Abstract
Nighttime blood pressure strongly predicts cardiovascular events (CVEs). Further, a preliminary trial has shown decreased CVEs from evening vs morning dosing of antihypertensive therapy. Is there additional evidence for evening dosing? The authors systematically classified all hypertension trials as evening dosing trials (EDTs) or usual dosing trials (UDTs). Meta‐analyses provided standardized hazard ratios for CVEs for EDTs (HREDT s) and UDTs (HRUDT s). HREDT s/HRUDT s gave the relative risk (RR) from evening vs usual dosing. Among 175 trials, 5 EDTs were discovered. The RR for CVEs (95% confidence limits) from evening vs usual dosing was 0.63 (0.43–0.92; P=.016). After adjustment for drug class, the RR was 0.54 (0.34–0.85; P=.008). Unlike other EDTs, the Heart Outcomes Prevention Evaluation (HOPE) study administered its entire antihypertensive dose prior to sleep and gave the greatest risk reduction. This study provides a third type of evidence suggesting a beneficial effect from evening dosing of antihypertensive therapy. Head‐to‐head, multicenter trials are needed to test this strategy.
More than 40 years ago, the US Veterans Administration conducted two small placebo‐controlled trials in men with very high clinic blood pressures (BPs). These trials demonstrated striking reductions (73 to 95%) in major cardiovascular events (CVEs) for patients randomized to receive antihypertensive therapy vs placebo.1, 2 Since then, there has been increasing interest in the use of home, 24‐hour ambulatory, and central arterial BP measurements. Nonetheless, 4 decades after these landmark trials, clinic BP remains the primary target for reducing cardiovascular risk from hypertension.3, 4, 5
Two types of observations suggest that targeting nighttime BP may be more relevant than targeting clinic, home, or daytime ambulatory BP. (1) In a longitudinal study of individuals randomly sampled from the general population, simultaneous adjustment for daytime and nighttime systolic blood pressures led to sustained prognostic value from nighttime but less so for daytime systolic blood pressure;6 in a longitudinal study of 13,844 patients with hypertension from 9 regions, simultaneous adjustment for clinic, daytime, and nighttime systolic blood pressures led to sustained prognostic value for nighttime systolic blood pressure while clinic and daytime systolic blood pressures lost their prognostic value entirely.7 (2) Hermida and colleagues8 have reported that patients randomized to receive ≥1 antihypertensive agent in the evening compared with patients receiving all antihypertensive agents in the morning had substantial reductions in CVEs and total mortality. However, as pointed out by its authors, this trial had important limitations because of its relatively modest sample size, lack of blinding, and limitation to a single center.
Because data are unavailable that directly compare timing of dosing regimens in large, multicenter, double blind trials, we compared the risk reduction in CVEs in trials whose protocols specifically dictated evening dosing of an antihypertensive with the risk reduction in CVEs in trials without this requirement.
Methods
A systematic review was conducted for trials examining the effects of BP reduction on CVEs (“BP difference trials”). For trials reported from 1967 (the year of the first report of an antihypertensive trial with CVEs as outcomes) through December 31, 2007, the comprehensive review of Law and colleagues9 was used. For trials published from January 01, 2008, through December 31, 2013, PubMed and Cochrane databases were searched using terms provided in the Data S1, Section 1. The BP difference trials were classified as either evening dosing trials (EDTs) or usual dosing trials (UDTs) (see Data S1, Section 2 for further details). Additional EDTs were sought from trials comparing 2 or more drugs with each other (“drug comparison trials”).
