Abstract
The purpose of this 2‐year multicentric, randomized, placebo‐controlled study was to evaluate the long‐term effects and adverse effects of spironolactone on chronic dialysis patients. A total of 253 non–heart failure dialysis patients with end‐stage renal disease were randomly assigned to 2‐year treatment with spironolactone (25 mg once daily, n=125) or a matching placebo (n=128) as add‐on therapy. The primary outcome was a composite of death from cardiocerebrovascular (CCV) events, aborted cardiac arrest, and sudden cardiac death, and the secondary outcome was death from all causes. Other CCV‐related indexes such as left ventricular mass index, left ventricular ejection fraction, heart rate variability, vascular endothelial function, and blood pressure–lowering effect were analyzed for patients who completed the whole 2‐year follow‐up study. Sociodemographic, clinical, and relevant laboratory data were also collected. During the 2‐year follow‐up, the primary outcome occurred less frequently in the spironolactone group vs the control group (7.2% vs 18.0%; adjusted hazard ratio [HR], 0.42; 95% confidence interval [CI], 0.26–0.78). Death from CCV events occurred in 4.0% of patients in the spironolactone group and in 11.7% of patients in the control group. Neither aborted cardiac arrest nor sudden cardiac death was significantly reduced by spironolactone treatment. The secondary outcome occurred less frequently in the spironolactone group vs the control group (9.6% vs 19.5%; adjusted HR, 0.52; 95% CI, 0.29–0.94). Other CCV‐related indexes except for heart rate variability were significantly improved. This study demonstrates that use of low‐dose spironolactone in non–heart failure dialysis patients can effectively reduce the risks of both CCV morbidity and mortality with few side effects. Moreover, the beneficial effect was mediated through improving the endothelial function or reducing left ventricular size independent of blood pressure changes, rather than mediation through changes in salt or potassium handling in the kidney.
End‐stage renal disease (ESRD) is recognized as a rapidly‐growing global health burden. At present, there are two effective renal replacement methods for treatment of ESRD, including chronic dialysis and renal transplant. Growing evidence suggests that patients with chronic kidney disease or dialysis have higher risks and severity of cardiocerebrovascular (CCV) diseases compared with the general population.1, 2, 3 One reason is attributed to several traditional and nontraditional risk factors and even uremia‐ or dialysis‐related factors. Farraginous factors will cause ill‐defined pathophysiologic processes (eg, persistent inflammation, endothelial dysfunction, oxidative stress, autonomic dysfunction, and vascular calcification) that are associated with the development of uremic cardiomyopathy or uremic vascular disease.4 CCV diseases account for more than 50% of deaths among dialysis patients with ESRD. Thus, management of CCV diseases is of particular importance in such patients because of their substantial impacts on prognosis. Clinically effective doses of spironolactone, a potassium‐sparing diuretic, can block the effect of aldosterone at the mineralocorticoid receptor, which reduces sodium reabsorption and promotes potassium excretion in distal renal tubular acidosis. Thus, spironolactone can therapeutically affect intravascular volume and electrolyte content and plays an important role in cardiovascular homeostasis. Apart from its hormonal function on renal handling of sodium, spironolactone as a pleiotropic hormone can activate mineralocorticoid receptors (MRs) in nonepithelial tissues, therefore affecting a variety of tissues (eg, myocardium, endothelium, and vascular smooth muscles). Recently, spironolactone was satisfactorily used in patients with refractory hypertension and in dialysis patients with reduced CCV morbidity and mortality.5, 6, 7
Spironolactone is not widely studied in dialysis populations, however, because of potential side effects (hyperkalemia and nonphysiologic gynecomastia). To address this clinical concern, we conducted a prospective randomized trial to evaluate its long‐term effects on survival rate, CCV protection, and various clinical parameters in dialysis patients with ESRD.
Methods
Study Design
This 2‐year randomized and placebo‐controlled study was conducted at three dialysis centers and approved by local ethics committees (approval No. 2011078). All patients gave informed consent before participation. Participants were assigned into two groups via block randomization with a block size of six with an allocation ratio of 1:1 after they completed baseline assessments. The allocation sequence was generated independently by a nurse and concealed in opaque envelopes. Both investigators and participants were not aware of the allocations. Both groups received conventional and chronic dialysis therapy.
Lifestyle modification such as potassium intake <1.5 g/d was advised. Measures were taken to reduce the effects of excessive interdialytic weight gain (defined as weight gain between two consecutive dialysis sessions, which is primarily dependent on the patient's fluid and sodium intake) and chronic volume overload on hyperkalemia, hypertension, chronic heart failure, and death rate.8, 9 Specifically, we strictly controlled the interdialytic weight gain (<3.0% of dry weight) by dietary guidance and reviewed volume overload (assessed by body weight) every 4 weeks. Antihypertensive (AHT) drugs were adjusted to stabilize blood pressure. No significant difference in blood pressure level was found before or after dialysis or between the two groups.
Safety, adherence to spironolactone treatment, clinical outcomes, and side effects were all assessed during the study. Adherence was evaluated by the ratio of actual taking the study medication, and good adherence was defined as ratio ≥80%. Study medication was withheld upon the occurrence of life‐threatening hyperkalemia (plasma potassium level >6.5 mEq/L), nonphysiologic gynecomastia, or breast tenderness, or when any condition wherein discontinuation of spironolactone treatment was deemed medically necessary by the physician in charge. Patients who moved to other dialysis facilities outside the three centers or who switched to renal transplant were all discontinued from the study.
