Abstract
The authors provided a systematic review of the clinical and population health impact of increased dietary salt intake during 1 year. Randomized controlled trials or cohort studies or meta‐analyses on the effect of sodium intake were examined from Medline searches between June 2013 to May 2014. Quality indicators were used to select studies that were relevant to clinical and public health. A total of 213 studies were reviewed, of which 11 (n=186,357) were eligible. These studies confirmed a causal relationship between increasing dietary salt and increased blood pressure and an association between several adverse health outcomes and increased dietary salt. A new association between salt intake and renal cell cancer was published. No study that met inclusion criteria found harm from lowering dietary salt. The findings of this systematic review are consistent with previous data relating increased dietary salt to increased blood pressure and adverse health outcomes.
In 2010, increased blood pressure (BP) caused an estimated 18% of all global deaths and 7% of global disability‐adjusted life years lost. Habitual excess salt consumption, in turn, is attributed to about one third of hypertension cases1 and is a key determinant of increased BP.2, 3 Increased dietary salt also contributes to a range of other adverse health outcomes such as gastric cancer and osteoporosis.4 In view of this and the cost‐effectiveness of population strategies to reduce salt, the World Health Organization (WHO) has identified salt reduction as a high priority intervention.5 A 30% reduction in mean population salt intake by 2025 has been included as one of the targets of the “25 by 25” United Nations/WHO initiative for the control of noncommunicable diseases.5
Background
Despite the large body of research and evidence supporting salt reduction, several studies have been published in recent years that challenge the benefits of reducing dietary salt.6, 7 This has generated much media attention and has resulted in some health organizations, policy makers, and consumers to question recommended salt reduction targets. In response, international health and scientific organizations including the Pan American Health Organization/WHO Technical Advisory Group have requested regular scientific updates. While reviews of the science have been published, there were previously no ongoing processes to keep the science related to dietary salt up‐to‐date and accessible.
Aims
The aim of this review is to update recent evidence on the impact of dietary salt on public health using the existing Science of Salt search methodology. The Science of Salt utilizes “quality” criteria to ensure that studies are relevant to clinical and population health and to ensure that low‐quality or irrelevant research designs do not skew review findings.
Methods
Search Strategy and Selection Criteria
This review used the existing automated search strategy (Table 1) set up for the weekly Science of Salt eNewsletter publication. This undertakes weekly Medline via Ovid searches to identify studies on dietary sodium according to quality criteria. The criteria were adapted from the systematic reviews conducted to develop WHO dietary salt recommendations. Studies were included from searches conducted between June 2013 to May 2014.
Table 1.
Included Studies (N=11)
| Author | Country | Outcome | Population | Intervention/Follow‐Up | Measurement Method | Results | Comments |
|---|---|---|---|---|---|---|---|
| Surrogate markers (2 randomized controlled trials [RCTs], 1 systematic review, 2 cohort [total of 5 studies]) | |||||||
| Dickinson and colleagues30 | Australia | Endothelial function, endothelin‐1 | 25 overweight or obese normotensive participants | 6 weeks of “low” sodium 6 g salt (sodium 2400 mg)/24 h and 6 weeks of “usual” sodium 9 g salt (sodium 3600 mg)/24 h. 12‐week crossover | 24‐h urinary sodium excretion; flow‐mediated dilatation (FMD) | Endothelin‐1 decreased by 14% after 6 weeks (P<.05) and this was associated with 45% increase in FMD (from 3.5 to 5.6; P<.001) | No changes to blood pressure (BP) between RCC cases and subcohort members. FMD not a hard outcome |
| Jablonkski (2013) | United States | Systolic BP (SBP), urinary marinobufagenin (MBG), and arterial stiffness | 11 participants with SBP within 130–159 mm Hg and diastolic BP (DBP) <99 mm Hg | 5 weeks on a low‐sodium diet with 4.4±0.5 g salt (sodium 1760±200 mg)/24 h and 5 weeks on a normal‐sodium diet with 8.2±0.5 g salt (sodium 3280±200 mg)/24 h. 10‐week intervention | 24‐h urinary sodium excretion; urine and plasma MBG excretion; aortic pulse wave velocity | urinary MBG excretion, SBP, DBP, and aortic pulse wave velocity were lower during the low‐ vs normal‐sodium intake (25.4 vs 30.7 pmol/kg per day; 127 mm Hg vs 138 mm Hg and 77 mm Hg vs 81 mm Hg; and 700 cm/s vs 843 cm/s; P<.05) | |
| Aburto and colleagues32 | Australia, Belgium, Finland, France, Germany, the Netherlands, Norway, New Zealand, Sweden, United Kingdom, United States, Japan, Scotland, Taiwan. One cohort study included participants from 40 countries. | Cholesterol: total, low‐ and high‐density lipoprotein; triglycerides; urinary nor/adrenaline; plasma nor/adrenaline; urinary protein excretion protein:creatinine ratio; creatinine clearance; serum creatinine; glomerular filtration rate | 5508 participants | Decreased sodium intake | Sodium intake was estimated to equal 24‐h urinary sodium excretion | Reduced sodium intake had no significant adverse effect on total cholesterol, low‐ and high‐density lipoprotein cholesterol, or triglyceride levels (mean difference 0.02 mmol/L, 0.03 mmol/L, −0.01 mmol/L, and 0.04 mmol/L, respectively). No effect of reduced sodium intake on urinary nor/adrenaline (mean difference −13.10 pg/mL and 17.13 pg/mL, respectively). No effect of reduced sodium on catecholamine levels (adrenaline 6.90 pg/mL, noradrenaline 8.23 pg/mL); nonsignificantly lower urinary protein excretion with reduced sodium intake (−76.61 μmol/L); lower urinary protein excretion with lower sodium intake; beneficial effect of lower sodium intake on renal function | |
| Larsen and colleagues26 | Denmark | Body weight, waist circumference, body fat, and fat free mass (FFM) | 215 participants | Changes in body weight, waist circumference, body fat, and FFM were calculated after 6 years of follow‐up | 24‐h urinary sodium excretion |
Increase in body fat of 0.24 kg (P=.015, confidence interval, 0.05–0.43) per 6 g of salt (sodium 2400 mg)/24 h; decrease in FFM of −0.21 kg (P=.041, confidence interval, −0.40 to −0.001) per 6 g of salt (sodium 2400 mg)/24 h. No significant associations between 24‐h urinary sodium and change in body weight or waist circumference |
|
