Abstract
Stem cells have great clinical significance in many cardiovascular diseases. However, there are limited data regarding the involvement of mesenchymal stem cells (MSCs) in the pathophysiology of arterial hypertension. The aim of this study was to investigate the circulation of MSCs in patients with essential hypertension. The authors included 24 patients with untreated essential hypertension and 19 healthy individuals. Using flow cytometry, MSCs in peripheral blood, as a population of CD45−/CD34−/CD90+ cells and also as a population of CD45−/CD34−/CD105+ cells, were measured. The resulting counts were translated into the percentage of MSCs in the total cells. Hypertensive patients were shown to have increased circulating CD45−/CD34−/CD90+ compared with controls (0.0069%±0.012% compared with 0.00085%±0.0015%, respectively; P=.039). No significant difference in circulating CD45−/CD34−/CD105+ cells was found between hypertensive patients' and normotensive patients' peripheral blood (0.018%±0.013% compared with 0.015%±0.014%, respectively; P=.53). Notably, CD45−/CD34−/CD90+ circulating cells were positively correlated with left ventricular mass index (LVMI) (r=0.516, P<.001). Patients with essential hypertension have increased circulating MSCs compared with normotensive patients, and the number of MSCs is correlated with LVMI. These findings contribute to the understanding of the pathophysiology of hypertension and might suggest a future therapeutic target.
In recent years there has been growing interest in the role of adult stem cells in the pathophysiology of cardiovascular diseases. Although it used to be believed that mammalian cardiomyocytes cease replication soon after birth and that the subsequent growth of the heart was attributable only to cardiomyocyte hypertrophy, newer studies have demonstrated a small degree of cardiogenesis and cardiomyocyte turnover that occurs throughout life.1, 2 These findings led to further research into the contribution of stem cells to the pathophysiology of cardiovascular disorders that has raised the hope of developing new therapeutic approaches. Stem cells have the potential for self‐renewal and differentiation and are the origin cells of various mature cells.
Mesenchymal stem cells (MSCs) are also known to have a highly plastic differentiation potential that includes not only adipogenesis, osteogenesis, and chondrogenesis, but also endothelial, cardiovascular,3 and neovascular differentiation.4, 5, 6 Although present in only very small numbers in peripheral blood, in recent years stem and progenitor cells have been implicated in ventricular remodeling and are thought to be of great clinical significance in the pathophysiology of heart failure and atheromatosis. Previous studies have indicated that MSCs derived from peripheral blood, apart from their multilineage potential, can also be used for cellular and gene therapies.7 Human MSCs isolated from adult bone marrow provide a model for the development of stem cell therapeutics and could find application in the cardiovascular system—although this is still under investigation.8
Under normal conditions, endogenous cardiac progenitor cells are responsible for homeostasis in the heart.9 However, it appears that under conditions of stress, this may change, with stem cells from extra‐cardiac sources also playing a role. An interesting experimental study has shown that an increase in preload results in the mobilization of progenitor cells from the bone marrow for use in neovascularization, which plays an important role in cardiac hypertrophy.10 There are indications that the recruitment of bone marrow–derived cells is involved in cardiac myocyte hypertrophy and maintenance of function in response to pressure overload.11 A recent study from our department has shown increased expression of myocardin and GATA4 genes in the peripheral blood mononuclear cell fraction of hypertensive patients, implying the presence of mesenchymal progenitor cells in the peripheral blood that could possibly be intended to differentiate into cells of the cardiac series.12 Interestingly, in the patients in that study, myocardin and GATA4 expression was associated with both blood pressure (BP) levels and left ventricular hypertrophy (LVH).
