Abstract
This prespecified subgroup analysis of a phase III study examined the effect of adding hydrochlorothiazide (HCTZ) to olmesartan (OLM)/amlodipine (AML) in patients with moderate to severe hypertension stratified by age, sex, body mass index, and hypertension severity. A total of 2690 patients, aged 18 years and older, with seated blood pressure (SeBP) ≥160/100 mm Hg received placebo or OLM/AML 20/5 mg, 40/5 mg, or 40/10 mg during a 2‐week, double‐blind, run‐in period, after which they were allocated to one of eight treatment groups with the same OLM/AML dose or with HCTZ 12.5 mg or 25 mg added for 8 weeks. By week 10, greater reductions in SeBP were observed in each OLM/AML/HCTZ group (P<.05, respectively) compared with the corresponding dual dose. Adding HCTZ increased blood pressure–lowering efficacy in all subgroups, with a higher proportion of blood pressure goal achievement vs dual therapy. OLM/AML/HCTZ reduced SeBP to a greater extent than OLM/AML in patients with moderate to severe hypertensive; this was unaffected by baseline hypertension severity, age, sex, and obesity.
The strong, continuous link between blood pressure (BP) and cardiovascular (CV) risk1 underpins the importance of controlling BP in hypertensive patients. In particular, BP control is vital in patients who are at increased risk of CV disease, such as patients with severe hypertension, those with excess body weight, and the elderly.2, 3, 4
Current US and European treatment guidelines state that two or more drugs are needed in the majority of patients to achieve BP control.5, 6, 7 In patients with moderate or severe hypertension (Europe) or a BP that is 20/10 mm Hg above goal (United States), combining agents from two different drug classes is recommended as the first treatment step. Combining two drugs has been shown to significantly increase BP lowering, and if BP control remains inadequate then adding a third drug leads to further reductions in BP and CV risk.8 Indeed, the benefits of BP lowering with a triple combination are well established. In the 1960s, a combination of hydrochlorothiazide (HCTZ), reserpine, and hydralazine was used in a landmark study by the US Veterans Administration to treat patients with severe hypertension and was shown to be associated with a significant reduction in morbidity.9
The current European Society of Hypertension/European Society of Cardiology (ESH‐ESC) treatment guidelines for patients who require three drugs to control their BP recommend a renin‐angiotensin system blocker, calcium channel blocker (CCB), and diuretic combination.6 The ESH‐ESC guidelines also recommend the use of single‐pill, fixed‐dose combinations because these increase adherence, which improves BP control and reduces the risk of CV outcomes, chronic heart failure, coronary artery disease, and cerebrovascular disease.6, 10, 11, 12, 13, 14
One recent development in antihypertensive therapy is the availability of single‐pill triple combinations containing an angiotensin receptor blocker (ARB) with a CCB and a diuretic. The Safety and Efficacy Study of a Triple Combination Therapy in Subjects With Hypertension (TRINITY) demonstrated that the triple combination of the ARB olmesartan medoxomil (OLM), the CCB amlodipine (AML), and the diuretic HCTZ produced significantly larger BP reductions than each of the component dual combinations.15 Furthermore, this was shown to be true for patients with severe hypertension (seated systolic BP [SeSBP] ≥180 mm Hg or seated diastolic BP [SeDBP] ≥110 mm Hg) as well as those with moderate hypertension (SeSBP<180 and SeDBP<110 mm Hg) at baseline. In addition, a factorial study in patients with moderate to severe hypertension investigated the addition of HCTZ 12.5 mg and 25 mg to OLM/AML treatment and found it provided statistically significant improvements in BP reduction and achievement of BP thresholds (ClinicalTrials.gov identifier: NCT00923091).16 This multinational, phase III, randomized, double‐blind study provides an opportunity to assess the efficacy of triple‐combination therapy in higher‐risk subgroups of hypertensive patients, including those with severe hypertension and other risk factors.
This paper presents the results of a prespecified analysis of the BP‐lowering effect of adding HCTZ 12.5 mg and 25 mg to a range of OLM/AML doses in patients with either moderate or severe hypertension who were included in the study reported by Volpe and colleagues.16 The ability of this triple‐combination therapy to lower BP in patients with a range of elevated BP levels was also investigated in a post‐hoc analysis of the hypertension severity profile of patients in the triple OLM/AML/HCTZ therapy compared with dual OLM/AML therapy groups. In addition, to obtain insights into the efficacy of triple‐combination therapy in other important groups of hypertensive patients, prespecified analyses of the efficacy and safety of adding HCTZ to OLM/AML were performed in subgroups defined by baseline age, sex, and obesity status.
Methods
Details of the methodology for this study are described elsewhere16 and are briefly summarized here. The study was conducted in accordance with the Declaration of Helsinki and was approved by the independent ethics committee for each center. Patients gave their written informed consent prior to entering the study.
Study Population
Patients aged 18 years and older were included if they were naive to antihypertensive therapy and satisfied the following BP inclusion criteria at two consecutive prerandomization visits (separated by 7 days): mean SeSBP ≥160 mm Hg, mean SeDBP ≥100 mm Hg, and a difference in mean seated BP of <20/10 mm Hg between visits. Patients receiving antihypertensive therapy underwent a 3‐week washout period and were included if they met the BP inclusion criteria, stated above, at two consecutive prerandomization visits (7 and 14 days after the last treatment dose).
Patients were excluded if they had serious cerebrovascular, CV, renal, or hepatic disorders or had a mean SeSBP ≥200 mm Hg, mean SeDBP ≥115 mm Hg, or bradycardia (heart rate <50 beats per minute at rest). A total of 3195 patients were screened and 2690 were eventually randomized.
