Abstract
Resistant hypertension (RH) is associated with organ damage and cardiovascular risk. Evidence suggests the involvement of matrix metalloproteinase 2 (MMP‐2) and tissue inhibitor of metalloproteinase 2 (TIMP‐2) in hypertension and in cardiovascular remodeling. The aim of this study was to assess the levels of MMP‐2 and TIMP‐2 in RH and its relation with organ damage, including arterial stiffness and cardiac hypertrophy. MMP‐2 and TIMP‐2 levels were compared among 19 patients with normotension (NT), 116 with nonresistant hypertension (HTN) and 116 patients with resistant HTN (RH). MMP‐2 levels showed no differences among NT, HTN, and RH groups, while TIMP‐2 levels were higher in RH compared with HTN and NT groups (90.0 [76.1–107.3] vs 70.1 [57.7–88.3] vs 54.7 [40.9–58.1] ng/mL, P<.01), respectively. MMP‐2/TIMP‐2 ratio was reduced in the RH group compared with the HTN and NT groups (2.7 [1.9–3.4] vs 3.3 [2.6–4.2] vs 4.9 [4.5–5.3], P<.01), respectively. No associations were found between MMP‐2 levels, TIMP‐2, and MMP‐2/TIMP‐2 ratio with cardiac hypertrophy and arterial stiffness in the RH and HTN groups. Finally, in a regression analysis, reduced MMP‐2/TIMP‐2 ratio and increased TIMP‐2 levels were independently associated with RH. The present findings provide evidence that TIMP‐2 is associated with RH and might be a possible biomarker for screening RH patients.
Resistant hypertension (RH) is a multifactorial condition associated with mortality,1 greater risk of cardiovascular events,2 and higher incidence of target organ damage.3, 4 Target organ damage in RH is closely associated with lack of blood pressure (BP) control and cardiovascular remodeling.3, 5 It requires the activation of intracellular pathways that lead to (1) proliferation, migration, and apoptosis of cells; and (2) synthesis and degradation of extracellular matrix (ECM) proteins of cardiac and vascular tissues.6, 7, 8
Closely linked to the ECM reorganization, the matrix metalloproteinases (MMPs) are zinc‐dependent endopeptidases that degrade several proteins in cardiac and vascular tissues. The activity of the MMPs is affected by endogenous inhibitors, such as tissue inhibitors of metalloproteinases (TIMPs) and α2‐macroglobulin (A2M), a nonspecific inhibitor of circulating proteases. Therefore, the balance between MMPs and inhibitors are essential for the extracellular matrix remodeling in tissues.9
Gelatinases, such as MMP‐2 and MMP‐9, have been associated with HTN and target organ damage (TOD).10, 11 MMP‐2 expression is upregulated in cardiac cells in response to many elements such as angiotensin II, endothelin‐1, proinflammatory cytokines, hormones, and growth factors.12 It has a role in cleaving ECM molecules, elastin, and type IV collagen and digests interstitial collagen types I, II, and II.9 In addition, MMP‐2 is highly expressed in almost all cardiac cells and can be activated by reactive oxygen species.13 Therefore, MMP‐2 is one of the most studied MMPs in essential HTN. Nonetheless, the relation between MMP‐2 and TIMP‐2 levels in RH remains unclear. The aim of this study was to assess the levels of MMP‐2 and TIMP‐2 in RH and its association with TOD (arterial stiffness and cardiac hypertrophy).
Methods
Patients and Study Design
For this cross‐sectional study, patients with RH regularly followed at the Resistant Hypertension Clinic of the University of Campinas (Campinas, Brazil) and those with nonresistant hipertension (HTN) followed at the Hypertension Clinic of Valinhos (Brazil) were screened. The study was conducted at the aforementioned center, reviewed, and approved by the institutional review board from the School of Medical Sciences of University of Campinas, Campinas‐Brazil (approval number 188.161/2013) and conducted in accordance with the Declaration of Helsinki Principles. In addition, written informed consent was obtained from all participants.
Inclusion and Exclusion Criteria
The inclusion criteria were: (1) diagnosis of nonresistant or resistant HTN;14 (2) volunteer participation and informed consent signature; (3) regular follow‐up for at least 6 months; and (4) previous proven adherence to nonpharmacologic and pharmacologic treatment.