| Jain and colleagues25 | United States | Total‐body percentage fat (TBPF) | 2782 participants | Association of urinary sodium‐to‐potassium ratio with obesity; multiethnic cohort Dallas Heart study: race classified into African American (49.8%), white (30.8%), and Hispanic (17.2%) and other (2.2%); 12% diabetes mellitus and 36% hypertension | Ratio of sodium‐to‐potassium intake estimated from spot urine; TBPF measured by dual‐energy x‐ray absorptiometry; fasting venous blood | TBFP was 32%±10%; urinary sodium to potassium was 4.2±2.6; TBPF increased by 0.75% (95% confidence interval, 0.25–1.25; P=.003) and 0.43%(95% confidence interval, 0.15–0.72; P=.003) in unadjusted and adjusted models, respectively, for every 3‐unit increase in urinary sodium to potassium ratio | |
| Blood pressure (1 RCT, 2 COCHRANE, and 1 systematic review [total of 4 studies]) | |||||||
| Diaz and colleagues33 | United States | Visit‐to‐visit (VVV) BP variability | 1820 overweight participants with high‐normal DBP | TOHP II: Education and counselling on (1) weight loss, (2) sodium reduction, (3) combined weight loss, and sodium reduction vs (4) usual care. 36‐month follow‐up | 24‐h urinary sodium excretion | VVV of SBP was not significantly different among the 3 intervention groups vs usual care (7.2, 7.1, 6.9, and 6.9 mm Hg respectively). Similarly, maintained sodium reduction throughout follow‐up did not have an effect on VVV | The aim of the TOPH II trial was not to test VVV, rather the adherence to and effects of the sodium reduction intervention among overweight adults |
| He and colleagues34 | Multinational | BP | 3230 participants (34 RCTs) | Decreased sodium intake in participants with normal and elevated BP | 24‐h urinary sodium excretion | Elevated BP: sodium reduction 4.4 g of salt (sodium 1760 mg)/24 h and reduction of 5.39 mm Hg SBP and 2.82 mm Hg DBP. Normal BP: sodium reduction 4.4 g salt (sodium 1760 mg)/24 h and BP reduction of 2.42 mm Hg SBP and 1.00 mm Hg DBP | |
| Aburto and colleagues32 | Australia, Belgium, Finland, France, Germany, the Netherlands, Norway, New Zealand, Sweden, United Kingdom, United States, Japan, Scotland, and Taiwan. One cohort study included participants from 40 countries | BP | 5508 participants | Decreased sodium intake | Sodium intake was estimated to equal 24‐h urinary sodium excretion | Sodium intake of <5 g of salt (sodium <2000 mg) vs >5 g of salt (sodium >2000 mg)/24 h reduced SBP by 3.39 mm Hg and DBP by 1.54 mm Hg | |
| Rees and colleagues35 | Multinational | Cardiovascular disease risk | 18,175 participants/clusters in 44 RCTs (11 on BP and sodium: 6406 participants) | Dietary advice vs no advice or minimal advice | 24‐h urinary sodium excretion | BP reduced by 2.61 mm Hg SBP and 1.45 mm Hg DBP. 24‐h urinary sodium excretion reduced by 2.3 g salt (sodium 940 mg) after 3–36 months. Serum cholesterol reduced; mean high‐density lipoprotein cholesterol and triglyceride unchanged | |
| Substantive patient outcomes (3 cohort and 1 systematic review [total of 4 studies]) | |||||||
| Deckers (2014) | The Netherlands | Renal cell cancer (RCC) | 120,852 participants 55–69 years from The Netherlands Cohort Study (485 RCC cases included in case‐cohort analysis) | Follow‐up of 17.3 years | Food frequency questionnaire to assess sodium, potassium, and fluid intake; observed incidence of RCC cases | Sodium intake increased RCC risk (P trend=0.03). High‐sodium and low‐fluid intake increase RCC risk (P interaction=.02). Potassium intake and RCC risk was not associated | Exposure was only assessed at baseline. Food frequency questionnaire shows weak estimate of sodium intake |
| Cook and colleagues27 | United States | Cardiovascular disease or cardiovascular disease death | 2275 participants (30–54 years) from the Trials of Hypertension Prevention (TOHP) I and II who were not in sodium‐reduction intervention arm | Follow‐up of 10 years after end of TOHP I and 5 years after the end of TOHP II | 24‐h urinary sodium excretion; review of medical records on notification of primary end point | There was a 17% increase in risk of cardiovascular disease per 2.5 g of salt (sodium 1000 mg)/24 h (P<.054) | Residual confounding of other healthy lifestyle behaviors |
| He and colleagues28 | United Kingdom | BP, stroke, and ischemic heart disease | 31,672 participants from the Health Survey for England from 2003 to 2011 | Follow‐up of 9 years during the implementation of United Kingdom's National Salt Reduction Program | 24‐h urinary sodium excretion in a random sample of 1589 participants 19–64 years; review of national statistics on deaths from ischemic heart disease and cardiovascular disease | Stroke mortality decreased by 42% (P<.001); ischemic heart disease decreased by 40% (P<.001); BP decreased by 3.0±0.33/1.4±0.20 mm Hg (P<.001); salt intake decreased by 1.4 g of salt (sodium 560 mg)/24 h (P<.001) | Does not quantify salt reduction contribution to stroke, and ischemic heart disease mortality “likely to play and important role” |
| Aburto and colleagues32 | Australia, Belgium, Finland, France, Germany, the Netherlands, Norway, New Zealand, Sweden, United Kingdom, United States, Japan, Scotland, Taiwan. One cohort study included participants from 40 countries | All‐cause mortality; cardiovascular disease; stroke; coronary heart disease | 5508 participants | Decreased sodium intake | Sodium intake was estimated to equal 24‐h urinary sodium excretion | Sodium intake was associated with an increased risk of stroke, stroke mortality, and coronary heart disease mortality (risk ratio: 1.24, 1.63, and 1.32, respectively) | |
Inclusion/Exclusion Criteria
For the purposes of this review, inclusion criteria were any randomized controlled trials (RCTs) or cohort studies in nonacutely ill adults that examined sodium intake (excluding studies with participants who had type II diabetes, chronic kidney disease, or chronic heart failure) and were published in English. Specifically, RCTs evaluating the association between sodium intake and BP, or any substantive adverse health outcome, were included if the following criteria were met: there was a minimum intervention period of 4 weeks; the intervention was composed of at least one group receiving decreased sodium intake compared with a control group; there was a difference of at least 2.3 g of salt (sodium 920 mg) per 24 hours between the intervention and the control; and sodium intake was measured by 24‐hour urinary excretion. RCTs were excluded if additional cointerventions were part of the study (including nondrug interventions, antihypertensives, or other drugs), thus preventing the independent assessment of the effects of reducing dietary salt. Cohort studies evaluating the association between sodium intake and any substantive adverse health outcome were included if they were prospective in design for at least 1 year or more and measured sodium intake in any way. Substantive adverse health outcomes of interest were all‐cause mortality, cardiovascular disease (CVD), stroke, coronary heart disease (CHD), kidney disease, bone disease, and cancer. Surrogate markers such as endothelium‐dependent dilation, urinary marinobufagenin (MBG) excretion, aortic pulse wave velocity (aPWV), plasma and urinary nitrate/nitrite, osteoporosis, renal stones, weight gain and obesity, and heart rate were assessed.