To identify EDTS, we reviewed trials with more than 20 coronary artery disease (CAD) events and more than 20 strokes, representing 98.4% of all outcomes. Of these, the methodology section of each trial was examined for an evening dosing protocol. When the primary reference cited an earlier paper pertaining to the methods, we reviewed the earlier paper for dosing regimens. A trial with twice‐daily, thrice‐daily, or 4‐times‐daily dosing was deemed to be insufficient to label it an EDT and was included as a UDT for the following reasons: (1) It was not clear that the last dose of the day was given in the evening; (2) twice‐daily, thrice‐daily, and 4‐times‐daily dosing would give the equivalent of one half, one third, and one fourth of the standard dose, respectively, for the last dose of the day; (3) in cross‐sectional data, twice‐daily dosing and exclusively morning dosing have been associated with similar reductions in nondipping, microalbuminuria, and chronic kidney disease, and both of these types of dosings were inferior to evening dosing with respect to these conditions.10
The hazard ratio (HR) of each trial was standardized to a 10 mm Hg reduction in systolic and a 5 mm Hg reduction in diastolic BP. For CAD, the pooled HRs for EDTs and UDTs were obtained by meta‐analysis11 (random‐effects models were used throughout), yielding HREDTs and HRUDTs, respectively. The relative risk (RR) from evening dosing vs usual dosing was obtained by computing the ratio of the two HRs (HREDTs/HRUDTs) and its 95% confidence limits.12 An analogous RR was computed for stroke. The two RRs were pooled to obtain the overall RR. The I 2 statistic was used to assess the extent to which the pooled RRs differed from one another (0%=no detectable heterogeneity, 100%=extreme heterogeneity). A two‐sided P value of <.05 was chosen as the level of statistical significance. Analyses were executed by RevMan, Version 5.2, of the Cochrane Collaboration (Oxford, England).
A second type of analysis removed the potentially beneficial, non–BP‐related effects specific to a drug class by repeating the above analyses within each drug class and then pooling the RRs across drug classes.
Two supplemental types of analyses were undertaken: (1) Sensitivity analyses were conducted in which ≥1 of the EDTs was excluded, and the remaining EDTs were used in the meta‐analysis; (2) the methods described above yielded RRs based on total events. However, both CAD and stroke could occur in the same individual (although this is uncommon, occurring in our sample in about 1% to 8% of all individuals with CVEs). Because trials typically report risks for individuals with CVEs rather than for the CVEs themselves, we estimated the RRs for CVEs based on affected individuals according to the method provided in Data S1, Section 3, a method that is highly conservative with respect to the evening dosing hypothesis.
Results
Trial Ascertainment
Trial ascertainment is shown in Figure 1. For trials published from January 1, 2008, to December 31, 2013, miscellaneous sources provided 5 trials, Cochrane 94, and PubMed 991. After screening titles and reading abstracts and journal articles, 21 primary references remained, of which 13 were BP difference trials and 8 were drug comparison trials (see Data S1 Sections 4 and 5 for the references for these trials). To these, the corresponding trials published from 1967 through December 31, 2007, were added, yielding 121 BP difference trials and 54 drug comparison trials for review.
Figure 1.
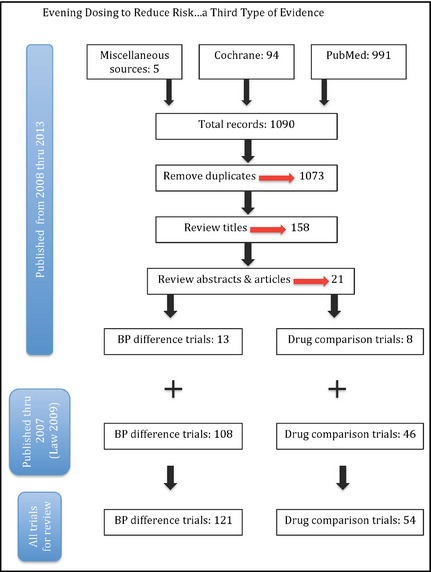
Number of articles at each step of systematic review beginning with miscellaneous sources, the Cochrane Registry of Randomized Trials, and the PubMed database leading to 13 blood pressure (BP) difference trials and 8 drug comparison trials published from January 1, 2008, to December 31, 2013. These were added to the trials from the systematic review by Law and colleagues,9 which covered trials published from 1967 to December 31, 2007, to yield 121 BP difference trials and 54 drug comparison trials for review.