To date, most published reports concerning the cardiovascular applications of stem cells have focused on their role in myocardial infarction and in heart failure. Very little work has been done on arterial hypertension, and most has concerned endothelial progenitor cells. The role and behavior of MSCs in patients with essential hypertension is unknown. In a recent animal study, it was shown that the degree to which angiotensin II increased neointima formation was statistically correlated with the increased incorporation of fluorescent bone marrow–derived smooth muscle cells, and that this was inhibited by angiotensin‐1 receptor antagonism.13 Based on the hypothesis that MSCs participate in pathophysiological processes that contribute to hypertension, and on the assumption that the behavior of MSCs is altered in hypertensive patients, we carried out the first flow cytometric analysis of CD45−/CD34−/CD90+ and CD45−/CD34−/CD105+ in the peripheral blood of those patients compared with healthy individuals.
Methods
Study Population
We prospectively enrolled 24 patients with untreated essential hypertension (aged 61±9 years) and no indications of other organic heart disease. The study population was recruited from the cardiology outpatient department and the diagnosis of hypertension was based on three outpatient measurements of BP >140/90 mm Hg at intervals of no longer than 2 weeks, according to the recommendations of the European Society of Hypertension/European Society of Cardiology.14 Participants with BP >140/90 mm Hg on the final visit underwent 24‐hour ambulatory BP monitoring. To be eligible for inclusion in the study, a mean 24‐hour BP >130/80 mm Hg was required. A physical examination and routine laboratory tests were performed before inclusion. The patients had not previously taken any hypertensive medication and did not take any other drugs for 3 weeks before the studies. Patients with any of the following characteristics were excluded: smokers, diabetics, pregnant or lactating women or women potentially childbearing, or patients with a previous history of or medication for hypertension; grade 3 hypertension or secondary hypertension, tachyarrhythmias or bradyarrythmias, or coronary artery disease; cerebrovascular, liver, or renal disease; albumin excretion rate >200 μg/min; history of drug or alcohol abuse; any chronic inflammatory or other infectious disease during the past 6 months; thyroid gland disease; body mass index >40 kg/m2; personal history of anemia; thrombocytopenia; or any other hematological disease. In addition, vascular, metabolic, or neoplastic conditions were ruled out by careful examination of the history and routine laboratory tests. Height and weight were also measured, and a full echocardiographic examination was performed in all participants.
We also selected a control group of 19 healthy volunteers (aged 57±9 years) without symptoms or signs of cardiovascular disease and with no major cardiovascular risk factors, such as diabetes, hypertension, or familial history of coronary artery disease. This group consisted of patients who came to the emergency department with atypical chest pain, but whose clinical and laboratory examination findings were normal.
After a rest of 20 minutes, blood was obtained from all participants, from a superficial brachial vein via a 21‐gauge needle with care to avoid stasis, hemolysis, and contamination by tissue fluids or exposure to glass.
The study complies with the Declaration of Helsinki and was approved by the hospital's ethics committee. All institutional guidelines were followed and all participants gave written informed consent.