Study Design
This is a post‐hoc analysis of a phase III study that has been published previously.16 The study consisted of a double‐blind safety run‐in (weeks 0–2: period I), double‐blind (weeks 3–10: period II), single‐blind (weeks 10–18), and open‐label treatment periods (weeks 18–54). The single‐blind and open‐label treatment periods are beyond the focus of the current report and are not described here.
Schedule of Interventions
On day 1, patients were randomized to treatment sequences for periods I and II, and stratified by age group (<65 years and ≥65 years), diabetes status, and study site. During period I, patients were randomized to receive one of three dual OLM/AML treatments (OLM/AML 20/5 mg, OLM/AML 40/5 mg, or OLM/AML 40/10 mg) or placebo for 2 weeks. In period II, patients were allocated to one of eight treatment groups. Patients who received OLM/AML 20/5 mg in period I continued with OLM/AML 20/5 mg or changed to OLM/AML/HCTZ 20/5/12.5 mg; OLM/AML 40/5 mg recipients in period I continued with OLM/AML 40/5 mg or were switched to either OLM/AML/HCTZ 40/5/12.5 mg or OLM/AML/HCTZ 40/5/25 mg; and OLM/AML 40/10 mg recipients in period I continued with OLM/AML 40/10 mg or were switched to either OLM/AML/HCTZ 40/10/12.5 mg or OLM/AML/HCTZ 40/10/25 mg.
Schedule of Assessments
Patients were assessed at screening, randomization (week 0), and every 2 weeks until week 10. At each visit, seated BP and heart rate were measured after patients had rested for at least 5 minutes. A total of three BP measurements were taken after a resting interval of 1 minute and the mean value was calculated. BP was assessed using a standardized model of a calibrated, validated sphygmomanometer (Greenlight 300 Sphygmomanometer; Accoson, Harlow, Essex, UK).
Efficacy Assessments
The primary efficacy endpoint of this study was the change in mean SeDBP from baseline to week 10 in groups with HCTZ added to OLM/AML, compared with the corresponding dual OLM/AML therapy, as previously reported.16 This present paper focuses on subgroup analyses of the effects of treatment on BP changes and BP goal rate achievement (for which the latter was defined as SeBP <140/90 mm Hg or <130/80 mm Hg for patients with diabetes, chronic renal disease, or chronic CV disease, based on the recommendations of the 2007 ESH‐ESC guidelines17) in patients stratified by hypertension severity or other risk factors. It should be noted that the study design was developed before the ESH revised their previous recommendation for a lower goal BP of <130/80 mm Hg for patients with diabetes and increased CV risk.18
Effects of Treatment in Patients With Different Classifications of Elevated BP/Hypertension
A prespecified analysis investigated the effects of treatment in patients stratified according to their level of hypertension severity at baseline. Mild/moderate hypertension was defined as an SeSBP from 140 mm Hg to <180 mm Hg and an SeDBP from 90 mm Hg to <110 mm Hg. Severe hypertension was defined as an SeSBP ≥180 mm Hg or SeDBP ≥110 mm Hg.
A post‐hoc analysis compared the hypertension severity profile of all patients at baseline and week 10, and also in patients taking triple OLM/AML/HCTZ or dual OLM/AML therapy at baseline and week 10. Patients’ hypertension severity profile was assessed using BP criteria based on the 2007 ESH‐ESC guidelines, which required both DBP and SBP thresholds to be fulfilled.17 BP severity was classified as follows: normal BP 120/80 mm Hg to <130/85 mm Hg; high‐normal BP 130/85 mm Hg to <140/90 mm Hg; grade 1 hypertension 140/90 mm Hg to <160/100 mm Hg; grade 2 hypertension 160/100 mm Hg to <180/110 mm Hg; grade 3 hypertension ≥180/≥110 mm Hg.
Effects of Treatment in Patients With Other Risk Factors
A prespecified analysis was also performed in patient subgroups defined by the following baseline factors: age (<65 years or ≥65 years), sex (male or female), and obesity status (body mass index [BMI] <30 kg/m2 or BMI ≥30 kg/m2).
Safety Assessments
Safety variables that were assessed during the study included physical examinations, adverse events (AEs), clinical laboratory evaluations, vital signs, and electrocardiograms (ECGs). Patients underwent a complete physical examination at screening and during the study at the discretion of the investigator. AEs were monitored from screening until 14 days after the last dose of study medication. Laboratory tests were measured at screening and at weeks 0 and 10. Vital signs including seated BP and heart rate measurements were assessed at all visits. ECGs were performed at screening, at week 10, and at the follow‐up visit if considered necessary.
Statistical Methods
Comparisons of changes in mean SeDBP and SeSBP were performed using the least‐squares (LS) method. The full analysis set included all randomized patients who received at least one dose of double‐blind study medication during periods I and II and provided at least one SeDBP measurement after randomization in periods I or II.
Treatment comparisons were performed between triple combinations and their corresponding dual combinations using an analysis of covariance model. The model included baseline BP as a covariate and treatment, age group (<65 years or ≥65 years), and diabetic status as fixed effects.
The safety set included all patients who received at least one dose of double‐blind study medication in periods I or II.
To simplify and facilitate effective presentation of data in the prespecified analyses of patients with other risk factors, changes in LS mean SeDBP and SeSBP from baseline to week 10 (using the last‐observation‐carried‐forward method), are reported for the following six treatment groups, which included those with the highest dose of HCTZ used in the study: OLM/AML 20/5 mg; OLM/AML/HCTZ 20/5/12.5 mg; OLM/AML 40/5 mg; OLM/AML/HCTZ 40/5/25 mg; OLM/AML 40/10 mg; and OLM/AML/HCTZ 40/10/25 mg. Therefore, efficacy data for patients receiving OLM/AML/HCTZ 40/5/12.5 mg and OLM/AML/HCTZ 40/10/12.5 mg are not reported in this present article but are available in Tables S1 and S2 and Figures S1 to S4.