Trial Characteristics
Five EDTs were discovered. Two drug comparison trials used evening dosing in one arm: Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) and Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET).13, 14 Blood pressure difference trials (all placebo controlled) using evening dosing were Heart Outcomes Prevention Evaluation (HOPE), Systolic Hypertension in China (Syst‐China), and Systolic Hypertension in Europe (Syst‐Eur).15, 16, 17 See Table 1 . There were a total of 35,075 participants in the EDTs and 312,057 in the UDTs. In the EDTs, there were 2320 CVEs, of which 1445 were CAD and 875 were stroke. The corresponding numbers for the UDTs were 18,129, 11,044, and 7085, respectively. The purpose of each EDT is given in the footnotes to Table 1. The mean ages of patients in the 5 trials were similar. Table 2 shows the 7 features of the EDTs that pertain to study quality. The HOPE trial had the highest quality with 6 of the 7 features, while CONVINCE, Syst‐China, and Syst‐Eur had 5 features, and FACET had 4 features.
Table 1.
Characteristics of Trials with Evening Dosing
| Trial (Author and Year) | N, Population | Target Population | Major Exclusions | Mean Age | Percent with the Cardiovascular Risk Factor | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | CAD | Stroke | CVD | DM | Smokers | |||||
| CONVINCE (Black 2003)13,a | 16,602. 661 centers in the Americas and Europe | Age 55+, Elevated office BP, 1+ CVD risk factor | BP > 190/110, CHF, arrhythmias, secondary hypertension, CKD, night or evening worker, recent MI or stroke | 66 | 44 | 8 | 5 | 17 | 20 | 23 |
| FACET (Tatti 1998)14,b | 380. Outpatient diabetes clinic, Italy | Elevated office BP | CAD, CVA, CKD, comorbidity with poor prognosis, albuminuria, lipid lowering drugs, aspirin | 63 | 41 | 0 | 0 | 0 | 100 | 6 |
| HOPE (HOPE 2000)15,c | 9297. The Americas and Europe | Age 55+, Atherosclerotic disease or DM, 1 other cardiovascular risk factor | CHF, EF < 40%, Recent MI or CVA, uncontrolled BP | 66 | 73 | 80 | 11 | 38 | 14 | |
| Syst‐China (Liu 1998)16,d | 2394. 31 Centers in China | Age 60+, Clinic BP 160+ | <60 years old, Secondary hypertension, CHF, Creatinine >180 mmol/L, severe disease | 67 | 64 | 10 | 2 | 31 | ||
| Syst‐Eur (Staessen 1997)17,d | 4695. 198 centers in Europe | Ditto | Ditto | 70 | 33 | 30 | 7 | |||
Abbreviations: BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; CVD, cardiovascular disease; CHF, congestive heart failure; EF, left ventricular ejection fraction; MI, myocardial infarction.
Blank cells indicate that this characteristic was not reported.
aCONVINCE tested whether controlled onset extended release verapamil when given in the evening would be more effective in reducing CVEs than atenolol or hydrochlorothiazide given in the morning. bFACET examined whether amlodipine was less effective in reducing CVEs than fosinopril. cHOPE tested whether an angiotensin converting enzyme inhibitor can improve outcomes in high risk patients without CHF. dSyst‐China and Syst‐Eur examined whether treatment with nitrendipine can reduce CVEs in older patients with isolated systolic hypertension.
Table 2.
Quality Measures of Trials with Evening Dosing
| Trial | Control | Double Blind | Precision in Specifying Evening Dosingb | Allocation Concealment | Complete Follow up | Independent Adjudication of Outcomes | Intention to Treat Analysis |
|---|---|---|---|---|---|---|---|
| CONVINCE | Alternate drug | Yes | “prior to sleep” | Yes | 80% | Yes | Yes |
| FACET | Alternate drug | No | “evening” | Yes | 99% | Yes | Yes |
| HOPE | Placeboa | Yes | “prior to sleep” | Yes | Not reported | Yes | Yes |
| Syst‐China | Placeboa | Yes | “evening” | Not reported | 90% | Yes | Yes |
| Syst‐Eur | Placeboa | Yes | “evening” | Not reported | 95% | Yes | Yes |
aA trial whose comparator arm was another drug, while not necessarily an inferior feature in the original trial, was considered a drawback for the purposes of our study. bDefining “evening” as “prior to sleep” was deemed to be more precise and of higher quality than “evening.”