After 6 months of follow‐up, patients were properly diagnosed as having RH or HTN. RH was defined as BP that remained above the target pressure (≥140/90 mm Hg) despite the use of three or more different classes of antihypertensive drugs at optimal doses or controlled BP with the use of four or more antihypertensive drugs.15 To identify true resistance to treatment and exclude white‐coat HTN, pseudoresistance, lack of medication adherence, and secondary forms of HTN, RH patients underwent drug therapy optimization and pill counts, office BP, ambulatory BP monitoring (ABPM) measurements,5 and imaging and biochemical examinations. The HTN patients were defined as all hypertensive patients without resistance to treatment.14
The following points were taken into account for exclusion criteria: (1) medical history or clinical symptoms of heart failure (ejection fraction <50%); (2) presence of cardiomyopathy; (3) valvular or pericardial heart diseases; (4) diagnosis of cerebrovascular disease or peripheral vascular disease; (5) nephropathy; (6) liver dysfunction; (7) autoimmune diseases; and (8) pregnancy or declared intention to become pregnant.
All hypertensive volunteers were recruited consecutively and were divided into two groups: HTN (n=116) and RH (n=116), according to the above‐mentioned criteria (Figure 1). Nineteen patients with normotension (NT) were randomly selected to be included as the control group (n=19).
Figure 1.
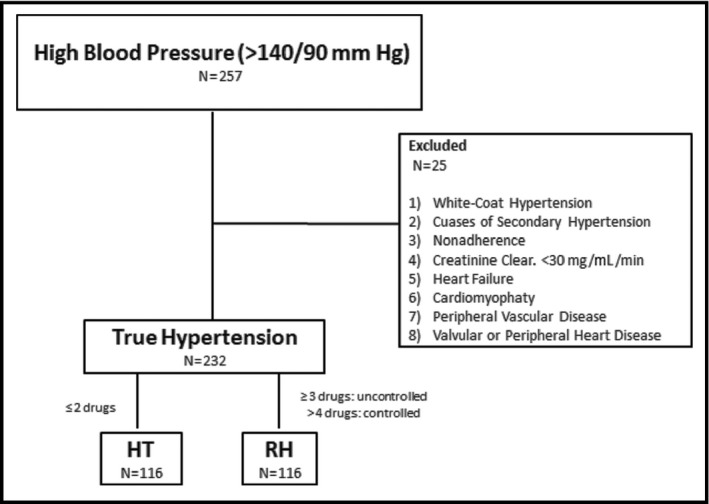
Screening flow and final sample size of resistant hypertension (RH) and nonresistant hypertension (HTN) patients.
BP Measurements
To determine office systolic BP (SBP) and diastolic BP (DBP) a trained health professional performed at least three measurements with a 3‐minute interval. Patients were monitored in a seated position after a 10‐minute rest, at approximately 8 am, using a digital BP monitor (OMRON Healthcare Inc, Bannockburn, IL).
ABPM was used to exclude pseudoresistance HTN through 24‐hour BP monitoring by an automatic oscillometric device (Spacelabs 90207, Spacelabs Inc, Redmond, WA). BP was measured automatically at 20‐minute intervals during a 24‐hour period and patients were instructed to keep their normal daily activities and take notes on their sleep period in a personal diary.16
Aortic PWV Measurement
Pulse wave velocity (PWV) measurements were obtained with the Sphygmocor system (Artcor, Sidney, Australia) and used to estimate arterial stiffness. Pulse waves were obtained via transcutaneous using a tonometer at the right common carotid and femoral arteries. PWV was calculated as the ratio of the distance (m)/Δt (seconds) between the femoral recording site and the suprasternal notch minus the distance from the suprasternal notch to the carotid recording site.17 Three consecutive readings were collected to determine the PWV average value that was used in analyses.
Echocardiography
The dimensions of the left ventricular (LV) diastolic and systolic diameters and interventricular septal and posterior wall thicknesses were measured at the end of diastole using the two‐dimensional targeted M‐mode, and LV mass was calculated by the Devereux formula, according to the recommendations of the American Society of Echocardiography.18 LV mass index (LVMI) was calculated by dividing the LV mass by the body surface area. Echocardiographic measurements were performed by two blinded investigators using a cardiovascular ultrasound machine (Siemens Acuson CV70, Munich, Bavaria, Germany) with multifrequency sector transducer (2–4 MHz).
Biochemical Measurements
Blood samples were collected at early morning after an overnight fast. Plasma aldosterone was measured by radioimmunoassay (Immunotech SAS, Marseille, France) according to the manufacturer's instructions. Urine albumin‐to‐creatinine ratio (mg/g) was used to evaluate microalbuminuria. Patients presenting with values <30 mg/g were classified as normal and microalbuminuria was classified at values between 30 and 300 mg/g.
MMP‐2 and TIMP‐2 Measurements
Heparin‐anticoagulated blood samples were obtained after an overnight fasting period by venipuncture. The blood samples were centrifuged and plasma samples were immediately stored at −80°C. MMP‐2 and TIMP‐2 were measured by enzyme‐linked immunosorbent assay (ELISA) (R&D Systems, Inc., Minneapolis) according to the manufacturer's instructions.