Echocardiography
Standard M‐mode and 2‐dimensional echocardiography was performed using a Vivid 7 (General Electric, Horten, Norway) ultrasound device with a 1.5‐MHz to 3.6‐MHz wide‐angle phased‐array transducer (M4S) according to the recommendations of the American Society of Echocardiography and the European Association of Echocardiography,15, 16 in order to measure left atrial (LA) diameter, left ventricular end‐diastolic diameter (LVEDD), left ventricular posterior wall in diastole (LVPW), interventricular septum in diastole (IVSD), and left ventricular ejection fraction (LVEF). Relative wall thickness (RWT) was calculated as 2 × LVPW/LVEDD and left ventricular wall thickness (LVWT) as IVSD+LVPWD. LV mass was calculated according to the Penn convention and expressed as left ventricular mass index (LVMI).17
MSC Detection by Flow Cytometry
For each participant, 200 μL of peripheral blood (PB) were stained with fluorescence‐conjugated mouse anti‐human monoclonal antibodies. The antibodies used for staining were CD45‐R Phycoerythrin‐Cyanine 7 (PC7), CD34‐R Phycoerythrin‐Cyanine 5.1 (PC5), CD90‐Fluorescein isothiocyanate (FITC), and CD90‐R Phycoerythrin (PE) (all by Beckman Coulter, Marseille, France). For each sample, an isotype control was prepared in order to monitor background staining using mouse anti‐human CD45‐PC7 negative control in conjunction with the mouse IgG1 fluorescence‐labeled antibodies IgG1‐PC5, IgG1‐FITC, and IgG1‐PE (all by Beckman Coulter). Staining was performed according to the manufacturer's instructions. Following staining, samples and isotype controls were lysed to remove the red cells and fixed by paraformaldehyde using the Q‐prep reagent system (Coulter, Luton, UK). Immediately after fixing, samples and isotype controls were subjected to flow cytometry using the Beckman Coulter Cytomics FC500 apparatus (Beckman Coulter, Inc, Fullerton, CA) and analyzed by the associated CXP software (Beckman Coulter, Inc). Approximately 0.5×106 cells were passed through the flow cytometer chamber in each experiment. Two populations of MSCs were identified as cells negative for CD45‐PC7 and CD34‐PC5 and positive for either CD90‐FITC or CD105‐PE (labeled in text as CD45−/CD34−/CD90+ and as CD45−/CD34−/CD133+). The percentage of MSCs in relation to the total live cells was calculated for each sample and was further normalized by subtracting the percentage of the relevant isotype control.
Statistical Methods
Summary descriptive statistics are presented as mean (standard deviation) or frequency (percentage), as appropriate. Comparisons between the hypertensive and normotensive groups were performed using a two‐sided independent samples t test. Associations between parameters of interest were assessed by the Pearson correlation coefficient. Partial correlations were also calculated to estimate the association of certain parameters after controlling for systolic BP. All comparisons were performed at the two‐sided 5% level of significance. The SPSS 20 (IBM, Armonk, NY) statistics package was used.
Results
The clinical characteristics of the participants are presented in the Table. Although there were no significant differences in basic biochemical parameters, hypertensive patients had significantly higher systolic and diastolic BP, LA diameter, LVMI, and body surface area compared with the control group.
Table 1.
Participants' Clinical Characteristics
| Hypertensive Patients (n=24) | Control Group (n=19) | P Value | |
|---|---|---|---|
| Sex, male/female | 12/12 | 9/10 | NS |
| Smokers, % | 44 | 45 | NS |
| Age, y | 61±9 | 57±9 | NS |
| Cholesterol, mg/dL | 227±50 | 242±52 | NS |
| Glucose, mg/dL | 101±11 | 96±9 | NS |
| Uric acid, mg/dL | 6.2±3.9 | 6.7±4.9 | NS |
| Creatinine, mg/dL | 1.1±0.3 | 1.0±0.3 | NS |
| Heart rate, beats per min | 69±12 | 72±19 | NS |
| Systolic blood pressure, mm Hg | 159±11 | 128±6 | <.001 |
| Diastolic blood pressure, mm Hg | 90±7 | 77±6 | <.001 |
| Pulse wave velocity, m/s | 11.1±2 | 10.1±2.6 | NS |
| Left atrium diameter, mm | 40±5 | 34±4 | .001 |
| Body surface area, m2 | 1.99±0.17 | 1.84±0.15 | .006 |
| Left ventricular mass index, g/m2 | 101.5±32 | 77.7±15 | .005 |
Abbreviation: NS, not significant.
Quantification of MSCs cells from peripheral blood by flow cytometry has shown that they represent <0.04% of mononuclear cells in the healthy population. Interestingly, there was a significant difference in the number of MSCs between the groups. Patients with essential hypertension were shown to have higher proportions of circulating CD45−/CD34−/CD90+ compared with the control group (0.0069%±0.012% vs 0.00085%±0.0015%, respectively; P=.039). In contrast, no statistically significant difference was found between hypertensive and normotensive patients in the circulating CD45−/CD34−/CD105+ cells in peripheral blood (0.018%±0.013% vs 0.015%±0.014%, respectively; P=.53) (Figure 1).