Table 3 gives the dosing schedules. With the exception of FACET, it was clear that the investigators reporting the EDTs had a specific interest in evening dosing. In CONVINCE, the hypothesis behind the evening dosing was that controlled‐onset extended‐release verapamil (COER verapamil) would blunt the early morning surge in BP, coming at a time when there is a higher rate of CVEs than in other segments of the 24‐hour period. COER verapamil begins its release after 4 to 5 hours so there would be an effect from the drug towards the end of the period for nocturnal BP. The HOPE trial used just one antihypertensive, ramipril, which was given prior to sleep. A small substudy of HOPE (N=38) showed statistically significant declines in nighttime and 24‐hour BP (17/8 mm Hg and 10/4 mm Hg, respectively), but not in clinic or daytime BP (8/2 mm Hg and 6/2 mm Hg, respectively).18 In the Syst‐China protocol, it was noted that patients in the intervention arm were “examined at trough levels” on clinic days. In the Syst‐Eur trial, in addition to dosing with nitrendipine initially in the evening, the step 2 drug, enalapril, was dosed only in the evening.
Table 3.
Blood Pressures in mm Hg, Interventions, Median or Mean Years of Follow up, Number of Events, and Hazard Ratios (95% Confidence Intervals)
| Trial | Baseline BP | Target BP | Intervention Arm (Drug Doses are in Milligrams) | Length of Follow up | Difference in Achieved BP | Number of Events | Hazard Ratio(95% Confidence Limits) | ||
|---|---|---|---|---|---|---|---|---|---|
| CAD | Stroke | CAD | Stroke | ||||||
| CONVINCE | 150/87 | <140/90 | Controlled onset verapamil 180 prior to sleepa | 3.0 | 0.1/0.7 | 299 | 251 | 0.82 (0.65–1.03) | 1.15 (0.90–1.48) |
| FACET | 171/95 | <140/90 |
(1) Amlodipine 10 in the eveningb (2) Fosinopril 20 in the am (given in 26% of those in the amlodipine arm) |
2.5–3.5 | 6/0 | 23 | 14 | 1.30 (0.57–2.94) | 2.56 (0.81–8.33) |
| HOPE | 139/79c | Not applicable | Ramipril 10 prior to sleep | 4.0–5.3 | 3.0/1.0 | 1029 | 382 | 0.80 (0.70–0.90) | 0.68 (0.56–0.84) |
| Syst‐China | 170/86 | Decrease in SBP by 20+ to less than 150 |
(1) Nitrendipine 10 at evening, then 20 at evening, then 20 BID (2) Captopril or HCTZ 12.5 in am escalating to 12.5–25 BID |
3.0 | 8.55/3.1 | 16 | 104 | 1.06 (0.39–2.84) | 0.62 (0.42–0.91) |
| Syst‐Eur | 174/86 | Ditto |
(1) Nitrendipine 10 at evening, then 10 BID, then 20 BID (2) Enalapril 5–20 at evening or HCTZ 12.5–25 in am |
2.0 | 10.1/4.5 | 78 | 124 | 0.70 (0.44–1.09) | 0.58 (0.40–0.83) |
Abbreviation: CAD, coronary artery disease.
aThe control arm received either 50 mg of atenolol or 12.5 mg of hydrochlorothiazide in the morning. bThe control arm received fosinopril 20 mg in the morning, and, if BP was not controlled, amlodipine was given at a time not specified; this occurred in 31% in the fosinopril arm. c47% were diagnosed with hypertension.
In FACET, there was no explanation as to why amlodipine was given in the evening. An interesting feature of FACET was the use of fosinopril at step 2 in the amlodipine arm and the use of amlodipine at step 2 in the fosinopril arm. In neither instance was the time of day for the step 2 drug specified.