Statistical Analysis
Descriptive statistics were described as mean±standard deviation or median and first–third quartiles, according to data distribution. The data distribution was assessed by Shapiro‐Wilk test. Continuous variables in all groups were compared by one‐way analysis of variance. Mann‐Whitney test was performed to compare nonparametric continuous variables, and Fisher test for categorical variables. Correlation analyses were performed by Spearman's correlation coefficient. Logistic regression analyses evaluated the association of MMP‐2, TIMP‐2, and MMP‐2/TIMP‐2 ratio, adjusted for potential confounders, with the presence of resistance to treatment. Finally, a given probability of type I error (α) of 0.05 was considered and a power (1−β) of 0.9 for TIMP‐2 analyses.
Results
The general characteristics and biochemical parameters of NT, HTN, and RH groups are shown in Table 1 and Table 2, respectively. RH patients presented with higher left ventricular mass index, microalbuminuria, cholesterol and low‐density lipoprotein levels, glycated hemoglobin, glucose, microalbuminuria, C‐reactive protein, and aldosterone levels and lower PWV compared with the HTN patients. The NT group had lower biochemical levels compared with the HTN group. Correlation analyses revealed no associations between aldosterone/renin ratio and TIMP‐2, MMP‐2, and MMP‐2/TIMP‐2 in either all hypertensive groups (RH+HTN) or the RH group (data not shown).
Table 1.
General Characteristics of Normotensive, Nonresistant Hypertensive, and Resistant Hypertensive Patients
| Variables | NT (n=19) | HTN (n=116) | RH (n=116) | P Value |
|---|---|---|---|---|
| Age, y | 52±5 | 62±9a | 60±10a | <.01 |
| Female sex % | 47 | 60 | 66 | .25 |
| Black race % | – | 15 | 43b | <.01 |
| Diabetes, % | – | 35 | 52b | .02 |
| BMI, kg/m2 | 25.0 (23.1–27.0) | 27.7 (24.9–31.7)a | 31.2 (27.0–34.9)a,b | <.01 |
| Waist/hip ratio | – | 0.91 (0.86–0.97) | 0.94 (0.88–0.99)b | .04 |
| Office SBP, mm Hg | 119 (111–134) | 139 (130–148)a | 146 (134–158)a,b | <.01 |
| Office DBP, mm Hg | 80 (72–86) | 82 (78–86) | 82 (76–91) | .19 |
| Office HR, beats per min | 64 (60–70) | 67 (61–77) | 66 (58–74) | .19 |
| ABPM SBP, mm Hg | – | 127 (121–135) | 129 (116–141) | .40 |
| ABPM DBP, mm Hg | – | 77 (72–82) | 76 (70–84) | .75 |
| LVMI, g/m2 | – | 96.3 (86.4–113.0) | 115.1 (95.0–142.0)b | <.01 |
| PWV, m/s | 7.1 (6.5–7.7) | 9.4 (8.4–11.3)a | 9.1 (7.6–11.1)a | <.01 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; BMI, body mass index; DBP, diastolic blood pressure; HR, heart rate; HTN, nonresistant hypertension; LVMI, left ventricular mass index; NT, normotension; PWV, pulse wave velocity; RH, resistant hypertension; SBP, systolic blood pressure. Data expressed as mean±standard deviation or median (first–third quartiles). a P<.05 vs NT. b P<.05 vs HTN.
Table 2.
Biochemical Parameters of Normotensive, Nonresistant Hypertensive, and Resistant Hypertensive Patients
| Variables | NT (n=19) | HTN (n=116) | RH (n=116) | P Value |
|---|---|---|---|---|
| Total cholesterol, mg/mL | 193 (179–215) | 165 (136–186)a | 180 (148–208)a,b | <.01 |
| LDL, mg/mL | 112 (93–133) | 85 (66–107)a | 97 (77–127)b | <.01 |
| HDL, mg/mL | 47 (40–56) | 49 (42–58) | 45 (38–53)b | .02 |
| HbA1c, % | 5.2 (5.1–5.3) | 6.0 (5.7–6.4)a | 6.3 (6.0–7.8)a,b | <.01 |
| Triglycerides | 134 (97–154) | 115 (80–157) | 130 (95–192) | .08 |
| Glucose, mg/mL | 82.2 (80.0–88.0) | 97 (90–107)a | 101 (89–136)a | <.01 |
| Creatinine, mg/mL | 0.9 (0.8–1.0) | 0.9 (0.8–1.1) | 1.0 (0.8–1.2) | .08 |
| Microalbuminuria, mg/g | 6.7 (3.5–9.3) n=19 | 5.8 (3.8–12.6) n=34 | 13.2 (7.6–45.9) n=74a,b | <.01 |
| C‐reactive protein, mg/dL | 0.09 (0.07–0.1) | 0.3 (0.1–0.6)a | 0.4 (0.1–0.6)a | <.01 |
| Aldosterone, pg/mL | 69.6 (63.5–73.0) | 67.5 (42.5–114.5) | 98 (60.7–176.9)a,b | <.01 |
| Aldosterone/renin | – | 1.68 (0.77–4.92) (n=72) | 3.99 (1.29–9.49) (n=17) | .08 |
Abbreviations: HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; HTN, nonresistant hypertension; LDL, low‐density lipoprotein; NT, normotension; RH, resistant hypertension. Data are expressed as median (first–third quartiles). a P<.05 vs NT. b P<.05 vs HTN.