Results
Study Population
Of 3195 patients who were screened, 2690 were randomized and 2543 patients (94.5%) completed the study to the end of period II (week 10). The demographic and baseline characteristics of patients in the full analysis set by treatment group are summarized in Table 1. A total of 926 patients were men (45.9%) and 99.9% were Caucasian. The mean age was 56.3 years (21.9% aged ≥65 years) and the mean weight was 89.1 kg. In terms of concomitant diseases, 14.8% were diabetic, 28.9% had CV disease, and 2.0% had chronic kidney disease. Overall, the population was moderately obese (mean BMI 31.0 kg/m2), the mean duration of hypertension was 8.4 years, 90.0% of patients had mild or moderate hypertension, and 10.0% had severe hypertension. Overall, there were no major differences among treatment groups in terms of baseline characteristics.
Table 1.
Patient Demographics and Baseline Characteristics According to Treatment Subgroup (Full Analysis Set)a
| OLM/AML 20/5 mg (n=337) | OLM/AML/HCTZ 20/5/12.5 mg (n=335) | OLM/AML 40/5 mg (n=337) | OLM/AML/HCTZ 40/5/25 mg (n=336) | OLM/HCTZ 40/10 mg (n=336) | OLM/AML/HCTZ 40/10/25 mg (n=336) | Total (N=2017) | |
|---|---|---|---|---|---|---|---|
| Age, y | 56.1 (±10.2) | 56.0 (±10.8) | 56.4 (±10.4) | 56.5 (±11.0) | 57.0 (±9.7) | 55.9 (±11.1) | 56.3 (±10.5) |
| ≥65, % | 22.0 | 21.5 | 22.0 | 22.0 | 22.0 | 21.7 | 21.9 |
| Male sex, % | 45.1 | 45.7 | 49.6 | 45.2 | 42.9 | 47.0 | 45.9 |
| Caucasian/white, % | 100.0 | 99.7 | 100.0 | 99.7 | 99.7 | 100.0 | 99.9 |
| Weight, kg | 87.4 (±17.7) | 90.0 (±17.5) | 91.0 (±17.1) | 88.2 (±17.9) | 87.8 (±16.7) | 90.0 (±19.1) | 89.1 (±17.7) |
| BMI, kg/m2 | 30.5 (±5.3) | 31.4 (±5.5) | 31.0 (±5.1) | 30.9 (±5.6) | 30.7 (±5.0) | 31.2 (±5.7) | 31.0 (±5.4) |
| ≥30, % | 51.0 | 53.1 | 53.4 | 51.2 | 50.3 | 54.8 | 52.3 |
| Diabetes, % | 14.8 | 14.0 | 14.8 | 15.2 | 15.2 | 14.6 | 14.8 |
| Nonsmoker, % | 73.0 | 67.8 | 70.6 | 73.8 | 73.2 | 70.8 | 71.5 |
| Cardiovascular disease, % | 30.6 | 28.1 | 30.3 | 26.8 | 26.8 | 30.7 | 28.9 |
| Chronic kidney disease, % | 2.1 | 1.8 | 1.5 | 0.9 | 2.4 | 2.7 | 1.9 |
| Duration of hypertension, y | 8.6 (±8.1) | 8.8 (±8.9) | 7.8 (±7.0) | 8.5 (±7.1) | 8.5 (±7.3) | 8.3 (±8.3) | 8.4 (±7.8) |
Abbreviations: AML, amlodipine; BMI, body mass index; HCTZ, hydrochlorothiazide; OLM, olmesartan. aContinuous variables are mean (±standard deviation).
Efficacy
Effects of Treatment in Patients With Different Classifications of Elevated BP/Hypertension
BP Changes in patients with mild/moderate and severe hypertension
In all treatment groups, patients with mild/moderate and severe hypertension had significant LS mean reductions in SeDBP and SeSBP by week 10 compared with baseline (P<.0001 for all comparisons) (Figure 1a,b). In patients with mild/moderate and severe hypertension, the addition of HCTZ to OLM/AML increased BP‐lowering efficacy. Patients with mild/moderate hypertension who received triple‐combination therapy showed significantly larger SeDBP and SeSBP reductions than those in the corresponding dual‐therapy groups (P<.05 for all comparisons). In addition, patients with severe hypertension had similar SeDBP reductions and slightly larger SeSBP reductions than those with mild/moderate hypertension.
Figure 1.