Overall RR From Evening vs Usual Dosing
The EDTs and UDTs lowered risk for CAD by 45% and 14%, respectively (RR=0.64, P=.061) (Figure 2), while EDTs and UDTs lowered risk for stroke by 57% and 29%, respectively (RR=0.61, P=.129). Pooling the RRs for CAD and stroke gave an overall RR for CVEs of 0.63 (95% confidence interval [CI], 0.43–0.92; P=.016). There was no detectable heterogeneity between the RRs for CAD and stroke (I 2=0%).
Figure 2.
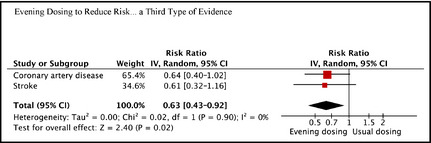
Relative risks from evening dosing vs usual dosing for coronary artery disease, stroke, and all cardiovascular events. P values are .061, .129, and .016, respectively. IV indicates inverse variance weighting; CI, confidence interval.
Drug‐Adjusted Analysis
Within the angiotensin‐converting enzyme (ACE) inhibitor drug class, the RR was 0.38 (95% CI, 0.21–0.69; P=.001) (Figure 3). Within the calcium channel blocker drug class, the corresponding RR was 0.77 (95% CI, 0.53–1.13; P=.184). The overall RR was 0.54 (95% CI, 0.34–0.85; P=.008), with moderate heterogeneity of the RRs between the drug classes (I 2=57%).
Figure 3.
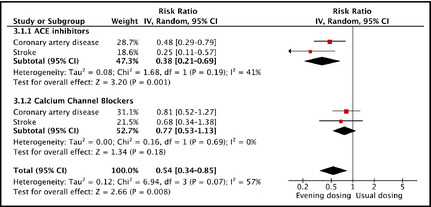
Relative risks from evening dosing vs usual dosing for all cardiovascular events within drug classes and the overall relative risk adjusted for drug class. IV indicates inverse variance weighting; CI, confidence interval; ACE, angiotensin‐converting enzyme; CAD, coronary artery disease; CVA, cerebrovascular accident.
Based on individuals affected by CVEs (as opposed to the events themselves), the RR for the drug‐adjusted analysis was 0.55 (95% CI, 0.35–0.89; P=.014). Table 4 shows the effect of withdrawing each EDT and computing the RR for the remaining EDTs vs the UDTs. The 4 largest trials (CONVINCE, HOPE, Syst‐China, and Syst‐Eur) each contributed to lowering the risk, with HOPE having the greatest effect on the overall RR from evening dosing. When excluding the 2 drug comparison trials, CONVINCE and FACET, and limiting the analysis to BP difference trials, results again favored evening dosing (RR=0.64 [95% CI, 0.45–0.92]; P=.014).
Table 4.
Withdrawal of an Evening Dosing Trial (s) and Computation of the Relative Risk from Evening Versus Usual Dosing for the Remaining Evening Dosing Trials
| Trial (s) Withdrawn | RR | Low CL | High CL | P |
|---|---|---|---|---|
| None | 0.63 | 0.43 | 0.92 | .016 |
| CONVINCE | 0.64 | 0.44 | 0.93 | .020 |
| FACET | 0.63 | 0.44 | 0.91 | .013 |
| HOPE | 0.78 | 0.54 | 1.12 | .180 |
| Syst‐China | 0.59 | 0.37 | 0.93 | .025 |
| Syst‐Eur | 0.54 | 0.30 | 0.97 | .040 |
| CONVINCE and FACETa | 0.64 | 0.45 | 0.92 | .014 |
Abbreviations: CL, 95% confidence limit; RR, relative risk.
This limits the analysis to the 3 blood pressure difference trials.
Discussion
The present meta‐analysis adds a third type of evidence suggesting a benefit from evening dosing of antihypertensive agents. Evening dosing trials achieved RRs for CVEs that were significantly lower than those achieved by usual dosing trials.