As expected, a higher number of antihypertensive drugs were used in the RH (4±1) compared with the HTN (2±1) group (Table 3. No statistical differences in MMP‐2 levels were observed among the NT, HTN, and RH groups (240.1 [191.2–308.4] vs 237.0 [197.2–276.6] vs 228.9 [203.9–293.5] ng/mL, P=.65), respectively (Figure 2a). On the other hand, increased TIMP‐2 levels were detected in the RH compared with the HTN and NT groups (90.0 [76.1–107.3] vs 70.1 [57.7–88.3] vs 54.7 [40.9–58.1] ng/mL, P<.01), respectively (Figure 2b). In addition, a lower MMP‐2/TIMP‐2 ratio was found in the RH group compared with the HTN and NT (2.7 [1.9–3.4] vs 3.3 [2.6–4.2] vs 4.9 [4.5–5.3], P<.01), respectively (Figure 2c).
Table 3.
Antihypertensive Drugs, Statins, and Acetylsalicylic Acid Use in Both Hypertensive Groups
| Drugs, % | HTN (n=116) | RH (n=116) | P Value |
|---|---|---|---|
| Diuretics | 65 | 90 | <.01 |
| Spironolactone | 02 | 38 | <.01 |
| β‐Blockers | 14 | 68 | <.01 |
| Angiotensin‐converting enzyme inhibitors | 17 | 38 | <.01 |
| Angiotensin receptor blockers | 73 | 55 | <.01 |
| Calcium channel blockers | 41 | 85 | <.01 |
| Sympatholytic drugs | 00 | 28 | <.01 |
| Statins | 73 | 58 | .01 |
| Acetylsalicylic acid | 16 | 59 | <.01 |
Abbreviations: HTN, nonresistant hypertension; RH, resistant hypertension.
Figure 2.
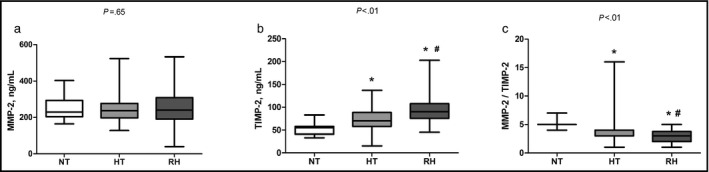
Plasma concentrations of matrix metalloproteinase 2 (MMP‐2) and tissue inhibitor of metalloproteinase 2 (TIMP‐2) levels in patients with normotension (NT), nonresistant hypertension (HTN), and resistant hypertension (RH).(a) MMP‐2 levels; (b) TIMP‐2 levels; (c) MMP‐2/TIMP‐2 ratio. The box and whisker plots show range and quartiles. *P<.05 vs NT. # P<.05 vs HTN.
Due to the higher prevalence of TOD in patients with RH and its association with increased mortality, we evaluated MMP‐2 and TIMP‐2 levels in both HTN groups and the association of plasma levels with cardiac hypertrophy and arterial stiffness (well‐known HTN‐induced TOD). However, after correlation analysis, no associations were found between PWV and LVMI (reflecting arterial stiffness and cardiac hypertrophy) with MMP‐2, TIMP‐2, and MMP‐2/TIMP‐2 ratio in HTN or RH groups (Table 4). On the other hand, correlation analysis combining both hypertensive groups (HTN+RH) revealed a positive correlation between TIMP‐2 and LVMI (Table 4).
Table 4.
