The prevalence of microalbuminuria in adult hypertensive patients without diabetes varies among studies, with reported ranges from below 10% up to about 25%.1 The development of microalbuminuria in primary hypertension likely reflects glomerular injury, a point reinforced by a recent study which demonstrated that the risk of development of microalbuminuria was related to the presence and evolution of glomerular hyperfiltration.2 The presence of microalbuminuria, even in nondiabetic patients, increases the risk of cardiovascular disease, and there seems to be a relationship between not only the presence of microalbuminuria, but also its severity in determining total cardiovascular risk.1, 3 There is also a strong association between microalbuminuria and inflammatory markers such as high‐sensitivity C‐reactive protein, suggesting a pathophysiologic role of inflammation and vascular dysfunction in the development of kidney damage.4 Given these clear relationships, current guidelines for assessment of hypertensive adults include screening for microalbuminuria,5 and treatment to reduce microalbuminuria has been shown to reduce cardiovascular risk in large‐scale clinical trials.1
In children, however, extensive data on microalbuminuria and its relationship with blood pressure (BP) are mostly lacking, so current clinical practice guidelines are less clear. The National High Blood Pressure Education Program Working Group stated that there was insufficient evidence to recommend routine assessment of microalbuminuria in hypertensive children and adolescents,6 whereas the more recent European Society of Hypertension pediatric guidelines recommend that microalbuminuria be assessed, but also note that there is no evidence to guide the practitioner in using this information.7 The 2011 Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents from the National Heart, Lung, and Blood Institute8 did not even mention microalbuminuria. However, the recently issued pediatric guideline from the Canadian Hypertension Education Program9 does recommend inclusion of urine microalbumin excretion in order to assess for the presence of target organ damage in children with confirmed hypertension; this recommendation was based on the results of one single‐center study10 which will be discussed later. Thus, the practitioner who cares for hypertensive children and adolescents has conflicting guidance from consensus organizations on whether to routinely screen for microalbuminuria, or what to do with the results of such testing if performed anyway.
Definitions
The most widely accepted definition for microalbuminuria is urinary albumin excretion of 30 to 299 mg/24 h in an adult, which typically correlates to a microalbumin: creatinine ratio of >20 to 30 mg/g creatinine on a spot urine specimen. This is a level of urinary protein excretion below the limit of detection of a standard urine dipstick, thus more specific assays are typically used.11
Pediatric‐specific definitions of microalbuminuria have not been developed because of the lack of robust data on urinary microalbumin excretion rates in normal healthy children.12 Population‐based studies such as the National Health and Nutrition Evaluation Survey (NHANES) have generally adopted similar definitions for pediatric patients as in adults.13 A large study conducted in nearly 2000 healthy Chinese children14 established upper ranges of 14.7 mg/g creatinine for boys and 19.8 mg/g creatinine for girls, suggesting that it is reasonable to use the adult definitions of microalbuminuria in pediatric patients as well.
Microalbuminuria in Healthy Children
There are few large‐scale studies available on urinary microalbumin excretion in healthy children. The studies that have been conducted have generally used timed urine collections, and have demonstrated mean microalbumin excretion rates between 2 and 6 μg/min, with a slight increase in age until adolescence.15 Age, sex, maturity, body size, and fasting insulin levels16 have been shown to correlate with urine microalbumin excretion, although findings between studies have not been consistent. The relationship with various cardiovascular risk factors has also been unclear, with at least one large‐scale study16 failing to show a relationship between microalbuminuria and cardiovascular risk factors such as systolic and diastolic BP. However, in the Chinese study mentioned earlier,14 systolic but not diastolic BP was positively correlated with albumin excretion rate, and children with systolic BP of 111 to 130 mm Hg had significantly higher albumin/creatinine ratios than those with systolic BP ≤90 mm Hg.