Figure 1.
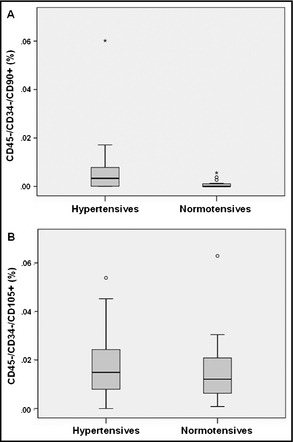
Percentages of CD45−/CD34−/CD90+ and CD45−/CD34−/CD105+ in hypertensive patients' peripheral blood compared with normotensive patients'. Data are presented as mean±standard error of the mean.*P < .05.
Notably, Pearson correlation revealed a strong association between the number of circulating CD45−/CD34−/CD90+ cells and LVMI (r=0.516, P<.001), indicating that hypertensive patients with LVH have increased numbers of circulating MSCs (Figure 2). This correlation remained strong when the hypertensive and normotensive groups were analyzed separately (r=0.47, P=.018 and r=0.475, P=.046, respectively). In addition, there was a less strong but still statistically significant correlation with levels of systolic BP (r=0.375, P=.013) and a weak correlation with diastolic BP (r=0.289, P=.06). No significant correlations were found between the examined clinical parameters and the number of circulating CD45−/CD34−/CD105+ cells. It should be noted that the correlation between the number of CD45−/CD34−/CD90+ and LVMI remained statistically significant after controlling for systolic BP (r=0.42, P=.005).
Figure 2.
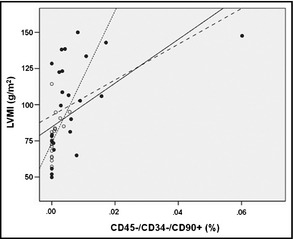
Association between the percentage of CD45−/CD34−/CD90+ in peripheral blood and left ventricular mass index. Solid circles, hypertensives; open circles, normotensives; solid line, all participants; dotted line, hypertensives; dashed line, normotensives.
Discussion
This is the first study to examine the mobilization of bone marrow–derived MSC populations in patients with essential hypertension. We examined two subpopulations of MSCs, CD45−/CD34−/CD90+ and CD45−/CD34−/CD105+ cells, and found that hypertensive patients have statistically significantly higher levels of CD45−/CD34−/CD90+ in their peripheral blood. This increased mobilization of MSCs might be implicated in the pathological process of essential hypertension. Notably, the number of those cells reveal a strong correlation with LVMI that was not dependent on the level of systolic BP, suggesting that they could be involved in the pathophysiology of cardiac hypertrophy and hypertension.
Stem cells have the potential for self‐renewal and differentiation and are the origin cells of various mature cells. Stem and progenitor cells have great clinical significance in the pathophysiology of hypertension, as well as in its progression to atheromatosis.18 Previous studies have shown that they are implicated in ventricular remodeling10 and have great clinical significance in the homeostasis of the heart,19 as well as in the pathophysiology of heart failure.20 In addition, mobilization of MSCs leads to intimal hyperplasia of the vessels and arteriopathy.21 However, there are limited data regarding the involvement of MSCs in the pathophysiology of essential hypertension, with or without target organ damage.