Data Extraction and Quality Assessment
Two reviewers (CJ and TSR) independently evaluated and excluded articles at the title/abstract review stage. Full‐text articles whose abstracts met the inclusion criteria were then reviewed. An Excel data extraction template was developed, tested, and then refined and populated articles that met the final inclusion criteria. For the RCTs, methodological quality was assessed using the Cochrane Risk of Bias Assessment Tool. Discrepancies in article inclusion, data extraction, and bias assessment were solved by team consensus.
Results
A total of 5534 studies were identified, of which 213 were selected for full review (Figure). Of these, 202 were excluded for the following reasons: 181 studies were cross‐sectional or other; five RCTs and two non‐RCTs included unhealthy populations (two populations with heart failure,8, 9 two involving patients with kidney disease,10, 11 two involving hypertensive patients,12, 13 and one involving participants with type II diabetes14). Seven cohort studies did not investigate an outcome of interest: five measured BP15, 16, 17, 18, 19 (one study measured environmental influences, such as sharing current residence, on sodium intake20 and one study investigated sex differences in salt sensitivity21). Additionally, seven RCTs were excluded (three did not investigate either BP or an outcome of interest nor did they measure sodium intake using 24‐hour urinary sodium excretion, three study interventions were for <4 weeks' duration,22, 23, 24 and one study did not investigate BP or an outcome of interest).
Figure 1.
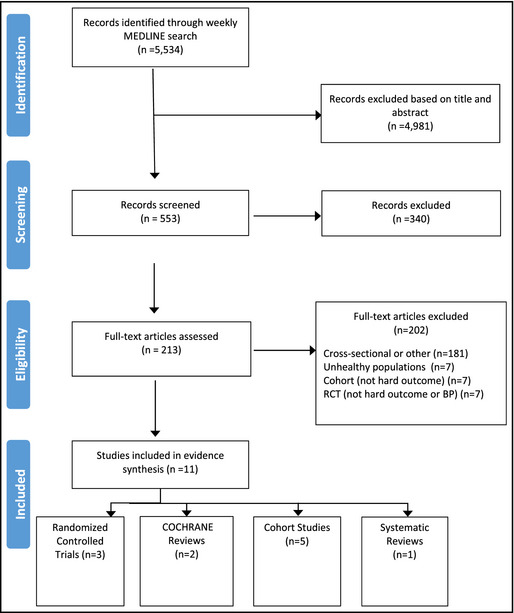
Included/excluded studies. RCT indicates randomized controlled trial.
The 11 studies included in the final review are summarized in Table 2. They include two RCTs on measures of surrogate markers including urinary MBG excretion and aPWV, flow‐mediated dilatation (FMD), pulse wave velocity (PWV), plasma and urinary nitrate/nitrite, asymmetric dimethylarginine (ADMA), renin, aldosterone, and endothelin‐1 and vascular adhesion molecules; one RCT on BP; five cohort studies including two studies on measures of surrogate markers including obesity25 and change in weight26; three studies on substantive patient outcomes including CVD and/or mortality,27 stroke and ischemic heart disease (IHD) mortality,28 and renal cell cancer (RCC)29; and one systematic review and two Cochrane reviews including studies on all‐cause mortality, CVD, stroke, chronic heart failure, changes in blood lipids, catecholamine levels and renal function, BP, and CVD risk assessed as BP.
Table 2.
Weekly Medline (Ovid) Search Strategy Years: 1946 – Present
| Step | Search |
|---|---|
| 1 | exp Sodium Glutamate |
| 2 | (monosodium glutamate or msg).tw. |
| 3 | (salt or sodium).tw. |
| 4 | 1 or 2 or 3 |
| 5 | exp Diet |
| 6 | (diet* or food or intake).tw. |
| 7 | 5 or 6 |
| 8 | 4 and 7 |
| 9 | exp Sodium, Dietary |
| 10 | exp Diet, Sodium‐Restricted |
| 11 | 8 or 9 or 10 |
| 12 | limit 11 to English language |
| 13 | limit 12 to year=“2013–Current” |
| 14 | limit 13 to humans |
Weekly Medline (Ovid) Search Strategy on dietary sodium is a modified version of the search strategy developed by a Cochrane librarian to support the development of the Canadian Hypertension Education Program recommendations regarding dietary sodium.
The included RCTs and cohort studies were conducted in the United States, Australia, the United Kingdom, Denmark, and the Netherlands and systematic reviews were multinational including Australia, Belgium, Finland, France, Germany, the Netherlands, Norway, New Zealand, Sweden, the United Kingdom, the United States, and Japan.