Some studies have shown that urine microalbumin excretion may be higher in very young children than in adolescents. A study of Korean children showed that the urine microalbumin/creatinine ratio declined with age, with the highest values seen in children younger than 1 year.17 Another study conducted in the Netherlands examined microalbuminuria in toddlers between just 20 and 40 months of age, and compared urine microalbumin excretion with data from an adult study of risk factors for cardiovascular and renal disease.18 These investigators found comparable levels of microalbuminuria in the toddler group as adults, and also found a similar proportion of elevated urine microalbumin/creatinine ratios in the two populations. These studies suggest that there may be a maturational component to urine microalbumin excretion and highlight the importance of using similar‐aged controls in studies intended to establish microalbuminuria prevalence in other pediatric populations.
Race does appear to be a determinant of microalbuminuria in children, with higher microalbumin excretion rates seen in black children and adolescents compared with whites.15 A study conducted in Nigerian schoolchildren19 found that 33% had abnormal microalbuminuria levels by dipstick, with a higher prevalence in female compared with male children. Hypertensive children had a higher prevalence of microalbuminuria, as did those with a family history of either hypertension or diabetes. Unfortunately, the sickle hemoglobin status of the patients was unknown, and effects of orthostasis and physical activity were not examined.19 Among American children, at least one relatively large study confirmed a higher albumin excretion rate in normotensive black adolescents compared with white adolescents,20 as well as a positive relationship between BP and microalbumin excretion in blacks but not whites. Interestingly, there was also an association with impaired stress‐induced pressure natriuresis in the black adolescents, suggesting an increased risk of renal injury in this population.
Obesity status is another important factor affecting microalbuminuria in otherwise healthy children,12, 15 with increased body mass index (BMI) associated with microalbuminuria in several studies.14, 17, 19 Data from 2515 adolescents in the 1999–2004 NHANES cohort,21 however, showed a higher prevalence of microalbuminuria in nonobese participants than in those with elevated BMI. While microalbuminuria was not associated with other cardiovascular risk factors in the normal‐weight adolescents, there was a relationship with impaired fasting glucose, insulin resistance, and BP among overweight adolescents. One other large‐scale study also showed a higher prevalence of microalbuminuria in lean children compared with overweight children22 and suggested that perhaps this was related to greater physical activity. In two other studies of obese children only, there was a clear relationship between microalbuminuria and other components of the metabolic syndrome among 150 Egyptian children,23 but the prevalence of microalbuminuria was just 2.7% in 408 Dutch children,24 among whom there was no relationship between microalbuminuria and other cardiovascular risk factors. Thus, even in a population that should at least theoretically be at risk for development of microalbuminuria, data are contradictory.
Microalbuminuria in Children With Elevated BP
As noted, several studies suggest that there is a relationship between urine microalbumin excretion rate and BP in the pediatric age group14, 19, 20; therefore, the rationale for examining this question in hypertensive youth is clear. Unfortunately, the studies that have been conducted among hypertensive children and adolescents have tended to be smaller than the cross‐sectional studies discussed earlier, limiting the applicability of their findings.
The first investigators to examine microalbuminuria in hypertensive children and adolescents were Belsha and colleagues,25 who performed ambulatory BP monitoring (ABPM) in 33 normotensive and 29 hypertensive adolescents, and compared 24‐hour microalbumin excretion between the two groups, with no significant difference found. Only four of the 62 patients in that study had abnormal microalbumin excretion, and three of those were in the normotensive group. Sorof and colleagues26 also failed to demonstrate abnormal urine microalbumin excretion in two populations of hypertensive adolescents, one identified by school screening and the other referred by other providers. There was no difference in microalbumin/creatinine ratio in the two groups, despite a greater severity of ABPM‐confirmed hypertension in the referral group.