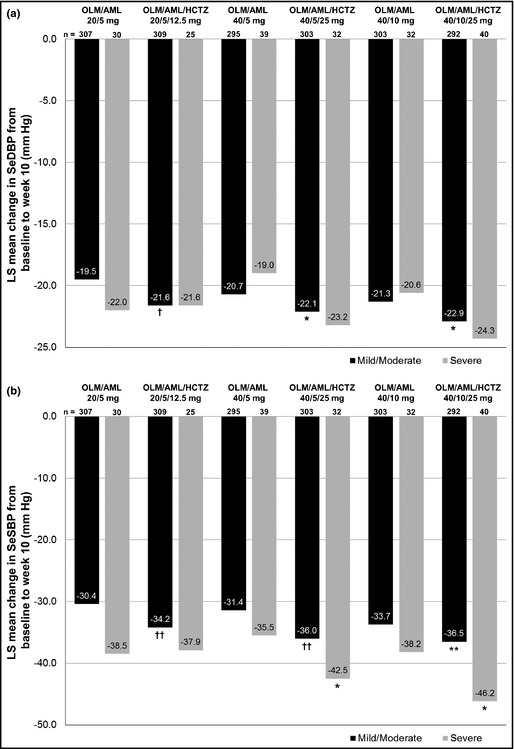
Changes from baseline to week 10 according to patient hypertension severity in SeDBP (a) and SeSBP (b). AML indicates amlodipine; HCTZ, hydrochlorothiazide; LS, least‐squares; OLM, olmesartan; SeSBP, seated systolic blood pressure; SeDBP, seated diastolic blood pressure. P<.0001 for mean change in SeDBP and SeSBP from baseline to week 10 in both hypertension severity subgroups for all six treatment groups. *P<.05; **P≤.01; † P<.001; †† P<.0001 for triple‐ vs dual‐combination comparison.
Mild/moderate and severe hypertensive patients taking triple therapy all had higher BP goal achievement rates than those in the corresponding dual‐therapy groups (Table 2). A statistically significant increase compared with dual therapy was observed with mild/moderate hypertensive patients in the OLM/AML/HCTZ 20/5/12.5‐mg and 40/5/25‐mg groups (P<.01 for both groups). Although patients with severe hypertension generally had larger BP reductions than mild/moderate hypertensive patients, they generally had lower BP goal achievement rates.
Table 2.
Achievement of Blood Pressure Goal From Baseline to Week 10 According to Patient Subgroup
| Patient Subgroup | OLM/AML 20/5 mg, % | OLM/AML/HCTZ 20/5/12.5 mg, % | OLM/AML 40/5 mg, % | OLM/AML/HCTZ 40/5/25 mg, % | OLM/AML 40/10 mg, % | OLM/AML/HCTZ 40/10/25 mg, % |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| <65 | 47.1 | 54.6 | 49.8 | 58.0 | 49.0 | 53.3 |
| ≥65 | 27.0 | 47.2a | 34.2 | 61.6b | 51.4 | 56.3 |
| Sex | ||||||
| Female | 46.5 | 55.5 | 52.4 | 61.4 | 52.9 | 55.9 |
| Male | 38.2 | 50.0a | 40.4 | 55.6b | 45.1 | 51.6 |
| Obesity status | ||||||
| BMI <30 kg/m2 | 46.7 | 62.4b | 47.7 | 67.5b | 54.5 | 64.9 |
| BMI ≥30 kg/m2 | 39.0 | 44.6 | 45.3 | 50.6 | 44.6 | 44.8 |
| Hypertension severity | ||||||
| Mild/moderatec | 44.3 | 55.0b | 49.5 | 61.4b | 52.5 | 55.5 |
| Severed | 26.7 | 28.0 | 23.1 | 34.4 | 21.9 | 42.5 |
Abbreviations: AML, amlodipine; BMI, body mass index; HCTZ, hydrochlorothiazide; OLM, olmesartan. a P<.05 and b P<.01 for triple vs dual combination comparison. cMild/moderate hypertension: seated systolic blood pressure from 140 mm Hg to <180 mm Hg and seated diastolic blood pressure from 90 mm Hg to <110 mm Hg. dSevere hypertension: seated systolic blood pressure ≥180 mm Hg or seated diastolic blood pressure ≥110 mm Hg.
Effects of Dual‐ and Triple‐Combination Therapy on Hypertension Severity Profile
The baseline hypertension severity profiles of patients who were allocated triple and dual therapy were similar (Figure 2a). However, by week 10 the hypertension severity profiles of the two groups (Figure 2b) were significantly different (P<.0001 using Cochran‐Mantel‐Haenszel test).
Figure 2.
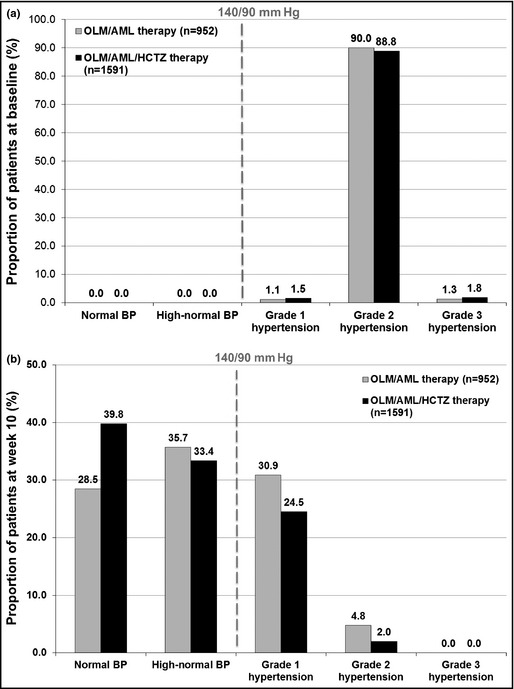
Hypertension severity profile of all patients taking dual and triple therapy at baseline (a) and week 10 (b). At week 10 there was a significant difference in severity profile of triple vs dual treatment using Cochran‐Mantel‐Haenszel test (P<.0001). Baseline uncategorized patients: 7.9% of triple therapy and 7.6% of dual therapy. Week 10 uncategorized patients: 0.13% of triple therapy and 0.11% of dual therapy. AML indicates amlodipine; BP, blood pressure; HCTZ, hydrochlorothiazide; OLM, olmesartan.