MMP‐2, TIMP‐2, and MMP‐2/TIMP‐2 Correlation With Target Organ Damage in Nonresistant and Resistant Hypertension
| MMP‐2 r (P Value) | TIMP‐2 r (P Value) | MMP‐2/TIMP‐2 r (P Value) | |
|---|---|---|---|
| HTN | |||
| PWV | −0.15 (.10) | −0.15 (.11) | 0.07 (.46) |
| LVMI | −0.01 (.93) | −0.09 (.34) | 0.05 (.61) |
| RH | |||
| PWV | 0.02 (.81) | −0.005 (.95) | 0.01 (.92) |
| LVMI | 0.10 (.30) | 0.17 (.07) | −0.05 (.62) |
| HTN+RH | |||
| PWV | −0.05 (.42) | −0.09 (.17) | 0.06 (.34) |
| LVMI | 0.06 (.34) | 0.16 (.02) | −0.09 (.18) |
Abbreviations: HTN, nonresistant hypertension; LVMI, left ventricular hypertrophy; MMP‐2, matrix metalloproteinase 2; PWV, pulse wave velocity; RH, resistant hypertension; TIMP‐2, tissue inhibitor of metalloproteinase 2.
A correlation analysis among MMP‐2, TIMP‐2, MMP‐2/TIMP‐2 ratio, and ambulatory BP measurements (systolic and diastolic) in the hypertensive groups (RH+HTN) were performed. While TIMP‐2 showed no significant correlation with systolic and diastolic ABPM values, a significant correlation between MMP‐2/TIMP‐2 and systolic ABPM (r=0.15, P=.03) was detected. In addition, a correlation between MMP‐2 levels with both systolic (r=0.24, P<.01) and diastolic (r=0.15, P=.04) ABPM was also found.
Finally, the different models of logistic regression demonstrated that TIMP‐2 levels and MMP‐2/TIMP‐2 ratio were independently associated with resistance to antihypertensive treatment, after adjustment for age, sex, aldosterone levels, presence of diabetes, body mass index, and race (Table 5). The same results were observed when the logistic regression was performed including the NT group (NT+HTN+RH) (TIMP‐2: odds ratio 1.04; 95% confidence interval, 1.02–1.05 [P<.01] and MMP‐2/TIMP‐2: odds ratio, 0.64; 95% confidence interval, 0.49–0.84 [P<.01]).
Table 5.
Logistic Regression for the Presence of Resistance to Treatmenta
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
| TIMP‐2 | 1.03 (1.02–1.05) | <.01 | MMP‐2/TIMP‐2 | 0.67 (0.51–0.87) | <.01 |
| BMI, kg/m² | 1.14 (1.07–1.22) | <.01 | BMI, kg/m² | 1.14 (1.07–1.21) | <.01 |
| Black race | 3.54 (1.63–7.68) | <.01 | Black race | 3.92 (1.84–8.31) | <.01 |
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio; MMP‐2, matrix metalloproteinase 2; TIMP‐2, tissue inhibitor of metalloproteinase 2. a Adjusted for age, sex, aldosterone levels, and presence of diabetes. The analysis was performed including resistant and nonresistant hypertension.
Discussion
In this study we found higher levels of TIMP‐2 and also lower MMP‐2/TIMP‐2 ratio in RH patients compared with HTN and NT patients. Interestingly, MMP‐2 levels were similar among all strata of HTN studied. In addition, high TIMP‐2 levels and low MMP‐2/TIMP‐2 ratio were independently associated with resistance to treatment in RH patients. Also, TIMP‐2 levels were correlated with LVMI in the total HTN group, but not in the RH group. A review of the literature was performed using PubMed and Web of Science and, to the best of our knowledge, this is the first study comparing MMP‐2 and TIMP‐2 levels among NT, HTN, and RH patients.
TIMP‐2 levels have been reported to be unchanged in HTN patients compared with controls.19, 20, 21 More conflicting findings have been reported on MMP‐2 levels in HTN. Some studies have found increased,22, 23 decreased,24, 25 and even unchanged MMP‐2 levels20, 21 in HTN. This inconsistency may be justified by the differences in (1) study design, (2) methods of measurement (ELISA, immunohistochemistry, or zymography), (3) study population and the presence of comorbidities and TOD,20, 26, 27 and (4) statistical power.