MSCs are present as a rare population of cells in bone marrow, representing perhaps 0.001% to 0.01% of the nucleated cells, and may express different surface molecules. These cells are characterized or defined using a set of cell surface markers. They are generally positive for CD44, CD90, and CD105 and are negative for hematopoietic markers CD34 and CD45. Evidence suggests that MSCs can also express phenotypic characteristics of endothelial, neural, and smooth muscle cells, skeletal myoblasts, and cardiac myocytes, and have a highly plastic differentiation potential.22 Previous studies have proved that a number of stem cells circulate in the peripheral blood and can be mobilized by cytokine stimulation under specific pathophysiological conditions.23, 24
The precise origin and characterization of cardiac progenitor cells remain unclear. Endogenous cardiac progenitor cells seem to be responsible for the homeostasis of the stem cell pool in hearts under physiological conditions.25 However, there are data from animal studies suggesting that these cardiac progenitor cells have a very low proliferating potency of cardiac stem cells, at least under normal circumstances.26 One previous study provided evidence that extra‐cardiac stem cells may originate from bone marrow but can transform into cardiac progenitor cells in response to cues from the myocardial environment.26 There are several indications that some cardiac progenitor cells might originate from bone marrow, since they have some characteristics of bone marrow stem cells,27, 28 and bone marrow stem cells can differentiate into cardiomyocytes in vivo and in vitro.29, 30, 31
A recent study from our department has shown increased expression of myocardin and GATA4 genes in the peripheral blood mononuclear cell fraction of hypertensive patients, implying the presence of mesenchymal progenitor cells in the peripheral blood that are possibly intended to differentiate into cells of the cardiac series.12 Interestingly, in the patients in that study, myocardin and GATA4 expression was associated with both BP levels and LVH. In a similar manner, we found altered expression of early cardiac marker genes and differentiation‐specific marker genes in the peripheral blood mononuclear cell fraction of patients with hypertrophic cardiomyopathy compared with control individuals, possibly reflecting changes in response to disease.32 The results of the present study add to these findings, demonstrating a mobilization of MSC populations in the peripheral blood that could possibly play a role in target organ damage. We found that the number of those cells in the peripheral blood was correlated with the degree of LVH. Despite intense efforts to determine the pathogenesis of LVH in arterial hypertension, this process remains poorly understood. Our findings indicate that the increased mobilization of bone marrow–derived MSCs may ultimately be targeted at structural changes in the hypertensive myocardium. However, whether the movement and homing of these cells into the myocardium is followed by differentiation into cardiomyocytes, leading to hypertrophy, remains to be proven by further research.
The presence of stem and progenitor cells in the peripheral blood of adult humans has clinical implications for the pathophysiology of cardiovascular disease and has evoked much hope regarding their possible application in treatment. However, several problems have arisen that need to be resolved before the clinical use of stem cells can become a part of regular practice. Mobilization of MSCs has been demonstrated in other pathological cardiovascular conditions, but most research has been done in acute ischemic syndromes. Iso and colleagues33 identified a population of CD271+ MSCs released into peripheral blood in acute myocardial infarction, reaching a peak number after 3 days and returning to levels comparable to those in healthy patients by 1 week.
An abnormal increase of left ventricular mass in cardiovascular diseases is an important prognostic indicator and therapeutic target. Hypertension is the most common cause of LVH. This hypertrophic process is initially adaptive, since it helps the heart to maintain normal pump performance in the face of the increased afterload. However, this leads to maladaptive changes, the most important being the growth in the fibrillar collagen network and abnormalities of excitation‐contraction coupling within the hypertrophied cardiomyocytes. Increased left ventricular mass predicts cardiovascular complications of hypertension and an increased risk for morbidity and mortality.34 However, there are limited data regarding the involvement of MSCs in the pathogenetic process of LVH. The increase in the myocardial mass of both ventricles is the result not only of the increase in protein synthesis and cell size, but also of the proliferating cardiac progenitor cells and the influx of bone marrow–derived cells developing into cardiomyocytes.35 It is known that an increase in preload leads to the mobilization of progenitor cells from bone marrow for use in neovascularization, which plays an important role in cardiac hypertrophy.10
The precise origin of cardiac progenitor cells is still unclear. Although their recruitment from bone marrow in essential hypertension with target organ damage is a likely scenario, further clinical and experimental studies will be needed to elucidate the significance of our findings and the role of MSCs in the pathophysiological changes that occur in hypertension. However, these findings agree with our previous understanding, according to which MSCs may be involved in ventricular or arterial remodeling and indicate possible new approaches aimed in that direction.