Surrogate Markers
Four studies assessed the relationship between salt intake and surrogate markers. In a 12‐week single‐blind randomized controlled crossover study undertaken in Australia, Dickinson and colleagues30 recruited a sample of 25 overweight/obese participants to examine the effects of a reduction of 3 g of salt (sodium 1200 mg) per 24 hours from a usual 9 g of salt (sodium 3600 mg) per 24 hours on measures of vascular function and explored mechanisms of effect in overweight and obese adults. Participants were randomized to a 6‐week diet with reduced 6 g of salt (sodium 2400 mg) per 24 hours and then crossed over to 6 weeks on their usual salt diet of 9 g salt (sodium 3600 mg) per 24 hours. FMD, 24‐hour BP, augmentation index, PWV, plasma and urinary nitrate/nitrite, ADMA, renin, aldosterone, and endothelin‐1 and vascular adhesion molecules were measured after 2 days and 6 weeks. Adherence to the diets was determined from two 24‐hour urinary excretions for measurement of sodium, potassium, creatinine, and nitrate/nitrite. The study showed that a diet of 9 g of salt (sodium 3600 mg) per 24 hours impairs FMD and increases endothelin‐1 and that these effects were reversed by salt reduction of approximately 3 g of salt (sodium 1200 mg) per 24 hours. Endothelin‐1 decreased by 14% after 6 weeks, which was associated with a 45% increase in FMD. The renin‐angiotensin‐aldosterone system was not altered by this change in salt intake. The authors say this study demonstrates a reduction in dietary salt intake of 3 g of salt (sodium 1200 mg) per 24 hours improves endothelial function in normotensive overweight and obese patients. There were no statistically significant changes in BP between the diets, which were consistent with the hypothesis that salt reduction has beneficial effects on endothelial function independently of BP. Additionally, there was no nonobese control group, and this affects the generalizability of the results.
Jablonski and colleagues31 conducted a randomized placebo‐controlled crossover study in the United States on a sample of 11 middle‐aged/older adults to investigate whether dietary sodium restriction would reduce urinary MBG as well as systolic BP (SBP) and aPWV (the gold standard measure of large elastic artery stiffness), hypothesizing that production of the natriuretic hormone MBG is increased in salt‐sensitive hypertension and contributes to the rise in SBP during sodium loading. Authors randomized participants to 5 weeks on a low‐sodium diet with 4.4±0.5 g of salt (sodium 1760±200 mg) per 24 hours and 5 weeks on a normal‐sodium diet with 8.2±0.5 g of salt (sodium 3280±200 mg) per 24 hours. Results showed that urinary MBG excretion (weekly measurements; 25.4±1.8 pmol/kg/d vs 30.7±2.1 pmol/kg/d), SBP (127±3 mm Hg vs 138±5 mm Hg), and aPWV (700±40 cm/s vs 843±36 cm/s) were lower during the low‐ vs normal‐sodium condition (P<.05, for all). Additionally, urinary MBG excretion was associated with SBP and sodium excretion, as assessed from all urine collections, and this association was found to be stronger in the diet with 4.4±0.5 g of salt (sodium 1760 ± 200 mg) per 24 hours but not stronger in the higher‐salt diet. The authors concluded that dietary sodium restriction reduces urinary MBG excretion and that MBG excretion is positively associated with SBP and aortic stiffness.
In a systematic review of the effect of lower sodium intake on health, Aburto and colleagues32 reviewed several estimates of effect of reduced sodium on surrogate markers such as total cholesterol, low‐ and high‐density lipoprotein cholesterol, and triglyceride levels. The review showed that reduced sodium intake had no significant effect on total cholesterol (mean difference, 0.02 mmol/L; 95% confidence interval [CI], −0.03 mmol/L to 0.07 mmol/L), low‐density lipoprotein cholesterol (0.03 mmol/L, −0.02 mmol/L to 0.08 mmol/L), high‐density lipoprotein cholesterol (−0.01 mmol/L, −0.03 mmol/L to 0.00 mmol/L), or triglyceride levels (0.04 mmol/L, −0.04 mmol/L to 0.09 mmol/L). Additionally, one RCT reporting urinary adrenaline (epinephrine) and two RCTs reporting urinary noradrenaline (norepinephrine) detected no effect of reduced sodium intake on these (mean differences, adrenaline −13.10 pg/mL, −29.24 pg/mL to 3.04 pg/mL; noradrenaline 17.13 pg/mL, −34.06 pg/mL to 68.33 pg/mL). Four RCTs reporting plasma adrenaline and seven RCTs reporting plasma noradrenaline also detected no effect of reduced sodium on catecholamine levels (adrenaline 6.90 pg/mL, −2.17 pg/mL to 15.96 pg/mL; noradrenaline 8.23 pg/mL, −27.84 pg/mL to 44.29 pg/mL). A meta‐analysis of three comparisons of renal function indicators showed a nonsignificant lower urinary protein excretion with reduced sodium intake (−76.61 μmol/L, −0.97 μmol/L to −154.2 μmol/L). Four additional RCTs (one using urinary protein excretion and three using urinary albumin excretion) also reported results consistent with a beneficial effect of lower sodium intake on renal function.
Larsen and colleagues26 undertook a longitudinal study in Denmark among 215 adults to examine the association between dietary salt and subsequent changes in weight, waist circumference, and body composition. Neither the crude nor the adjusted models in the analyses showed any statistically significant associations between sodium excretion and change in body weight or waist circumference. The study found that there was a significant increase in body fat of 0.24 kg (P=.015; CI, 0.05–0.43) per 6 g of salt (sodium 2400 mg) per 24 hours during the 6‐year period as well as a significant association with fat free mass of −0.21 kg (P=.041; CI, −0.40 to −0.01).
Jain and colleagues25 conducted a cohort study in the United States among a sample of 2782 multiethnic participants to evaluate the association between the ratio of dietary sodium‐to‐potassium intake and obesity. The investigators collected health‐related data, fasting venous blood, early‐morning first‐void urine sample, and total‐body percentage fat (TBPF), which was measured by dual‐energy x‐ray absorptiometry. An independent direct association between urinary sodium‐to‐potassium ratio and TBPF was revealed whereby TBPF increased by 0.75% (95% CI, 0.25–1.25; P=.003), respectively, for every three‐unit increase in the urinary sodium‐to‐potassium ratio. The association was present even after adjustment for BP, diabetes, and serum glucose and triglyceride concentrations.
