [DOI] [PubMed] [Google Scholar]
- 11. Cushman WC, Ford CE, Einhorn PT, et al. Blood pressure control by drug group in the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2008;10:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 13. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed‐dose combinations improve medication compliance: a meta‐analysis. Am J Med. 2007;120:713–719. [DOI] [PubMed] [Google Scholar]
- 14. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed‐dose combinations of antihypertensive agents a meta‐analysis. Hypertension. 2010;55:399–407. [DOI] [PubMed] [Google Scholar]
- 15. Sherrill B, Halpern M, Khan S, et al. Single‐pill vs free‐equivalent combination therapies for hypertension: a meta‐analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13:898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. [DOI] [PubMed] [Google Scholar]
- 17. d'Agostino RB. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 18. Malesker M, Hilleman D. Comparison of amlodipine/valsartan fixed‐dose combination therapy and conventional therapy. Manag Care. 2010;19:36. [PubMed] [Google Scholar]
- 19. Taylor AA, Shoheiber O. Adherence to antihypertensive therapy with fixed‐dose amlodipine besylate/benazepril HCl versus comparable component‐based therapy. Congest Heart Fail. 2003;9:324–332. [DOI] [PubMed] [Google Scholar]
- 20. Wu PH, Yang CY, Yao ZL, et al. Relationship of blood pressure control and hospitalization risk to medication adherence among patients with hypertension in Taiwan. Am J Hypertens. 2010;23:155–160. [DOI] [PubMed] [Google Scholar]
- 21. Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. [DOI] [PubMed] [Google Scholar]
- 22. Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836. [DOI] [PubMed] [Google Scholar]
- 23. Hepke KL, Martus MT, Share DA. Costs and utilization associated with pharmaceutical adherence in a diabetic population. Am J Manag Care. 2004;2:144–151. [PubMed] [Google Scholar]
- 24. Lang K, Meyers J, Korn J, et al. Medication adherence and hospitalization among patients with schizophrenia treated with antipsychotics. Psychiatr Serv. 2010;61:1239–1247. [DOI] [PubMed] [Google Scholar]
- 25. Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598–1605. [DOI] [PubMed] [Google Scholar]
- 26. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. [DOI] [PubMed] [Google Scholar]
- 27. Shin S, Song H, Oh S‐K, et al. Effect of antihypertensive medication adherence on hospitalization for cardiovascular disease and mortality in hypertensive patients. Hypertens Res. 2013;36:1000–1005. [DOI] [PubMed] [Google Scholar]
- 28. Corrao G, Parodi A, Nicotra F, et al. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens. 2011;29:610–618. [DOI] [PubMed] [Google Scholar]
- 29. Sicras Mainar A, Galera Llorca J, Munoz Orti G, Navarro Artieda R. Influence of compliance on the incidence of cardiovascular events and health costs when using single‐pill fixed‐dose combinations for the treatment of hypertension. Med Clin. 2011;136:183–191. [DOI] [PubMed] [Google Scholar]
- 30. Thom S, Poulter N, Field J, et al. Effects of a fixed‐dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA. 2013;310:918–929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient baseline costs and resource use.
Table S2. Patient post‐index costs.
Table S3. Multivariate analysis of total health care and pharmacy costs.
Table S4. Multivariate analysis of medication persistence and proportion of days covered.
Table S5. Number of patients with major adverse cardiovascular events.