Assadi27 studied 55 adolescents from a single center with primary hypertension diagnosed based on office BP measurements who required antihypertensive treatment. All underwent echocardiography, determination of urine microalbumin excretion, and measurement of other biochemical parameters. Microalbuminuria was found to correlate with the severity of hypertension and also with the presence of left ventricular hypertrophy. After 1 year of treatment with enalapril plus hydrochlorothiazide (nine children also received losartan), the level of microalbuminuria was significantly reduced and the reduction in microalbuminuria was associated with regression of left ventricular hypertrophy. While provocative, the findings from this study are limited due to the single‐center study design, diagnosis of hypertension based on office BP measurements, and lack of an untreated control group.
In another study, Seeman and colleagues28 retrospectively studied 52 children and adolescents with either primary or white‐coat hypertension recruited from three centers; all had elevated office BPs and either elevated (primary hypertension group) or normal (white‐coat hypertension group) ambulatory BP on 24‐hour ABPM. A total of 20% of children with confirmed primary hypertension had microalbuminuria, defined as microalbumin/creatinine ratio >3.2 mg/mmol creatinine, whereas none of the children with white‐coat hypertension had microalbuminuria. The median level of urinary microalbumin excretion in the primary hypertension group was also significantly greater than in the white‐coat hypertension group (Figure). The applicability of these data to clinical practice are limited by the retrospective study design, the small sample size, and by the lack of repeat measurements of urinary microalbumin excretion.
Figure 1.
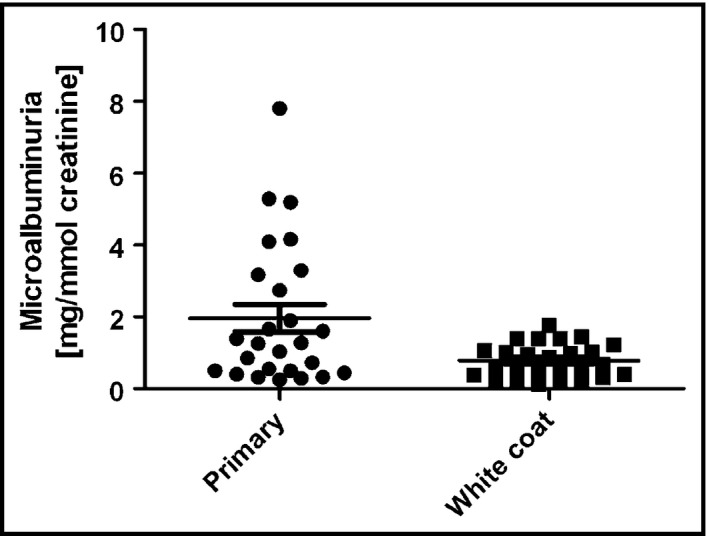
Urinary albumin excretion in children with primary and white‐coat hypertension. Each filled symbol represents data from an individual patient; horizontal line represents the mean; bracketed lines, standard error of mean. P<.05 between primary and white‐coat hypertension. Reprinted with permission from Springer.28
More recently, two studies performed in Turkish children have added some new information on microalbuminuria in hypertensive children. Girişgen and colleagues29 examined both 24‐hour urinary microalbumin and urine N‐acetyl‐β‐D‐glucosaminidase, another potential marker of hypertensive renal damage, in 56 children with elevated BP and 29 children with normal BP. There was no significant difference in median urinary microalbumin excretion between the two groups. Abnormal microalbuminuria (not precisely defined) was reported in 9% of the hypertensive children and 25% of the normotensive children. Interestingly, a correlation was found between ABPM parameters and urine microalbumin excretion, although the correlations were relatively weak, with R values ranging from 0.339 for night systolic BP to 0.384 for day diastolic BP.29 However, no such relationship between ABPM parameters and microalbuminuria was found by Conkar and colleagues,10 whose study was cited by the Canadian pediatric BP guideline,9 and who studied 82 hypertensive children with ABPM and examined relationships between various ABPM parameters and markers of hypertensive target organ damage, including microalbuminuria and left ventricular hypertrophy. Despite a high rate of microalbuminuria (25.6%) in their study group, there were no relationships seen between microalbuminuria and any ABPM parameter.