Blood Pressure
Four studies assessed the relationship between salt intake and BP. Diaz and colleagues33 conducted an RCT investigating the effects of weight loss and salt reduction on visit‐to‐visit (VVV) BP variability. VVV of BP was defined as the standard deviation of BP across six follow‐up visits. The authors state that VVV of BP is associated with CVD events including stroke, CHD, and mortality. The study was a secondary analysis of Trials of Hypertension Prevention (TOHP) II and participants were randomly assigned to one of four treatment groups: weight loss alone (10.5 g of salt [sodium 4232 mg] per 24 hours), sodium reduction alone (10.6 g of salt [sodium 4278 mg] per 24 hours), combined weight loss and sodium reduction (10.2 g of salt [sodium 4117 mg] per 24 hours), or usual care (10.9 g of salt [sodium 4393 mg] per 24 hours) for 36 months. The results of this study found that there were no significant differences in VVV of SBP or diastolic BP (DBP) for participants in any of the active intervention groups compared with participants in the usual care group over a 36‐month follow‐up period (weight loss 7.2+3.1 mm Hg, sodium reduction 7.1+3.0 mm Hg, or combined 6.9+2.9 mm Hg, usual care group 6.9+2.9 mm Hg). It was also found that participants who maintained a reduced sodium intake throughout follow‐up did not have lower VVV of SBP compared with those who did not reduce their sodium intake (0.1+0.3 mm Hg). The findings suggest that weight loss and sodium reduction may not be effective interventions for lowering VVV of BP in individuals with high‐normal DBP.
A Cochrane systematic review and meta‐analysis of randomized trials conducted by He and colleagues34 aimed to examine the effects of longer‐term modest salt reduction on blood pressure, hormones, and lipids. The authors reviewed data from 34 trials of salt reduction over at least 4 weeks. The studies included 3230 participants. The results found that among hypertensive participants there was a reduction in urinary sodium of 4.4 g of salt (sodium 1760 mg) per 24 hours and a reduction in BP of 5.39 mm Hg systolic and 2.82 mm Hg diastolic. Among normotensive participants there was a reduction in urinary sodium of 4.4 g of salt (sodium 1760 mg) per 24 hours and a reduction in BP of 2.42 mm Hg systolic and 1.00 mm Hg diastolic. The authors conclude that a reduction in salt intake lowers BP both in individuals with elevated BP and in those with normal BP.
Rees and colleagues35 conducted a Cochrane systematic review to assess the effectiveness of providing dietary advice on sustained improved diets of healthy adults. The authors reviewed 18,175 participants or clusters that included 6406 participants from 11 studies on BP and salt intake. The results showed that serum cholesterol was reduced, mean high‐density lipoprotein cholesterol and triglyceride were unchanged, BP was reduced by 2.61 mm Hg systolic and 1.45 mm Hg diastolic, and 24‐hour urinary sodium excretion was reduced by 2.3 g of salt (sodium 940 mg) after 3 to 36 months. The authors conclude that dietary advice appears to be effective in bringing about modest beneficial changes in diet and cardiovascular risk factors over approximately 12 months, but longer‐term effects were not known. Additionally, the authors found that advice to reduce salt intake from 3 to 36 months of follow‐up was associated with a reduction in BP of 2.61 mm Hg systolic and 1.45 mm Hg diastolic.
Substantive Adverse Health Outcomes
Four studies examined the relationship between salt intake and adverse health outcomes. In a cohort study investigating whether sodium, potassium, and fluid intake were associated with RCC, Deckers and colleagues29 recruited 120,852 participants aged 55 to 69 years from the Netherlands Cohort Study (NLCS) to complete a baseline questionnaire on dietary habits and lifestyle. After 17.3 years of follow‐up, 485 RCC cases and 4438 subcohort members were available for analyses. The results indicated that higher sodium intake was associated with increased RCC risk whereas fluid and potassium intake was not. Significant increases were observed in RCC risk per increment of 1 g of salt (sodium 400 mg) per 24 hours (hazard ratio, 1.07; 95% CI, 1.00–1.15). Furthermore, there was a significant increase in risk across sodium intake quintiles (P trend=.03), with a hazard ratio of 1.40 (95% CI, 0.99–1.97) in the highest quintile compared with the lowest. The authors state that high sodium intake increased RCC risk, and for high sodium and low fluid intake combined, the RCC risk additionally increased (P interaction=.02).
Cook and colleagues27 conducted a reanalysis of a cohort study that investigated the relationship between sodium intake and CVD among 1855 prehypertensive individuals aged between 30 and 54 years from 1987 to 1990 and 1191 participants from 1990 to 1995 from phases 1 and 2 of the TOHP. TOHP I evaluated the effects of four supplement and three lifestyle interventions, including weight loss and sodium reduction interventions, on BP whereas TOHP II evaluated the effects of sodium reduction and weight loss on BP over a longer 3‐ to 4‐year follow‐up period. There were 193 CVD deaths during the extended observational follow‐up for CVD in 2000, 10 years after the end of TOHP I, and 5 years after the end of TOHP II. In this reanalysis, results showed that median sodium excretion was 9 g of salt (sodium 3600 mg) per 24 hours, with 1.4% of participants consuming <3.75 g of salt (sodium <1500 mg) per 24 hours and 10% of participants consuming <5.75 g of salt (sodium <2300 mg) per 24 hours, consistent with current dietary guidelines. The findings showed that risk of CVD for participants whose sodium intake was <5.75 g of salt (sodium <2300 mg) per 24 hours was 32% lower compared with those who had sodium intakes from 9 g of salt to <12 g (sodium 3600–<4800 mg) per 24 hours. There was a linear 17% increase in risk per 2.5‐g increase in salt (sodium 1000 mg) per 24 hours. The authors concluded that the study findings were consistent with overall health benefits of reducing sodium intake to between 3.75 g and 5.75 g of salt (sodium 1500–2300 mg) per 24 hours.