Among evening dosing trials, HOPE was the strongest contributor to this effect, had the highest study quality, and was the only trial wherein its entire antihypertensive dose was given prior to sleep. The rationale for using ramipril in HOPE was based on the non–BP‐related effects of ACE inhibitors (eg, antiproliferative, antiatherogenic, and hormonal influences). However, a HOPE substudy18 was consistent with ramipril's pharmaco‐kinetics19 and showed that ramipril with its evening dosing had a greater effect on nighttime and 24‐hour BP than on clinic and daytime BP. An important finding of our study is that the statistically significant advantage of HOPE persisted when possible non–BP‐related drug class effects were removed by limiting the UDT comparators to ACE inhibitor trials in the drug‐adjusted meta‐analysis. Our result is consistent with the finding from a comprehensive meta‐analysis that risk reduction from ACE inhibitors (as well as other drug classes) is due primarily to their ability to lower BP rather than due to class‐specific, non–BP‐related effects.9 Rather than the non–BP‐related, class‐specific effects of ramipril as the mechanism for HOPE's substantial risk reduction, the above facts argue for evening dosing as the best explanation for the striking results of this trial.18
Relative to usual dosing, evening dosing may lead to better 24‐hour BP control. There was substantial improvement in 24‐hour BP control in the small HOPE substudy reported above.18 Also, in patients with resistant hypertension, more than two thirds of whom are nondippers in the evening, 24‐hour systolic BP was improved by 9.4 mm Hg by randomizing 1 antihypertensive agent to be given in the evening compared with all antihypertensive agents in the morning.20 However, in the more usual hypertensive population, the improvement in 24‐hour control from evening dosing is a more modest 1.71 mm Hg systolic.21
Why would targeting nighttime BP produce greater risk reduction than targeting daytime or clinic BP? One possibility is the better 24‐hour control from evening dosing noted above. In addition, nighttime hypertension and nondipping of BP are often induced by the need for nocturnal sodium excretion22 and are common among patients with hypertension, diabetes, renal insufficiency, and cardiovascular disease. In one study, nondipping in hypertensive patients increased cardiovascular risk by 25% even after adjusting for 24‐hour BP and cardiovascular risk factors.6 Reductions in nighttime pressure and restoration of the normal nocturnal BP dipping pattern may be beneficial through sodium‐related physiologic mechanisms that are incompletely understood.
Further, nondipping in uncontrolled hypertension is associated with endothelial dysfunction,23, 24 which is a known cardiovascular risk factor.25 Perhaps in hypertensive patients, arteriolar tone is improperly regulated at night, exposing the microcirculation, brain, heart, and kidneys to hydrostatic insult when there is raised BP in the larger arteries. It has been proposed that restoration of the normal dipping pattern may improve endothelial function and thereby lower risk.24
Potential adverse effects from evening dosing must be considered, with the possibility of events such as falling at night. Already, physicians may be dosing ≥1 antihypertensive agent in the evening, as recommended, for example, in the 2014 American Diabetes Association guidelines on managing diabetes with the designation as level A evidence.26 From the present set of trials, no data were available on potential adverse effects from evening dosing. However, a systematic review of 21 randomized trials of evening dosing (none of which included CVEs as outcomes) found no increase in withdrawal from evening treatment (RR=0.53 [95% CI, 0.26–1.07]) and no increase in adverse events (types not specified) in the evening dosing arm (RR=0.78 [95% confidence limits, 0.37–1.65]).21 A concern regarding evening dosing is the potential for a “J‐curve” effect on CVEs from an impact on BP that is already at a lower level in the evening, but a J curve was not demonstrated on examination of observational data.27
Study Limitations