A larger proportion of patients allocated to triple therapy fulfilled the criteria for normal BP than those taking dual therapy (39.8% vs 28.5%). The proportion of patients who met the criteria for grade 1 hypertension was smaller among the triple‐therapy group than the dual‐therapy group (24.5% vs 30.9%). A total of 73.2% of patients taking triple therapy and 64.2% of patients taking dual therapy had either normal or high‐normal BP (120/80 mm Hg to <140/90 mm Hg) and 26.5% of patients taking triple therapy and 35.7% of patients taking dual therapy met the criteria for either grade 1 or 2 hypertension (140/90 mm Hg to <180/110 mm Hg).
Effects of Treatment on Patients With Other Risk Factors
Age (Younger vs Older Patients)
By week 10, all patients younger than 65 and those 65 years and older had significant LS mean reductions in SeDBP and SeSBP compared with baseline (P<.0001 for all comparisons) (Figure 3). In patients younger than 65 years, SeSBP reductions were significantly larger in every triple‐combination group compared with those who received the corresponding dual therapy (P≤.01 for all comparisons). A similar pattern was seen in patients 65 years and older with triple therapy consistently providing significantly larger SeSBP reductions than dual therapy (P<.02 for all comparisons).
Figure 3.
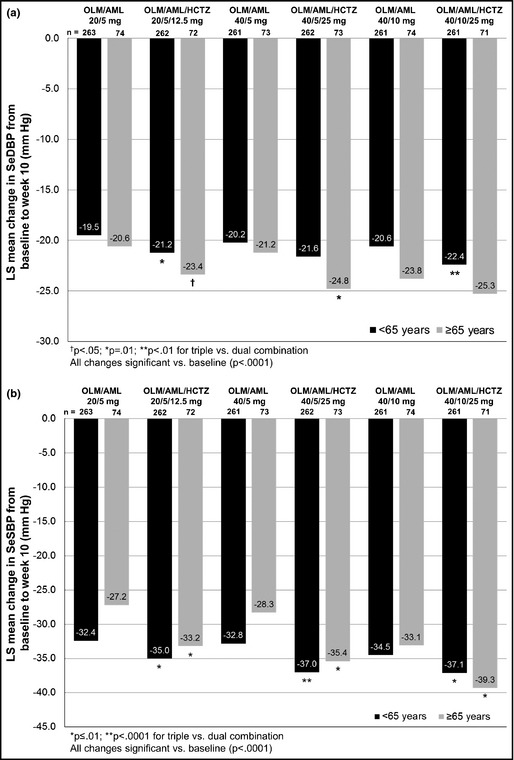
Blood pressure changes from baseline to week 10 according to age in seated diastolic blood pressure (a) and seated systolic blood pressure (b). AML indicates amlodipine; HCTZ, hydrochlorothiazide; LS, least‐squares; OLM, olmesartan; SeSBP, seated systolic blood pressure; SeDBP, seated diastolic blood pressure.
Patients younger than 65 or 65 years and older in the triple‐therapy groups consistently achieved a higher proportion of BP goal achievement than those taking dual therapy (Table 2). In addition, more than 50% of patients younger than 65 or 65 years and older in both the triple OLM/AML/HCTZ 40/5/25‐mg and 40/10/25‐mg groups achieved BP goal.
Sex (Male vs Female Patients)
Both men and women had significant LS mean reductions in SeDBP and SeSBP at week 10 compared with baseline (P<.0001 for all comparisons) (Figure 4). Also, the addition of HCTZ to OLM/AML made a positive contribution to BP‐lowering efficacy in both men and women. For men, SeSBP reductions in every triple‐combination therapy group and SeDBP reductions in the OLM/AML/HCTZ 40/5/25‐mg and 40/10/25‐mg groups were significantly larger than in the corresponding dual‐combination groups (P<.05 for all comparisons). Women in each treatment group had slightly larger BP reductions than men and all BP reductions in women taking triple therapy were larger compared with women taking dual therapy.
Figure 4.
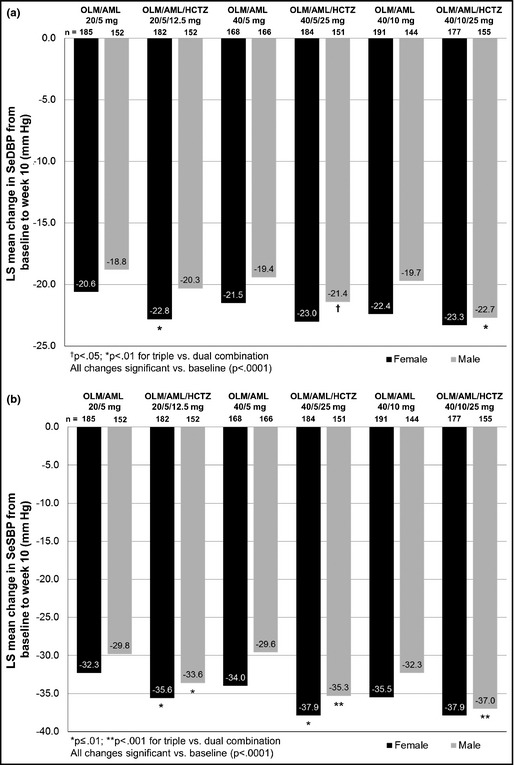
Blood pressure changes from baseline to week 10 according to sex in seated diastolic blood pressure (a) and seated systolic blood pressure (b). AML indicates amlodipine; HCTZ, hydrochlorothiazide; LS, least‐squares; OLM, olmesartan; SeSBP, seated systolic blood pressure; SeDBP, seated diastolic blood pressure.