MMP‐2 is expressed in almost all cardiac cells. It is activated by oxidative stress injury and in long‐term and chronic processes may transform into an irreversible injury. Recently, an interesting study showed the influence of MMP‐2 levels in both animal and human cardiovascular disease,28 suggesting MMPs as potential therapeutic targets in myocardial diseases.29 Although some data show increased MMP‐2 levels in myocardial diseases, one study found decreased levels in hypertensive patients with LV hypertrophy. The low levels support that such alteration may contribute to the phenotypic and structural changes in hypertensive heart disease.20
Experimental and clinical studies have shown the influence of TIMP‐2 levels in cardiac remodeling. For instance, it has been shown that TIMP‐2 deficiency is related to exacerbated LV dilation and dysfunction.30, 31, 32 Such scarcity impairs cardiac function, since TIMP‐2 is required for optimal structure and function following biomechanical stress.33 On the other hand, it was reported that TIMP‐2 increases collagen synthesis34 and also stimulates cardiac fibroblast proliferation and differentiation into myofibroblasts, contributing to the mature collagen matrix formation.35 In addition, increased TIMP‐2 levels were found in patients with hypertrophic cardiomyopathy,36 suggesting that TIMP‐2 levels may reflect an adaptive response to dampen the elevated proteolytic activity that occurs in heart disease.31
The literature shows controversial associations between MMP‐2 and TIMP‐2 levels and TOD. The high prevalence of TOD is a well‐known feature in RH; however, no associations were found between arterial stiffness and LVMI with MMP‐2, TIMP‐2, and MMP‐2/TIMP‐2 ratio in both antihypertensive groups analyzed separately (resistant and nonresistant). Interestingly, a positive correlation was observed between TIMP‐2 levels and LVMI in the total group (HTN+RH). It may suggest a compensatory response of CV remodeling in an attempt to control MMP‐2 activity and minimize CV damage in HTN. Although MMP‐2 levels were similar between RH and HTN groups, the decreased ratio observed in RT patients might reflect lower activity of MMP‐2. TIMP‐2 binds to MMP‐2, inhibiting its proteolytic activity, thus the MMP‐2/TIMP‐2 ratio is also an important index for assessing MMP‐2 activity. Interestingly, the MMP‐2/TIMP‐2 ratio was decreased in RH patients, which might indicate a reduced global action of the MMP‐2 enzyme.
Logistic regression analysis revealed that TIMP‐2 levels, but not MMP‐2 levels, are significantly independently associated with RH after adjustment for risk factors for RH (age, sex, race, diabetes, body mass index, aldosterone levels). Moreover, the RH group was divided into white and nonwhite and obese and nonobese groups to analyze the influence on TIMP‐2 levels. No differences were found in TIMP‐2 levels according to race and body mass index, which strengths the association between high TIMP‐2 levels and RH found in the current study.
We have shown in this study that TIMP‐2 is a significant predictor for RH. Considering the lack of correlation between TIMP‐2 and ambulatory BP (RH+HTN) this finding may not represent a continuum of BP, and TIMP‐2 levels may represent an important role in the pathophysiology of RH. Although resistant and nonresistant HTN represent distinct conditions, systolic and diastolic ABPM values were similar between HTN groups in this study. Interestingly, MMP‐2 levels and MMP‐2/TIMP‐2 were positively correlated with ABPM, suggesting that MMP‐2 is associated with BP in a continuum manner but not with RH phenotype. Although it might sound paradoxical, these findings lead to the interpretation that RH represents a distinct hypertensive phenotype. TIMP‐2 levels are associated with RH phenotype and might be explored as a possible biomarker of this condition in further studies.
Study Strengths and Limitations
In spite of reaching interesting findings, our study has some drawbacks. First, the possible influence of multiple antihypertensive drugs is an intrinsic limitation of studies on resistant HTN. Some antihypertensive drugs may affect MMP concentrations,37, 38 as well as statins,39, 40 acetylsalicylic acid,41, 42 and some diuretics such as spironolactone and hydrochlorothiazide.43 Multiple linear regression showed no association between TIMP‐2 levels and antihypertensive medication, statins, acetylsalicylic acid, and antidiabetics. Thus, the medication might not have affected our results. Second, since the analysis of aldosterone/renin ratio was performed only in a subset of RH (n=72) and HTN (n=17) patients, our negative finds might be underestimated. Third, the sample size was not large enough to ensure more robust inferences. Fourth, there was a low power of the study in MMP‐2 analyses. Finally, this was a cross‐sectional study and a cause‐effect relationship cannot be inferred. Therefore, further studies with different designs, such as randomized controlled trials or cohorts, are needed to clarify some points and also to strength our findings.
Our regression analysis model included well‐known factors that contribute to increased risk for RH. In spite of that, we were still able to detect a significant association between TIMP‐2 and RH.
Conclusions
RH was associated with higher TIMP‐2 levels and low MMP‐2/TIMP‐2 ratio, suggesting that these regulators of ECM remodeling play a key role in BP control in this high‐risk subset of hypertensive individuals. These findings provide evidence that TIMP‐2 is associated with RH and might be a candidate risk biomarker for RH.