We wish to emphasize that this study in no way provides conclusive evidence of the evening dosing hypothesis. (1) Random effects meta‐analyses must estimate between‐study heterogeneity and may be unreliable when there are a small number of studies (e.g., 3 to 5 or less). However, there is no observable heterogeneity in Figure 2. Furthermore, in Figure 3 the individual component effects for CAD and stroke favor the evening dosing hypothesis, and, in the case of the ACE inhibitor trials, these individual components were each statistically significant. Additionally, the overall effect in Figure 3 had a p value of 0.008, substantially below the significance level of .05. (2) Only CONVINCE and HOPE specified that the evening medication should be taken “prior to sleep,” and only HOPE measured the effect of evening dosing on nocturnal BP. However, with respect to the Syst‐China and Syst‐Eur trials, nitrendipine undoubtedly produced an effect on nighttime BP because the onset of its antihypertensive effect begins at 1 hour and peaks at 5 hours after ingestion.28 Thus, it is clear that HOPE, Syst‐China, and Syst‐Eur all had effects on nighttime BP from evening dosing, and these 3 trials by themselves demonstrated a statistically significant reduction in CVEs from evening vs usual dosing, as shown in sensitivity analysis. (3) Although there were a substantial number of CVEs (2320), there were only 5 EDTs to examine this question. Nonetheless, when HOPE was excluded, the point estimate for the RR from evening dosing still showed a benefit (RR=0.78), although the reduction in risk was no longer statistically significant. Also, as noted above, the features of HOPE and its greater contribution to risk reduction from evening dosing are consistent with the evening dosing hypothesis. (4) The usual dosing trials did not specify per protocol morning dosing and their classification relies on the assumption that antihypertensive agents are generally not taken in the evening. However, to the extent that some patients in these trials took their drugs in the evening, the result has been biased towards finding no effect from evening dosing, and therefore these results may be viewed as conservative with respect to the evening dosing hypothesis. (5) EDTs may have differed from UDTs on variables other than dosing time (eg, age and sex). However, the analysis herein is methodologically superior to case‐control and cohort studies, because, in the present analysis, variables such as age are not potential “confounders” in the usual sense. For such a variable to distort these results requires that (1) EDTs and UDTs differ substantially on that variable, (2) the variable is a strong effect modifier (which is by itself uncommon), and (3) conditions 1 and 2 must be in the appropriate direction. Because all 3 conditions would rarely prevail simultaneously, this consideration is unlikely to have affected the results.
Of the three types of evidence suggesting the value of evening dosing of antihypertensive agents, the present study is more relevant than data suggesting a superior ability of nocturnal BP to predict CVEs. On the other hand, our evidence is clearly less compelling than the Ambulatory Blood Pressure Monitoring for Prediction of Cardiovascular Events (MAPEC) trial, which showed substantially greater reduction in CVEs in those randomized to evening vs morning dosing.8 To their credit, the MAPEC authors have carefully delineated their study's limitations. Extending and modifying their design would provide a clearer understanding of the potential benefits (and possible hazards) from an evening dosing strategy.
Conclusions
These meta‐analyses add a third type of evidence favoring nocturnal BP as the most relevant target to reduce CVEs. This concept needs to be tested in large, multicentered, randomized trials with blind ascertainment of outcome. Ideally, two types of protocols would be undertaken, one with blinding of both patients and doctors and another with an open‐label design. The former may be considered a “pure” test of the hypothesis, while the latter represents a more “real‐world” assessment of the evening dosing strategy.
Supporting information
Data S1. Search Algorithm for Systematic Review, Statistical Considerations, and Ancillary References.
Disclosure
There were no grants or conflicts of interest to disclose.
J Clin Hypertens (Greenwich). 2014;16:561–568. ©2014 Wiley Periodicals, Inc.
References
- 1. Veterans Administration . Effects of dosing on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202:1028–1034. [PubMed] [Google Scholar]
- 2. Veterans Administration . Effects of dosing on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143–1152. [PubMed] [Google Scholar]
- 3. National Institute for Health and Clinical Excellence . Hypertension: Clinical management of primary hypertension in adults. Newcastle Guideline Development and Research Unit, National Clinical Guideline Center, and the British Hypertension Society, August 2011.