As in the age subgroup, all men and women taking triple therapy had higher levels of BP goal achievement than those taking dual therapy (Table 2). For men, this difference was statistically significant in the OLM/AML/HCTZ 20/5/12.5‐mg and 40/5/25‐mg groups (P<.05 and P<.01, respectively).
Obesity (Obese vs Non‐obese Patients)
By week 10, all patients with a BMI <30 kg/m2 or ≥30 kg/m2 had significant LS mean reductions in SeDBP and SeSBP compared with baseline (P<.0001 for all comparisons) (Figure 5).
Figure 5.
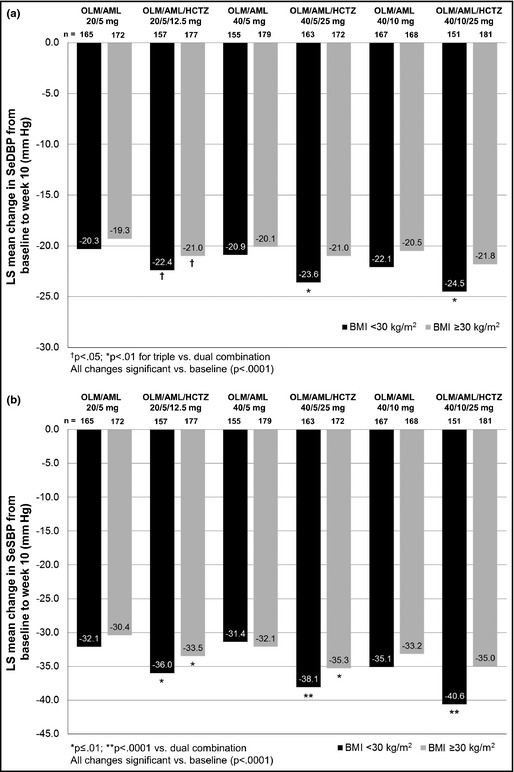
Blood pressure changes from baseline to week 10 according to obesity state in seated diastolic blood pressure (a) and seated systolic blood pressure (b). AML indicates amlodipine; HCTZ, hydrochlorothiazide; BMI, body mass index; LS, least‐squares; OLM, olmesartan; SeSBP, seated systolic blood pressure; SeDBP, seated diastolic blood pressure.
For patients with a BMI <30 kg/m2, all triple‐combination treatment groups had significantly larger SeDBP and SeSBP reductions than the corresponding dual‐combination groups (P≤.01 for all comparisons). In addition, patients with a BMI ≥30 kg/m2 taking the triple combination had larger reductions in SeDBP and SeSBP than those taking the corresponding dual therapy, but these changes were not always statistically significant.
The addition of HCTZ to OLM/AML therapy increased BP goal achievement in all treatment groups and all patients with a BMI <30 kg/m2 or ≥30 kg/m2 (Table 2). Among patients with a BMI <30 kg/m2, these increases were statistically significant in the OLM/AML/HCTZ 20/5/12.5‐mg and 40/5/25‐mg groups (P<.01 for both groups).
Tolerability and Safety
Treatment was well tolerated across the triple‐ and dual‐combination therapy groups, with a similar frequency of discontinuations as a result of an AE, treatment‐emergent AE (TEAE), or drug‐related TEAE.16
Discussion
In this study, the addition of HCTZ to a range of OLM/AML doses increased BP‐lowering efficacy compared with dual OLM/AML therapy in patients with moderate to severe hypertension. The increase in efficacy was unaffected by hypertension severity. In addition, the hypertension severity profile of patients taking OLM/AML/HCTZ therapy was significantly better than that of patients taking dual OLM/AML therapy by week 10. Taken together, these findings demonstrate that adding HCTZ to dual OLM/AML therapy substantially increases BP reductions across patients with a range of elevated BPs. Furthermore, the efficacy of triple‐combination therapy was unaffected by baseline age, sex, and obesity status and therefore offers an effective and convenient way to control BP across a range of high‐risk patients. The results from these subgroup and post‐hoc analyses extend the findings described by Volpe and colleagues16 in the initial report from this study.
The findings from these analyses provide further insights into the BP‐lowering effect of triple‐combination therapy in higher‐risk subgroups of hypertensive patients. A total of 10% of the study population had severe hypertension; however, the additional BP‐lowering efficacy of adding HCTZ to OLM/AML was not affected by hypertension severity. Compared with dual‐therapy recipients, a larger proportion of patients with severe hypertension who received triple therapy achieved BP goal although the differences were not significant. Higher rates of BP goal achievement were also seen in patients with moderate to severe hypertension who received triple therapy than in those treated with dual therapy, and these differences were significant for some between‐group comparisons. These results are similar to those previously observed with triple OLM/AML/HCTZ therapy in the TRINITY study, where both moderate and severe hypertensive patients in the OLM/AML/HCTZ treatment group showed significantly larger BP reductions and higher goal achievement than patients in the dual‐combination groups.15 It was also notable that in each treatment group in the present study, a greater proportion of patients with mild/moderate hypertension reached BP goal compared with patients with severe hypertension. This effect is almost certainly related to the higher baseline BP levels in the severe hypertension group, and has been observed in previous studies.19, 20
The impact of treatment on hypertension severity was also examined in a post‐hoc analysis based on the ESH's classification system for BP/hypertension. This analysis revealed that, at baseline, the majority of patients had met the criteria for grade 2 or 3 hypertension. However, by the end of double‐blind treatment, a large shift in severity profile had occurred so that the majority of patients met the BP criteria for normal or high‐normal BP. This change was greatest in patients who received the triple combination, and more than 70% of these patients had BP levels within the normal or high‐normal range. Data from the open‐label extension phase of this study,21 and the TRINITY study,22 indicate that BP reductions and control rates with triple‐combination therapy are stably maintained over the long term. If the shift in severity profile seen here were to be maintained over the long term, then this would represent a substantial reduction in patients’ level of CV risk.