A cohort study was conducted in England by He and colleagues28 to determine the relationship between the reduction in population salt intake and fall in BP and mortality from stroke and IHD during 2003 to 2011. Authors analyzed the Health Survey for England data from 2003 (n=9183), 2006 (n=8762), 2008 (n=8974), and 2011 (n=4753) for participants aged 16 years and older. In all surveys, participants underwent BP measurement, anthropometric measures, 24‐hour dietary recall surveys, and 24‐hour urinary sodium excretion. Stroke and IHD mortality rates were calculated as the number of stroke or IHD deaths divided by the population. The study found that from 2003 to 2011 there was a decrease in mortality from stroke by 42% and from IHD by 40%. Similarly, there was a fall in BP of 3.0±0.33/1.4±0.20 mm Hg, a decrease in cholesterol of 0.4±0.02 mmol/L, a reduction in smoking prevalence from 19% to 14%, an increase in fruit and vegetable consumption of 0.2±0.05 portions per day, and an increase in body mass index (BMI) of 0.5±0.09 kg/m2. After adjusting for age, sex, ethnic group, education, household income, alcohol consumption, fruit and vegetable intake, and BMI in participants not treated for hypertension, there was a fall in BP of 2.7±0.34/1.1±0.23 mm Hg. Salt intake, as measured by 24‐hour urinary sodium, decreased by 1.4 g of salt (sodium 560 mg) per 24 hours between 2003 and 2011 (salt intake was 9.5 g of salt (sodium 3800 mg) per 24 hours in 2003, 9 g (sodium 3600 mg) per 24 hours in 2005, 8.64 g (sodium 3456 mg) per 24 hours in 2008, and 8.1 g (sodium 3240 mg) per 24 hours in 2011). The authors conclude that the reduction in salt intake was likely to be an important contributor to the fall in BP between 2003 and 2011 in England and, as a result, the decrease in salt intake could have played an important role in the reduction of stroke and IHD mortality during this period.
Aburto and colleagues32 conducted a systematic review and meta‐analysis to assess the effect of lower sodium intake on BP, all‐cause mortality, CVD, stroke, CHD, and potential adverse effects such as changes in blood lipids, catecholamine levels, and renal function in adults and on BP, blood lipids, and catecholamine levels in children. Inclusion and exclusion criteria were similar to the methods used in this review. However, in order to assess studies in children, exclusion criteria were changed to include duration of more than 3 weeks, any controlled design, any difference in sodium intake between intervention and control, and any method of measuring intake. Thirty‐six RCTs were included in the meta‐analyses in adults, which found that a reduction in sodium intake significantly reduced resting SBP by 3.39 mm Hg and resting DBP by 1.54 mm Hg. When sodium intake was <5 g of salt (sodium 2000 mg) per 24 hours, compared with >5 g of salt (sodium 2000 mg) per 24 hours, SBP was decreased by 3.47 mm Hg and DBP by 1.81 mm Hg. Data from RCTs on cardiovascular outcomes lacked sufficient power to detect a relationship between sodium intake and the outcomes of interest. Results from 10 cohort studies in a meta‐analyses found that increased sodium intake was associated with an increased risk of stroke, stroke mortality, and CHD mortality (risk ratio, 1.24, 1.63, and 1.32, respectively). Ten studies in children were included in the review: six randomized controlled trials, three non‐RCTs, and one cohort study. The findings suggest that a reduction in sodium intake in children would significantly reduce SBP by 0.84 mm Hg and DBP by 0.87 mm Hg. None of the studies that reached the inclusion criteria for this review reported the effect of lower sodium intake on blood lipids, catecholamine levels, or side effects in children. The meta‐analysis concluded at the evidence suggests most people would likely benefit from reducing sodium intake.
Discussion
This systematic review of studies published on salt during the past 12 months found 11 studies that associated increased dietary salt intake with adverse health outcomes. A meta‐analysis of RCTs did not have statistical power to assess increased dietary salt intake with substantive adverse outcomes. Findings from a meta‐analysis of cohort studies in healthy populations was consistent with higher dietary salt being associated with higher CVD rates and increased risk of all stroke, fatal stroke, and fatal CHD events. The meta‐analysis of cohort studies included studies with salt intake reduced to 5 g (sodium 2000 mg) per 24 hours. Examination of reductions of 1.4 g of salt (sodium 560 mg) per 24 hours in England were also consistent with expected reduced CVD and reduced BP, but, as is the case with observational studies of this nature, there were multiple confounding factors. The association with reduced cardiovascular events was also seen in one cohort study at levels of sodium intake of 3.75 to 5.75 g of salt (sodium 1500–2300 mg) per 24 hours. It is of note that the systematic review also identified a study that associated increased dietary salt with RCC.
Two meta‐analyses examined RCTs of salt reduction with changes in BP as an outcome. The meta‐analyses confirm decreases in BP with reduced dietary salt in a variety of settings including an impact on children, people with normal BP as well as hypertension, and people who eat a diet with <5 g of salt (sodium 2000 mg) per 24 hours. Two RCTs that assessed the impact of salt consumption on BP were also identified. One was a re‐analysis of a previously published study while the other should be included in subsequent meta‐analyses.13
Studies of the impact of dietary salt on surrogate endpoints showed that reduced dietary salt improved flow‐mediated vasodilation and aortic stiffness. Several of the studies examining surrogate endpoints also examined BP and should be incorporated into future meta‐analyses on the impact of salt on BP. Hence, the findings of this updated systematic review of studies that are relevant to clinical and population efforts are in support of current recommendations to reduce dietary salt and, specifically, the recommendations of the WHO, World Hypertension League, and International Society of Hypertension.36
It is notable that this systematic review did not identify studies that showed increases in adverse outcomes associated with lower dietary salt. This review applied a priori criteria to ensure that the included studies met minimum methodological standards, that the intervention had an impact on dietary salt, and that the studies were of a duration that had relevance to public health and clinical medicine. Low‐quality research methods have been identified as a critical factor in generating controversy over the impact of changing dietary salt on health.37 It is notable that reviews that included poor‐quality clinical designs (short duration, ineffective reduction in salt intake) and flawed assessments of intake and that included cohort studies in people with disease have resulted in conflicting conclusions on dietary salt.1, 38
The standards applied in this review should not be interpreted as being optimum for the conduct of clinical studies on dietary salt. The majority of the studies assessed salt intake based on a single day's urine collection despite day‐to‐day variability, meaning that multiple days of assessment are required to have a higher degree of accuracy. Further, studies should apply criteria for ensuring that 24‐hour urine collections were complete. Lastly, in cohort studies, we allowed any assessment of salt intake, which means that studies using inaccurate assessments of salt intake may have been included. The lack of ability to accurately classify an individual's salt intake is likely to result in underestimates of the impact of dietary salt on health and favor the null hypothesis. Where salt intake is estimated using a formula that includes parameters which predict outcomes (age, sex, creatinine), confounding may occur. Further, the impact of salt on BP may not be fully assessed using the criteria applied in this review, including studies as short as 4 weeks; optimally, studies should be much longer. Animal studies find irreversible, epigenetic, and long‐term changes in BP associated with salt ingestion that short (4‐week) studies of salt reduction would be unlikely to detect.39, 40 Studies are markedly limited by the challenges of substantively reducing dietary salt over long periods of time in the research setting, making large‐scale RCTs challenging at best. Nearly all of the methodological limitations favor the null hypothesis or even reverse causality. The World Hypertension League is leading an effort to develop recommended standards for the conduct of such research to improve reliability of findings.41
The fact that this is a systematic review of the literature for a single year is another potential limitation. Past evidence linking high dietary salt to gastric cancer (systematic review), kidney stones, osteoporosis, asthma, and other outcomes were not covered in this year's literature review. In addition, cohort studies conducted in people with chronic disease in which there is a high risk of the studies being influenced by reverse causality (where more severe disease results in lower food intake [and salt intake] resulting in false associations between low food [and salt] intake and increased adverse outcomes) were excluded. However, the inclusion of recent comprehensive systematic reviews on the impact of high dietary salt on cardiovascular patient outcomes, BP, and some surrogate markers and means it represents much of the relevant research.