Study Limitations
Our study has several limitations. We included only a small number of patients. However, our findings were clear and indicative. It is possible that the inclusion of more patients would have revealed further correlations in addition to those derived from the present analysis. Also, we did not provide a mechanistic explanation of our data and whether the MSC populations we studied differed between groups, not only in their degree of mobilization, but also in their functional properties. In addition, we did not establish whether the increased number of circulating CD45−/CD34−/CD90+ resulted in their recruitment and homing into the myocardium. In such a case, we cannot know whether those cells would differentiate into myocardial cells or into nonmyocyte cardiac cells. The determination of their precise role remains a subject for future extensive research. Finally, our study was not designed to investigate whether the effect of antihypertensive medication that leads to BP control and a possible improvement in LVH would also have an effect on the mobilization of MSCs from bone marrow.
Conclusions
Patients with essential hypertension exhibit an increased number of circulating CD45−/CD34−/CD90+ cells, making up a population of MSCs, in comparison with normotensive patients. This number is correlated with the degree of LVH, as expressed by the LVMI. The clinical significance of these findings will require further elucidation. However, our study is the first to provide indicative data from humans suggesting an increased mobilization of bone marrow–derived MSCs in patients with essential hypertension, which becomes more prominent in patients with LVH.
Disclosures
The authors declare no conflict of interest.
Acknowledgments
The study was supported by the European Commission (EC) support program Translational Potential (TransPOT; EC contract number 285948).
J Clin Hypertens (Greenwich). 2014;16:883–888. DOI: 10.1111/jch.12426. © 2014 Wiley Periodicals, Inc.
References
- 1. Mollova M, Bersell K, Walsh S, et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci USA. 2013;110:1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gojo S, Gojo N, Takeda Y, et al. In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp Cell Res. 2003;288:51–59. [DOI] [PubMed] [Google Scholar]
- 4. Kobayashi T, Hamano K, Li TS, et al. Enhancement of angiogenesis by the implantation of self bone marrow cells in a rat ischemic heart model. J Surg Res. 2000;89:189–195. [DOI] [PubMed] [Google Scholar]
- 5. Tomita S, Li RK, Weisel RD, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–II256. [DOI] [PubMed] [Google Scholar]
- 6. Sato T, Iso Y, Uyama T, et al. Coronary vein infusion of multipotent stromal cells from bone marrow preserves cardiac function in swine ischemic cardiomyopathy via enhanced neovascularization. Lab Invest. 2011;91:553–564. [DOI] [PubMed] [Google Scholar]
- 7. Tondreau T, Meuleman N, Delforge A. Mesenchymal stem cells derived from CD133‐positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. [DOI] [PubMed] [Google Scholar]
- 8. Noort WA, Oerlemans MI, Rozemuller H, et al. Human versus porcine mesenchymal stromal cells: phenotype, differentiation potential, immunomodulation and cardiac improvement after transplantation. J Cell Mol Med. 2012;16:1827–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anversa P, Kajstura J, Rota M, et al. Regenerating new heart with stem cells. J Clin Invest. 2013;123:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Müller P, Kazakov A, Semenov A, et al. Pressure‐induced cardiac overload induces upregulation of endothelial and myocardial progenitor cells. Cardiovasc Res. 2008;77:151–159. [DOI] [PubMed] [Google Scholar]
- 11. Sopko NA, Turturice BA, Becker ME, et al. Bone marrow support of the heart in pressure overload is lost with aging. PLoS One. 2010;5:e15187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kontaraki JE, Marketou ME, Zacharis EA, et al. Early cardiac gene transcript levels in peripheral blood mononuclear cells in patients with untreated essential hypertension. J Hypertens. 2011;29:791–797. [DOI] [PubMed] [Google Scholar]