Triple‐combination therapies that comprise an ARB, the CCB AML, and the diuretic HCTZ have been shown to be more effective than dual therapy.15, 23 The TRINITY study looked at patients with moderate to severe hypertension and assessed the efficacy of triple‐combination therapy comprising high doses of the ARB OLM, AML, and HCTZ. The study showed that triple therapy was more effective in reducing BP than the component dual therapies,15 and a subsequent open‐label extension showed that reductions in BP were maintained over the long term (52 weeks).22 Subgroup analyses from TRINITY have also shown that the improved efficacy of triple therapy was seen in major subgroups of patients, including those with diabetes, chronic kidney disease, or chronic CV disease,24, 25 as well as those of black or Hispanic/Latino origin,26, 27 those with advanced age,28 or those with obesity.29 The study by Volpe and colleagues16 showed that triple therapy is more effective than dual therapy at lowering BP across a range of doses. The present findings add to this by showing that the increased efficacy of triple therapy is seen across a range of patient subgroups.
Single‐pill fixed‐dose combinations allow patients to take multiple medications without increasing pill burden. The benefits of this are highlighted by the observation that patient adherence decreases as the number of concomitantly administered pills increases.30 This is of particular relevance for certain subgroups of hypertensive patients such as the elderly and obese who are typically at a higher CV risk than younger and non‐obese individuals and are also more likely to be receiving treatments for concomitant conditions.3, 31 Studies indicate that adherence to antihypertensive treatment can be significantly improved by the use of single‐pill, fixed‐dose combinations.32, 33 Thus, the single‐pill, fixed‐dose combination of OLM/AML/HCTZ has the potential to significantly reduce pill burden and improve compliance in a range of higher‐risk hypertensive patients for whom pill burden may be an issue. Furthermore, the additional reductions in BP and improvement in the hypertension severity profile of patients following the addition of HCTZ to OLM/AML suggest that further gains may be achieved in reducing CV risk.
Study Limitation
One limitation of the present study is that while the majority of the analyses were prespecified, the original clinical trial was not designed to compare differences between subgroups. However, each subgroup examined was quite large (>300 patients each) and therefore provides a reasonable insight into the efficacy of triple vs dual therapy in these subgroups.
Conclusions
In patients with moderate to severe hypertension, triple OLM/AML/HCTZ treatment reduced SeSBP and SeDBP to a greater degree than the corresponding dual OLM/AML combination and this was unaffected by baseline hypertension severity, age, sex, or obesity. Patients taking the triple combination also had a significantly better hypertension severity profile, and a greater percentage of patients achieved BP goal. All treatments were generally well tolerated.
Supporting information
Figure S1. Changes from baseline to week 10 according to patient hypertension severity in seated diastolic blood pressure (a) and seated systolic blood pressure (b).
Figure S2. Blood pressure changes from baseline to week 10 according to age in seated diastolic blood pressure (a) and seated systolic blood pressure (b).
Figure S3. Blood pressure changes from baseline to week 10 according to sex in seated diastolic blood pressure (a) and seated systolic blood pressure (b).
Figure S4. Blood pressure changes from baseline to week 10 according to obesity state in seated diastolic blood pressure (a) and seated systolic blood pressure (b).
Table S1. Patient Demographics and Baseline Characteristics According to Treatment Subgroup (Full Analysis Set)
Table S2. Achievement of Blood Pressure Goal From Baseline to Week 10 According to Patient Subgroup
Disclosures
Reinhold Kreutz has received honoraria for participating in advisory boards and lectures sponsored Bayer HealthCare, Berlin‐Chemie, Bristol‐Myers Squibb, Daiichi Sankyo, Merck, Menarini, Recordati Pharma, Servier, and Trommsdorff. Alejandro de la Sierra has received honoraria for participating in advisory boards and speaking at scientific meetings sponsored by Daiichi Sankyo, Menarini, and MSD. Bettina Ammentorp and Petra Laeis are current employees of Daiichi Sankyo Europe GmbH, Munich, Germany. This study was sponsored by Daiichi Sankyo Europe GmbH, Munich, Germany. Editorial assistance during the preparation of this manuscript was provided by Phil Jones, PhD, of inScience Communications, Springer Healthcare, Chester, UK, and funded by Daiichi Sankyo Europe GmbH.
J Clin Hypertens. 2014;16:729–740. DOI: 10.1111/jch.12217. © 2014 The Authors. Journal of Clinical Hypertension Published by Wiley Periodicals, Inc.