The review found insufficient statistical power in RCTs to assess the impact of high dietary salt on substantive adverse patient outcomes. The challenges of conducting large, long‐duration, randomized trials of salt reduction are substantive. A large RCT of a salt replacer (partial replacement of sodium with potassium) on substantive patient outcomes is currently being conducted with results expected in 2017 to 2018.42
Conclusions
A variety of modeling studies have estimated the impact of changes in BP related to recommended changes in dietary salt (and, in some, the association of increased dietary salt to gastric cancer). About one third hypertension cases have been attributed to increased dietary salt.1 In a recent updated analyses of the Global Burden of Disease Study, 1.65 million deaths in 2010 were attributed to dietary salt in excess of 5 g of salt (sodium 2000 mg) per 24 hours.43 The data in this updated systematic review are consistent with the data that were used in these modeling studies and outline the substantive impact that increased dietary salt is estimated to have based on best available evidence. The systematic review also extends the health concerns of increased dietary salt to RCC, which will require further study.
Disclosures
Ms Claire Johnson and Mr Thout Sudhir Raj have nothing to disclose.
Dr Norm Campbell does not have commercial conflicts of interests in the area of dietary sodium but did receive travel funds to attend a meeting in Russia from Novartis Russia in 2012. Dr Campbell is a member of World Action on Salt and Health, and several governmental and charitable organization committees related to hypertension and dietary salt. Dr Campbell receives <1% of his income in the form of honoraria for speaking to academic and charitable organizations on hypertension and diet.
Dr Jacqui Webster is Director of the WHO Collaborating Centre on Population Salt Reduction and currently receives funding from the National Health and Medical Research Council, the National Heart Foundation, the Victorian Health Promotion Foundation, and the WHO for work on salt reduction. She has previously received funding from Bupa Australia and the Australian Food and Grocery Council for related work.
Dr Simon Bacon does not have commercial conflicts of interests in the area of dietary sodium but has received consultancy fees from Kataka Medical Communication for the development and delivery of health behavior change continuing medical education programs and is supported by a salary award from the Fonds de Recherche du Quebec – Sante. In addition, Dr Bacon is Chair of the Health Behaviour subcommittee of the Canadian Hypertension Education Program recommendations committee, which has the responsibility of proposing dietary‐related recommendations.
Dr Luc Trudeau does not have any commercial conflicts of interest interests in the area of dietary sodium. Dr Trudeau is Chair of the sodium review and recommendations committee of Health Behaviour subcommittee of the Canadian Hypertension Education Program recommendations committee, which has the responsibility of proposing sodium‐related dietary recommendations.
Dr Padwal is supported by an alternative funding plan from the Government of Alberta and the University of Alberta and has no other relevant disclosures.
Acknowledgments
The Science of Salt is an initiative of the HSF CIHR Chair in Hypertension Prevention and Control (Canada) and is funded in part by the Canadian Stroke Network and the George Institute for Global Health. The initiative is supported by World Hypertension League, WHO Collaborating Centre on Population Salt Reduction, PAHO/WHO Technical Advisory Group on CVD Prevention through Dietary Sodium, and World Action on Salt and Health.
J Clin Hypertens (Greenwich). 2015;17:401–411. DOI: 10.1111/jch.12529. © 2015 Wiley Periodicals, Inc.
References
- 1. Institute of Medicine Committee on Public Health Priorities to Reduce and Control Hypertension, Population‐Based Policy and Systems Change Approach to Prevent and Control Hypertension. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 2. Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370:2044–2053. [DOI] [PubMed] [Google Scholar]
- 3. Elliott P, Stamler J, Nichols R, et al. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. BMJ. 1996;312:1249–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Reducing salt intake in populations: report of a WHO forum and technical meeting, 5–7 October 2006, Paris, France; 2007. [Google Scholar]
- 5. World Health Organization . Draft comprehensive global monitoring framework and targets for the prevention and control of noncommunicable diseases. 2013.http://apps.who.int/gb/ebwha/pdf_files/WHA66/A66_8-en.pdf Accessed February 24, 2015.