Disclosure
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
Acknowledgments
This study was supported by the Sao Paulo Research Foundation (FAPESP), Sao Paulo, Brazil; the National Council for Scientific and Technological Development (CNPq); and Coordination for the Improvement of Higher Education Personnel (Capes), Brazil.
J Clin Hypertens (Greenwich). 2016;18:969–975. DOI: 10.1111/jch.12865 © 2016 Wiley Periodicals, Inc.
References
- 1. Fatemi O, Goa C, Faselis C, et al. Improvement in all‐cause mortality with blood pressure control in a group of US veterans with drug‐resistant hypertension. J Clin Hypertens (Greenwich). 2016;18:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sabbatini AR, Faria AP, Barbaro NR, et al. Deregulation of adipokines related to target organ damage on resistant hypertension. J Hum Hypertens. 2014;28:388–392. [DOI] [PubMed] [Google Scholar]
- 4. Lotufo PA, Pereira AC, Vasconcellos PS, et al. Resistant hypertension: risk factors, subclinical atherosclerosis, and comorbidities among adults‐the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil). J Clin Hypertens (Greenwich). 2015;17:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martins LC, Figueiredo VN, Quinaglia T, et al. Characteristics of resistant hypertension: ageing, body mass index, hyperaldosteronism, cardiac hypertrophy and vascular stiffness. J Hum Hypertens. 2011;25:532–538. [DOI] [PubMed] [Google Scholar]
- 6. Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38(3 Pt 2):581–587. [DOI] [PubMed] [Google Scholar]
- 7. Louis SF, Zahradka P. Vascular smooth muscle cell motility: from migration to invasion. Exp Clin Cardiol. 2010;15:e75–e85. [PMC free article] [PubMed] [Google Scholar]
- 8. Weber KT, Anversa P, Armstrong PW, et al. Remodeling and reparation of the cardiovascular system. J Am Coll Cardiol. 1992;20:3–16. [DOI] [PubMed] [Google Scholar]
- 9. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. [DOI] [PubMed] [Google Scholar]
- 10. Zahradka P, Harding G, Litchie B, et al. Activation of MMP‐2 in response to vascular injury is mediated by phosphatidylinositol 3‐kinase‐dependent expression of MT1‐MMP. Am J Physiol Heart Circ Physiol. 2004;287:H2861–H2870. [DOI] [PubMed] [Google Scholar]
- 11. Fontana V, Silva PS, Gerlach RF, et al. Circulating matrix metalloproteinases and their inhibitors in hypertension. Clin Chim Acta. 2012;413:656–662. [DOI] [PubMed] [Google Scholar]
- 12. Kandasamy AD, Chow AK, Ali MA, et al. Matrix metalloproteinase‐2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res. 2010;85:413–423. [DOI] [PubMed] [Google Scholar]
- 13. Viappiani S, Nicolescu AC, Holt A, et al. Activation and modulation of 72kDa matrix metalloproteinase‐2 by peroxynitrite and glutathione. Biochem Pharmacol. 2009;77:826–834. [DOI] [PubMed] [Google Scholar]
- 14. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 15. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 16. Zakopoulos NA, Nanas SN, Lekakis JP, et al. Reproducibility of ambulatory blood pressure measurements in essential hypertension. Blood Press Monit. 2001;6:41–45. [DOI] [PubMed] [Google Scholar]
- 17. Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30:445–448. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 19. Yasmin, McEniery CM, Wallace S, et al. Matrix metalloproteinase‐9 (MMP‐9), MMP‐2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol 2005;25:372. [DOI] [PubMed] [Google Scholar]
- 20. Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. [DOI] [PubMed] [Google Scholar]
- 21. Fontana V, Silva PS, Belo VA, et al. Consistent alterations of circulating matrix metalloproteinases levels in untreated hypertensives and in spontaneously hypertensive rats: a relevant pharmacological target. Basic Clin Pharmacol Toxicol. 2011;109:130–137. [DOI] [PubMed] [Google Scholar]
- 22. Castro MM, Rizzi E, Figueiredo‐Lopes L, et al. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis. 2008;198:320–331. [DOI] [PubMed] [Google Scholar]
- 23. Marchesi C, Dentali F, Nicolini E, et al. Plasma levels of matrix metalloproteinases and their inhibitors in hypertension: a systematic review and meta‐analysis. J Hypertens. 2012;30:3–16. [DOI] [PubMed] [Google Scholar]
- 24. Zervoudaki A, Economou E, Pitsavos C, et al. The effect of Ca2+ channel antagonists on plasma concentrations of matrix metalloproteinase‐2 and ‐9 in essential hypertension. Am J Hypertens. 2004;17:273–276. [DOI] [PubMed] [Google Scholar]