Thus, it seems that although microalbuminuria has been reported more often in hypertensive children in recent studies, a clear relationship between urine microalbumin excretion and BP levels has not yet been established. While it appears that ABPM may be better correlated with microalbuminuria than casual BP levels, ABPM was not used in all studies, and the studies that did use ABPM10, 25, 28, 29 had conflicting results. In addition, all of the studies of microalbuminuria in hypertension were relatively small and were conducted in single centers, with the inherent flaws found in such research. A large, multicenter study, optimally utilizing both casual and ambulatory BP measurement is required to generate more reliable data on microalbuminuria in hypertensive children and adolescents.
Conclusions and Future Directions
Current data on microalbuminuria in children with primary hypertension remain scant and are subject to numerous limitations. While some cross‐sectional population studies have demonstrated a relationship between urine microalbumin excretion and BP in healthy children, similar conclusions cannot be drawn from the small single‐center studies that have been conducted to date in hypertensive children and adolescents. In addition, data from studies conducted in other high‐risk pediatric populations, specifically obese children and adolescents, remain inconsistent. Given this, there is still insufficient evidence to support routine screening for microalbuminuria in hypertensive children, and no specific evidence‐based recommendations regarding treatment can be made. Appropriate large‐scale, multicenter studies are needed to generate reliable evidence on the prevalence and predictors of microalbuminuria in children and adolescents with elevated BP. Such evidence should then be used to inform future clinical practice guidelines for the evaluation and management of elevated BP in the young.
Conflict of Interest
The author reports no specific funding in relation to this research and no conflicts of interest to disclose.
Financial Support
None received.
References
- 1. de Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol. 2006;17:2100–2105. [DOI] [PubMed] [Google Scholar]
- 2. Palatini P, Mos L, Ballerini P, et al; HARVEST Investigators . Relationship between GFR and albuminuria in stage 1 hypertension. Clin J Am Soc Nephrol 2013;8:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahfoud F, Ukena C, Pöss J, et al. Microalbuminuria independently correlates to cardiovascular comorbidity burden in patients with hypertension. Clin Res Cardiol. 2012;101:761–766. [DOI] [PubMed] [Google Scholar]
- 4. Kaplan NM, Victor RG. Primary hypertension: natural history and evaluation. In: Kaplan NM, Victor RG, eds. Kaplan's Clinical Hypertension, 10th ed. Philadelphia, PA: Lippincott‐Williams and Wilkins; 2009:122–160. [Google Scholar]
- 5. Tsioufis C, Dimitriadis K, Andrikou E, et al. ADMA, C‐reactive protein, and albuminuria in untreated essential hypertension: a cross‐sectional study. Am J Kidney Dis 2010;55:1050–1059. [DOI] [PubMed] [Google Scholar]
- 6. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555–576. [PubMed] [Google Scholar]
- 7. Lurbe E, Cifkova R, Cruickshank JK, et al. Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J Hypertens. 2009;27:1719–1742. [DOI] [PubMed] [Google Scholar]
- 8. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–S256. [DOI] [PMC free article] [PubMed]
- 9. Harris KC, Benoit G, Dionne J, et al; CHEP Guidelines Task Force . Hypertension Canada's 2016 CHEP guidelines for blood pressure measurement, diagnosis and assessment of risk of pediatric hypertension. Can J Cardiol 2016;32:589–597. [DOI] [PubMed] [Google Scholar]