- 6. Stolarz‐Skrzypek K, Kuznetsova T, Thijs L, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–1785. [DOI] [PubMed] [Google Scholar]
- 7. Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease: a meta‐analysis of randomized controlled trials (Cochrane review). Am J Hypertens. 2011;24:843–853. [DOI] [PubMed] [Google Scholar]
- 8. Philipson H, Ekman I, Forslund HB, Swedberg K, Schaufelberger M. Salt and fluid restriction is effective in patients with chronic heart failure. Eur J Heart Fail. 2013;15:1304–1310. [DOI] [PubMed] [Google Scholar]
- 9. Hummel S, Seymour EM, Brook RD, et al. Low‐sodium DASH diet improves diastolic function and ventricular‐arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail. 2013;6:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMahon E, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol. 2013;24:2096–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Telini L, de Carvalho Beduschi G, Caramori JC, et al. Effect of dietary sodium restriction on body water, blood pressure, and inflammation in hemodialysis patients: a prospective randomized controlled study. Int Urol Nephrol. 2014;46:91–97. [DOI] [PubMed] [Google Scholar]
- 12. Kitaoka K, Nagaoka J, Matsuoka T, et al. Dietary intervention with cooking instructions and self‐monitoring of the diet in free‐living hypertensive men. Clin Exp Hypertens. 2013;35:120–127. [DOI] [PubMed] [Google Scholar]
- 13. Lima ST, da Silva Nalin de Souza B, França AK, Salgado Filho N, Sichieri R. Dietary approach to hypertension based on low glycaemic index and principles of Dietary Approaches to Stop Hypertension (DASH): a randomised trial in a primary care service. Br J Nutr. 2013;110:1472–1479. [DOI] [PubMed] [Google Scholar]
- 14. Petersen K, Torpy DJ, Chapman IM, et al. Food label education does not reduce sodium intake in people with type 2 diabetes mellitus. Appetite. 2013;68:147–151. [DOI] [PubMed] [Google Scholar]
- 15. Batis C, Gordon‐Larsen P, Cole SR, et al. Sodium intake from various time frames and incident hypertension among Chinese adults. Epidemiology. 2013;24:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Du S, Batis C, Wang H, et al. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. Am J Clin Nutr. 2014;99:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi L, Krupp D, Remer T. Salt, fruit and vegetable consumption and blood pressure development: a longitudinal investigation in healthy children. Br J Nutr. 2014;111:662–671. [DOI] [PubMed] [Google Scholar]
- 18. Otsuka T, Kato K, Ibuki C, Kodani E, Kusama Y, Kawada T. Does subjective evaluation of the frequency of salty food intake predict the risk of incident hypertension? A 4‐year follow‐up study in a middle‐aged population. Intern Med J. 2013;43:1316–1321. [DOI] [PubMed] [Google Scholar]
- 19. Ohta Y, Iwashima Y, Hayashi S, et al. Trend of office and home blood pressure control in treated hypertensive patients: changes in antihypertensive medication and salt intake. Clin Exp Hypertens. 2014;36:103–107. [DOI] [PubMed] [Google Scholar]
- 20. Kho M, Lee JE, Song YM, et al. Genetic and environmental influences on sodium intake determined by using half‐day urine samples: the Healthy Twin Study. Am J Clin Nutr. 2013;98:1410–1416. [DOI] [PubMed] [Google Scholar]
- 21. Bursztyn M, Ben‐Dov IZ. Sex differences in salt‐sensitivity risk approximated from ambulatory blood pressure monitoring and mortality. J Hypertens. 2013;31:900–905. [DOI] [PubMed] [Google Scholar]
- 22. Bonfils PK, Taskiran M, Damgaard M, et al. The influence of high versus low sodium intake on blood pressure and haemodynamics in patients with morbid obesity. J Hypertens. 2013;31:2220–2229. [DOI] [PubMed] [Google Scholar]
- 23. DuPont JJ, Greaney JL, Wenner MM, et al. High dietary sodium intake impairs endothelium‐dependent dilation in healthy salt‐resistant humans. J Hypertens. 2013;31:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Allen A, Gullixson LR, Wolhart SC, et al. Dietary sodium influences the effect of mental stress on heart rate variability: a randomized trial in healthy adults. J Hypertens. 2014;32:374–382. [DOI] [PubMed] [Google Scholar]
- 25. Jain N, Minhajuddin AT, Neeland IJ, et al. Association of urinary sodium‐to‐potassium ratio with obesity in a multiethnic cohort. Am J Clin Nutr. 2014;99:992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Larsen SC, Ängquist L, Sørensen TI, Heitmann BL. 24 h urinary sodium excretion and subsequent change in weight, waist circumference and body composition. PLoS One. 2013;8:e69689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He FJ, Pombo‐Rodrigues S, MacGregor GA. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ Open. 2014;4:e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deckers I, van den Brandt PA, van Engeland M, et al. Long‐term dietary sodium, potassium and fluid intake; exploring potential novel risk factors for renal cell cancer in the Netherlands Cohort Study on diet and cancer. Br J Cancer. 2013;110:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dickinson KM, Clifton PM, Keogh JB. A reduction of 3 g/day from a usual 9 g/day salt diet improves endothelial function and decreases endothelin‐1 in a randomised cross–over study in normotensive overweight and obese subjects. Atherosclerosis. 2014;233:32–38. [DOI] [PubMed] [Google Scholar]
- 31. Jablonski KL, Fedorova OV, Racine ML, et al. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clin J Am Soc Nephrol. 2013;8:1952–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aburto NJ, Li J, Macgregor GA. Effect of lower sodium intake on health: systematic review and meta‐analyses. BMJ. 2013;4:CD004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diaz KM, Muntner P, Levitan EB. The effects of weight loss and salt reduction on visit‐to‐visit blood pressure variability: results from a multicenter randomized controlled trial. J Hypertens. 2014;32:840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He FJ, Li J, MacGregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta‐analysis of randomised trials. BMJ. 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 35. Rees K, Dyakova M, Wilson N, et al. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst Rev. 2013;12:CD002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Campbell NR, Lackland DT, Chockalingam A, et al. The International Society of Hypertension and World Hypertension League call on governments, nongovernmental organizations and the food industry to work to reduce dietary sodium. J Hypertens 2014;32:446–447. [DOI] [PubMed] [Google Scholar]
- 37. Campbell NR, Appel LJ, Cappuccio FP, et al. A call for quality research on salt intake and health: from the world hypertension league and supporting organizations. J Clin Hypertens (Greenwich). 2014;16:469–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Graudal N, Hubeck‐Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2011;11:CD004022. [DOI] [PubMed] [Google Scholar]
- 39. Van Vliet B, Montani J. The time course of salt‐induced hypertension, and why it matters. Int J Obes. 2008;32:S35–S47. [DOI] [PubMed] [Google Scholar]
- 40. Svitok P, Molcan L, Vesela A, et al. Increased salt intake during early ontogenesis lead to development of arterial hypertension in salt‐resistant Wistar rats. Clin Exp Hypertens. 2014. July 22;1–6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41. Gee ME, Campbell N, Sarrafzadegan N, et al. Standards for the uniform reporting of hypertension in adults using population survey data: recommendations from the World Hypertension League expert committee. J Clin Hypertens (Greenwich). 2014;16:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. A large‐scaled cluster randomised trial to determine the effects of sodium reduction on stroke: The China salt substitute and stroke study (SSaSS). http://clinicaltrials.gov/show/NCT02092090. Accessed February 24, 2015.
- 43. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]