- 4. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 5. James PA, Oparil S, Carter BL, et al. 2014 Evidence‐Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 6. Hansen TW, Li Y, Boggia J, et al. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10. [DOI] [PubMed] [Google Scholar]
- 7. The ABC‐H Investigators: Roush G, Fagard R, Salles G, Pierdomenico S, Reboldi G, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polognia J, de la Sierra A, Hermida R, Dolan E, Zamalloa H. Prognostic Impact from Clinic, Daytime, and Nighttime Systolic Blood Pressure in 9 Cohorts of 13,844 Patients with Hypertension. J Hypertension. 2014. (in press). [DOI] [PubMed] [Google Scholar]
- 8. Hermida RC, Ayala DE, Mojón A, Fernández JR. Influence of circadian time of hypertension dosing on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629–1651. [DOI] [PubMed] [Google Scholar]
- 9. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hermida RC, Ríos MT, Crespo JJ, et al. Hygia Project Investigators . Dosing‐time regimen of hypertension medications significantly affects ambulatory blood pressure and clinical characteristics of patients with resistant hypertension. Chronobiol Int. 2013;30:192–206. [DOI] [PubMed] [Google Scholar]
- 11. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. West Sussex, UK: JohnWiley & Sons; 2009. [Google Scholar]
- 12. Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first‐line agents: a network meta‐analysis. JAMA. 2003;289:2534–2544. [DOI] [PubMed] [Google Scholar]
- 13. Black HR, Elliott WJ, Grandits G, et al. CONVINCE Research Group . Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073–2082. [DOI] [PubMed] [Google Scholar]
- 14. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21:597–603. [DOI] [PubMed] [Google Scholar]
- 15. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. [DOI] [PubMed] [Google Scholar]
- 16. Liu L, Wang JG, Gong L, et al. Comparison of active dosing and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst‐China) Collaborative Group. J Hypertens. 1998;16(12 pt 1):1823–1829. [DOI] [PubMed] [Google Scholar]
- 17. Staessen JA, Fagard R, Thijs L, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Lancet. 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 18. Svensson P, de Faire U, Sleight P, et al. Comparative effects of ramipril on ambulatory and office blood pressures: a HOPE substudy. Hypertension. 2001;38:E28–E32. [DOI] [PubMed] [Google Scholar]
- 19. Heber ME, Brigden GS, Caruana MP, et al. First dose response and 24‐hour antihypertensive efficacy of the new once‐daily angiotensin converting enzyme inhibitor, ramipril. Am J Cardiol. 1988;62:239–245. [DOI] [PubMed] [Google Scholar]
- 20. Hermida RC, Ayala DE, Fernandez JR, Calvo C. Chronotherapy improves blood pressure control and reverts the nondipper pattern in patients with resistant hypertension. Hypertension. 2008;51:69–76. [DOI] [PubMed] [Google Scholar]
- 21. Zhao P, Xu P, Wan C, Wang Z. Evening versus morning dosing regimen drug therapy for hypertension (Review). Cochrane Database Syst Rev. 2011;CD004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sachdeva A, Weder AB. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension. 2006;48:527–533. [DOI] [PubMed] [Google Scholar]
- 23. Quinaglia T, Martins LC, Figueiredo VN, et al. Non‐dipping pattern relates to endothelial dysfunction in patients with uncontrolled resistant hypertension. J Hum Hypertens. 2011;25:656–664. [DOI] [PubMed] [Google Scholar]
- 24. Higashi Y, Nakagawa K, Kimura M, et al. Circadian variation of blood pressure and endothelial function in patients with essential hypertension: a comparison of dippers and non‐dippers. J Am Coll Cardiol. 2002;40:2039–2043. [DOI] [PubMed] [Google Scholar]
- 25. Lerman A, Zeiher AM. Endothelial dysfunction: cardiovascular events. Circulation. 2005;111:363–368. [DOI] [PubMed] [Google Scholar]
- 26. American Diabetes Association . Standards of medical care in diabetes—2014. Diabetes Care. 2014;37:S14–S80. [DOI] [PubMed] [Google Scholar]
- 27. Hermida RC, Ayala DE, Mojón A, Fernández JR. Role of time‐of‐day of hypertension treatment on the J‐shaped relationship between blood pressure and cardiovascular risk. Chronobiol Int. 2013;30:328–339. [DOI] [PubMed] [Google Scholar]
- 28. Santiago TM, Lopez LM. Nitrendipine: a new dihydropyridine calcium‐channel antagonist for the treatment of hypertension. DICP. 1990;24:167–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Search Algorithm for Systematic Review, Statistical Considerations, and Ancillary References.