- 10. Conkar S, Yılmaz E, Hacıkara Ş, et al. Is daytime systolic load an important risk factor for target organ damage in pediatric hypertension? J Clin Hypertens (Greenwich). 2015;17:767–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Busby DE, Bakris GL. Comparison of commonly used assays for the detection of microalbuminuria. J Clin Hypertens (Greenwich). 2004;6(11 Suppl 3):8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsioufis C, Mazaraki A, Dimitriadis K, et al. Microalbuminuria in the paediatric age: current knowledge and emerging questions. Acta Paediatr. 2011;100:1180–1184. [DOI] [PubMed] [Google Scholar]
- 13. Jones CA, Francis ME, Eberhardt MS, et al. Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39:445–459. [DOI] [PubMed] [Google Scholar]
- 14. Wu DQ, Yang HP, Luo J, et al. Age‐ and gender‐specific reference values for urine albumin/creatinine ratio in children of southwest China. Clin Chim Acta. 2014;431:239–243. [DOI] [PubMed] [Google Scholar]
- 15. Rademacher ER, Sinaiko AR. Albuminuria in children. Curr Opin Nephrol Hypertens. 2009;18:246–251. [DOI] [PubMed] [Google Scholar]
- 16. Rademacher ER, Mauer M, Jacobs DR, et al. Albumin excretion rate in normal adolescents: relation to insulin resistance and cardiovascular risk factors and comparisons to type 1 diabetes mellitus patients. Clin J Am Soc Nephrol. 2008;3:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwak BO, Lee ST, Chung S, Kim KS. Microalbuminuria in normal Korean children. Yonsei Med J. 2011;52:476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gracchi V, van den Belt SM, Küpers LK, et al. Prevalence and distribution of (micro)albuminuria in toddlers. Nephrol Dial Transplant. 2015; doi: 10.1093/ndt/gfv407. [DOI] [PubMed] [Google Scholar]
- 19. Okpere AN, Anochie IC, Eke FU. Prevalence of microalbuminuria among secondary school children. Afr Health Sci. 2012;12:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanevold CD, Pollock JS, Harshfield GA. Racial differences in microalbumin excretion in healthy adolescents. Hypertension. 2008;51:334–338. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen S, McCulloch C, Brakeman P, et al. Being overweight modifies the association between cardiovascular risk factors and microalbuminuria in adolescents. Pediatrics. 2008;121:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirschler V, Molinari C, Maccallini G, Aranda C. Is albuminuria associated with obesity in school children? Pediatr Diabetes. 2010;11:322–330. [DOI] [PubMed] [Google Scholar]
- 23. Sanad M, Gharib A. Evaluation of microalbuminuria in obese children and its relation to metabolic syndrome. Pediatr Nephrol. 2011;26:2193–2199. [DOI] [PubMed] [Google Scholar]
- 24. Radhakishun NN, van Vliet M, von Rosensteil IA, et al. Limited value of routine microalbuminuria assessment in multi‐ethnic obese children. Pediatr Nephrol. 2013;28:1145–1149. [DOI] [PubMed] [Google Scholar]
- 25. Belsha CW, Wells TG, McNiece KL, et al. Berry PL. Influence of diurnal blood pressure variations on target organ abnormalities in adolescents with mild essential hypertension. Am J Hypertens. 1998;11:410–417. [DOI] [PubMed] [Google Scholar]
- 26. Sorof JM, Turner J, Martin DS, et al. Cardiovascular risk factors and sequelae in hypertensive children identified by referral versus school‐based screening. Hypertension. 2004;43:214–218. [DOI] [PubMed] [Google Scholar]
- 27. Assadi F. Effect of microalbuminuria lowering on regression of left ventricular hypertrophy in children and adolescents with essential hypertension. Pediatr Cardiol. 2007;28:27–33. [DOI] [PubMed] [Google Scholar]
- 28. Seeman T, Pohl M, Palyzova D, John U. Microalbuminuria in children with primary and white‐coat hypertension. Pediatr Nephrol. 2012;27:461–467. [DOI] [PubMed] [Google Scholar]
- 29. Girişgen I, Sönmez F, Yenisey Ç, Kurt‐Omurlu I. Urinary markers of renal damage in hypertensive children diagnoses with ambulatory blood pressure monitoring. Turk J Pediatr. 2014;56:48–55. [PubMed] [Google Scholar]