- 25. Dorr O, Liebetrau C, Mollmann H, et al. Beneficial effects of renal sympathetic denervation on cardiovascular inflammation and remodeling in essential hypertension. Clin Res Cardiol. 2015;104:175–184. [DOI] [PubMed] [Google Scholar]
- 26. Belo VA, Lacchini R, Miranda JA, et al. Increased activity of MMP‐2 in hypertensive obese children is associated with hypoadiponectinemia. Obesity (Silver Spring). 2015;23:177–182. [DOI] [PubMed] [Google Scholar]
- 27. Zervoudaki A, Economou E, Stefanadis C, et al. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J Hum Hypertens. 2003;17:119–124. [DOI] [PubMed] [Google Scholar]
- 28. Iyer RP, de Castro Bras LE, Jin YF, et al. Translating Koch's postulates to identify matrix metalloproteinase roles in postmyocardial infarction remodeling: cardiac metalloproteinase actions (CarMA) postulates. Circ Res. 2014;114:860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DeCoux A, Lindsey ML, Villarreal F, et al. Myocardial matrix metalloproteinase‐2: inside out and upside down. J Mol Cell Cardiol. 2014;77:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kandalam V, Basu R, Abraham T, et al. TIMP2 deficiency accelerates adverse post‐myocardial infarction remodeling because of enhanced MT1‐MMP activity despite lack of MMP2 activation. Circ Res. 2010;106:796–808. [DOI] [PubMed] [Google Scholar]
- 31. Kandalam V, Basu R, Moore L, et al. Lack of tissue inhibitor of metalloproteinases 2 leads to exacerbated left ventricular dysfunction and adverse extracellular matrix remodeling in response to biomechanical stress. Circulation. 2011;124:2094–2105. [DOI] [PubMed] [Google Scholar]
- 32. Givvimani S, Kundu S, Narayanan N, et al. TIMP‐2 mutant decreases MMP‐2 activity and augments pressure overload induced LV dysfunction and heart failure. Arch Physiol Biochem. 2013;119:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moore L, Fan D, Basu R, et al. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail Rev. 2012;17:693–706. [DOI] [PubMed] [Google Scholar]
- 34. Lovelock JD, Baker AH, Gao F, et al. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2005;288:H461–H468. [DOI] [PubMed] [Google Scholar]
- 35. Vanhoutte D, Schellings M, Pinto Y, et al. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res. 2006;69:604–613. [DOI] [PubMed] [Google Scholar]
- 36. Noji Y, Shimizu M, Ino H, et al. Increased circulating matrix metalloproteinase‐2 in patients with hypertrophic cardiomyopathy with systolic dysfunction. Circ J. 2004;68:355–360. [DOI] [PubMed] [Google Scholar]
- 37. Rizzoni D, Porteri E, De Ciuceis C, et al. Effect of treatment with candesartan or enalapril on subcutaneous small artery structure in hypertensive patients with noninsulin‐dependent diabetes mellitus. Hypertension. 2005;45:659–665. [DOI] [PubMed] [Google Scholar]
- 38. Derosa G, Maffioli P, Ferrari I, et al. Different actions of losartan and ramipril on adipose tissue activity and vascular remodeling biomarkers in hypertensive patients. Hypertens Res. 2011;34:145–151. [DOI] [PubMed] [Google Scholar]
- 39. Li M, Li Z, Sun X. Statins suppress MMP2 secretion via inactivation of RhoA/ROCK pathway in pulmonary vascular smooth muscles cells. Eur J Pharmacol. 2008;591:219–223. [DOI] [PubMed] [Google Scholar]
- 40. Izidoro‐Toledo TC, Guimaraes DA, Belo VA, et al. Effects of statins on matrix metalloproteinases and their endogenous inhibitors in human endothelial cells. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:547–554. [DOI] [PubMed] [Google Scholar]
- 41. Nicolae M, Tircol M, Alexandru D. Inhibitory effect of acetylsalicylic acid on matrix metalloproteinase‐2 activity in human endothelial cells exposed to high glucose. J Cell Mol Med. 2005;9:953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bhatt LK, Veeranjaneyulu A. Enhancement of matrix metalloproteinase 2 and 9 inhibitory action of minocycline by aspirin: an approach to attenuate outcome of acute myocardial infarction in diabetes. Arch Med Res. 2014;45:203–209. [DOI] [PubMed] [Google Scholar]
- 43. Ceron CS, Castro MM, Rizzi E, et al. Spironolactone and hydrochlorothiazide exert antioxidant effects and reduce vascular matrix metalloproteinase‐2 activity and expression in a model of renovascular hypertension. Br J Pharmacol. 2010;160:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