References
- 1. Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153:598–615. [DOI] [PubMed] [Google Scholar]
- 2. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 3. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2011;123:2434–2506. [DOI] [PubMed] [Google Scholar]
- 4. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta‐analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. [DOI] [PubMed] [Google Scholar]
- 5. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 6. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 7. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Veterans Administration Cooperative Studies Program . Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202:1028–1034. [PubMed] [Google Scholar]
- 10. Bramley TJ, Gerbino PP, Nightengale BS, Frech‐Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm. 2006;12:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corrao G, Parodi A, Nicotra F, et al. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens. 2011;29:610–618. [DOI] [PubMed] [Google Scholar]
- 12. Kettani FZ, Dragomir A, Cote R, et al. Impact of a better adherence to antihypertensive agents on cerebrovascular disease for primary prevention. Stroke. 2009;40:213–220. [DOI] [PubMed] [Google Scholar]
- 13. Perreault S, Dragomir A, Roy L, et al. Adherence level of antihypertensive agents in coronary artery disease. Br J Clin Pharmacol. 2010;69:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perreault S, Dragomir A, White M, et al. Better adherence to antihypertensive agents and risk reduction of chronic heart failure. J Intern Med. 2009;266:207–218. [DOI] [PubMed] [Google Scholar]
- 15. Oparil S, Melino M, Lee J, et al. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the TRINITY multicenter, randomized, double‐blind, 12‐week, parallel‐group study. Clin Ther. 2010;32:1252–1269. [DOI] [PubMed] [Google Scholar]
- 16. Volpe M, Rump LC, Ammentorp B, Laeis P. Efficacy and safety of triple antihypertensive therapy with the olmesartan/amlodipine/hydrochlorothiazide combination. Clin Drug Investig. 2012;32:649–664. [DOI] [PubMed] [Google Scholar]
- 17. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 18. Mancia G, Laurent S, Agabiti‐Rosei E, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158. [DOI] [PubMed] [Google Scholar]
- 19. Schmieder RE, Bohm M. Efficacy and safety of olmesartan medoxomil plus amlodipine in age, gender and hypertension severity defined subgroups of hypertensive patients. J Hum Hypertens. 2011;25:354–363. [DOI] [PubMed] [Google Scholar]
- 20. Weycker D, Edelsberg J, Vincze G, et al. Blood pressure control in patients initiating antihypertensive therapy. Ann Pharmacother. 2008;42:169–176. [DOI] [PubMed] [Google Scholar]
- 21. de la Sierra A, Ammentorp B, Laeis P. Effect of long‐term treatment with the triple olmesartan (O)/amlodipine (A)/hydrochlorothiazide (H) combination on hypertension severity. J Hypertens. 2012;30(e suppl A):e276. [Google Scholar]
- 22. Kereiakes DJ, Chrysant SG, Izzo JL Jr, et al. Long‐term efficacy and safety of triple‐combination therapy with olmesartan medoxomil and amlodipine besylate and hydrochlorothiazide for hypertension. J Clin Hypertens (Greenwich). 2012;14:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Calhoun DA, Lacourciere Y, Chiang YT, Glazer RD. Triple antihypertensive therapy with amlodipine, valsartan, and hydrochlorothiazide: a randomized clinical trial. Hypertension. 2009;54:32–39. [DOI] [PubMed] [Google Scholar]
- 24. Chrysant SG, Izzo JL Jr, Kereiakes DJ, et al. Efficacy and safety of triple‐combination therapy with olmesartan, amlodipine, and hydrochlorothiazide in study participants with hypertension and diabetes: a subpopulation analysis of the TRINITY study. J Am Soc Hypertens. 2012;6:132–141. [DOI] [PubMed] [Google Scholar]
- 25. Kereiakes DJ, Chrysant SG, Izzo JL Jr, et al. Olmesartan/amlodipine/hydrochlorothiazide in participants with hypertension and diabetes, chronic kidney disease, or chronic cardiovascular disease: a subanalysis of the multicenter, randomized, double‐blind, parallel‐group TRINITY study. Cardiovasc Diabetol. 2012;11:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chrysant SG, Littlejohn T 3rd, Izzo JL Jr, et al. Triple‐combination therapy with olmesartan, amlodipine, and hydrochlorothiazide in black and non‐black study participants with hypertension: the TRINITY randomized, double‐blind, 12‐week, parallel‐group study. Am J Cardiovasc Drugs. 2012;12:233–243. [DOI] [PubMed] [Google Scholar]
- 27. Lewin AJ, Kereiakes DJ, Chrysant SG, et al. Triple‐combination treatment with olmesartan medoxomil/amlodipine/hydrochlorothiazide in Hispanic/Latino patients with hypertension: the TRINITY study. Ethn Dis. 2014;24:41–47. [PubMed] [Google Scholar]
- 28. Lewin AJ, Izzo JL Jr, Melino M, et al. Combined olmesartan, amlodipine, and hydrochlorothiazide therapy in randomized patients with hypertension: a subgroup analysis of the TRINITY study by age. Drugs Aging. 2013;30:549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roth EM, Oparil S, Melino M, et al. Olmesartan/amlodipine/hydrochlorothiazide in obese participants with hypertension: a TRINITY subanalysis. J Clin Hypertens (Greenwich). 2013;15:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gerbino PP, Shoheiber O. Adherence patterns among patients treated with fixed‐dose combination versus separate antihypertensive agents. Am J Health Syst Pharm. 2007;64:1279–1283. [DOI] [PubMed] [Google Scholar]
- 31. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity‐related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of the Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich). 2013;15:14–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sherrill B, Halpern M, Khan S, et al. Single‐pill vs free‐equivalent combination therapies for hypertension: a meta‐analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13:898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed‐dose combinations of antihypertensive agents: a meta‐analysis. Hypertension. 2010;55:399–407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Changes from baseline to week 10 according to patient hypertension severity in seated diastolic blood pressure (a) and seated systolic blood pressure (b).
Figure S2. Blood pressure changes from baseline to week 10 according to age in seated diastolic blood pressure (a) and seated systolic blood pressure (b).
Figure S3. Blood pressure changes from baseline to week 10 according to sex in seated diastolic blood pressure (a) and seated systolic blood pressure (b).
Figure S4. Blood pressure changes from baseline to week 10 according to obesity state in seated diastolic blood pressure (a) and seated systolic blood pressure (b).
Table S1. Patient Demographics and Baseline Characteristics According to Treatment Subgroup (Full Analysis Set)
Table S2. Achievement of Blood Pressure Goal From Baseline to Week 10 According to Patient Subgroup