- 13. Yamada T, Kondo T, Numaguchi Y, et al. Angiotensin II receptor blocker inhibits neointimal hyperplasia through regulation of smooth muscle‐like progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27:2363–2369. [DOI] [PubMed] [Google Scholar]
- 14. Mancia G, de Backer G, Dominiczak A, et al. Guidelines for the Management of Arterial Hypertension. The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1751–1762. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 16. Schiller N, Shah P, Crawford M, et al. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 17. Devereux R, Reichek N. Echocardiographic determination of left ventricular mass in man: anatomic validation of the method. Circulation. 1977;55:613–618. [DOI] [PubMed] [Google Scholar]
- 18. Marketou ME, Vardas PE. Stem cells and hypertension: another challenge for the future? Hellenic J Cardiol. 2011;52:289–292. [PubMed] [Google Scholar]
- 19. Torella D, Ellison GM, Méndez‐Ferrer S, et al. Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3:S8–S13. [DOI] [PubMed] [Google Scholar]
- 20. Koudstaal S, Jansen O, Lorkeers SJ, et al. Concise review: heart regeneration and the role of cardiac stem cells. Stem Cells Transl Med. 2013;2:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Oostrom O, Fledderus JO, de Kleijn D, et al. Smooth muscle progenitor cells: friend or foe in vascular disease? Curr Stem Cell Res Ther. 2009;4:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. [DOI] [PubMed] [Google Scholar]
- 23. Kucia M, Ratajczak J, Ratajczak MZ. Bone marrow as a source of circulating CXCR4 + tissue‐committed stem cells. Biol Cell. 2005;97:133–146. [DOI] [PubMed] [Google Scholar]
- 24. Wojakowski W, Tendera M, Michalowska A, et al. Mobilization of CD34 + /CXCR4 + , CD34‐/CD117 + , c‐met+ stem cells, and mononuclear cells expressing early cardiac muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. [DOI] [PubMed] [Google Scholar]
- 25. Mouquet F, Pfister O, Jain M, et al. Restoration of cardiac progenitor cells after myocardial infarction by self‐proliferation and selective homing of bone marrow‐derived stem cells. Circ Res. 2005;97:1090–1092. [DOI] [PubMed] [Google Scholar]
- 26. Li TS, Suzuki R, Ueda K, et al. Analysis of the origin and population dynamics of cardiac progenitor cells in a donor heart model. Stem Cells. 2007;25:911–917. [DOI] [PubMed] [Google Scholar]
- 27. Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. [DOI] [PubMed] [Google Scholar]
- 28. Pfister O, Mouquet F, Jain M, et al. CD31‐ but not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. [DOI] [PubMed] [Google Scholar]
- 29. Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. [DOI] [PubMed] [Google Scholar]
- 31. Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. [DOI] [PubMed] [Google Scholar]
- 32. Kontaraki JE, Parthenakis FI, Patrianakos AP, et al. Altered expression of early cardiac marker genes in circulating cells of patients with hypertrophic cardiomyopathy. Cardiovasc Pathol. 2007;16:329–350. [DOI] [PubMed] [Google Scholar]
- 33. Iso Y, Yamaya S, Sato T, et al. Distinct mobilization of circulating CD271+ mesenchymal progenitors from hematopoietic progenitors during aging and after myocardial infarction. Stem Cells Transl Med. 2012;1:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verdecchia P, Carini G, Circo A, et al; MAVI (MAssa Ventricolare sinistra nell'Ipertensione) Study Group. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol. 2001;38:1829–1835. [DOI] [PubMed] [Google Scholar]
- 35. Castellani C, Padalino M, China P, et al. Bone‐marrow–derived CXCR4‐positive tissue‐committed stem cell recruitment in human right ventricular remodeling. Hum Pathol. 2010;41:1566–1576. [DOI] [PubMed] [Google Scholar]