Patients and Methods
Participants were adults 18 years and older undergoing stable hemodialysis (HD) from outpatient dialysis units or peritoneal dialysis (PD) from PD centers in Southeast China for at least 3 months. All patients were diagnosed with ESRD by US criteria according to National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines.10 Patients were randomly assigned to a 2‐year treatment of spironolactone (25 mg daily once, the spironolactone group) or to a matching placebo (the control group) during the recruitment window from July 2011 to January 2012. The flow chart of the study protocol is depicted in Figure 1. Oral medication was administered following HD or in the morning for introduction of spironolactone or placebo.
Figure 1.
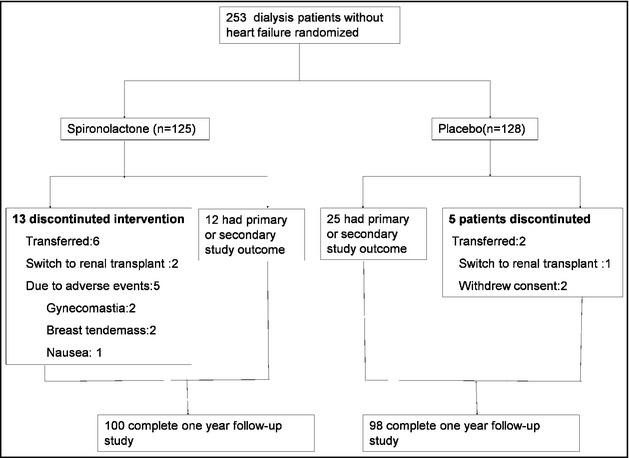
Flow chart of the study.
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (1) diagnosis of ESRD, reception of current conventional HD (three times per week and 4–4.5 hours per session) or maintenance PD (three exchanges a day and continuous ambulatory PD, CAPD); (2) older than 18 years; and (3) voluntary participation and signed consent form. Exclusion criteria were as follows: (1) hypotension, hepatic failure, and any life‐threatening disease other than ESRD; (2) congestive heart failure (ejection fraction [EF] ≤50%) in recent 6 months; (3) occurrence of an acute myocardial infarction or stroke within 6 months after the study initiation; and (4) prior use of spironolactone or potassium level >6.0 mmol/L.
Primary or Secondary Outcome
The primary outcome was a composite of death from CCV events, aborted cardiac arrest (ACA), and sudden cardiac death (SCD). CCV events were diagnosed according to a previous study,4 including new or exacerbated heart failure that was not improved by water removal through dialysis; malignant ventricular arrhythmias; new or recurrent acute myocardial infarction; new or exacerbated angina pectoris; dissecting aneurysm of the aorta (diagnosed by imaging techniques); stroke or intracerebral hemorrhage (diagnosed by computed tomography and/or magnetic resonance imaging); ACA; and SCD. According to a previous study,11 ACA was defined as resuscitation after cardiac arrest in a patient who remained alive 28 days later. According to the Hemodialysis (HEMO) study,12 SCD was defined as an unexpected death, with a preceding symptom duration <24 hours for witnessed deaths and greater than the interval since the last dialysis session for unwitnessed deaths. The secondary outcome was death from all causes.
Other Outcomes
Left ventricular (LV) mass index (LVMI), LV ejection fraction (LVEF), heart rate variability (HRV), vascular endothelial function (VEF), and number of AHT drugs were analyzed at baseline and at the end of the follow‐up among the patients who finished the whole 2‐year follow‐up. LVMI and LVEF were evaluated by Doppler echocardiography. In brief, interventricular septal thickness (IVS), posterior wall thickness (PWT), and LV internal dimension (LVID) were measured at end‐diastole and end‐systole according to American Society of Echocardiography (ASE) standards. LV mass was calculated as recommended by ASE13:
LVMI was defined as LV mass/height2.7. HRV was monitored by 24‐hour ambulatory Holter (Premier 12 [CardioScan 12] Holter, DM Software, Beijing, China). VEF was assessed by brachial artery flow‐mediated dilation (FMD) using an ultrasound/Doppler system equipped with a 7.5‐MHz vascular transducer (HDI5000 SonoCT, PHILIPS, Best, Netherlands) according to previous studies.14, 15 FMD was evaluated for all patients at room temperature. FMD is expressed as the change in arterial diameter from baseline to the end of reactive hyperemia.16, 17 This change is then expressed as a percentage as follows:
Measurements of Demographic and Laboratory Parameters and Assessment of Side Effects
Information of age, sex, primary kidney disease for renal failure, weight, smoking status, and number of AHT drugs was gathered from medical records or by asking the patients. Comorbidity was scored on the number of comorbid conditions using a comorbidity index.18 Basic laboratory parameters such as plasma levels of hemoglobin, potassium, sodium, intact parathyroid hormone, high‐sensitivity C‐reactive protein (hs‐CRP), brain natriuretic peptide (BNP), N‐terminal pro–B‐type natriuretic peptide (NT‐proBNP), and aldosterone were also measured at baseline. Blood samples were collected before dialysis from HD patients and on the morning of the day from PD patients. Measurements were done with standard methods. Plasma levels of potassium, BNP, NT‐proBNP, and aldosterone were also evaluated after the 2‐year follow‐up. Plasma potassium level was reviewed by monthly laboratory routine or measured anytime a suspected cardiac origin (eg, palpitation) occurred.
Side effects such as hyperkalemia, breast tenderness, nonphysiologic gynecomastia, and nausea were assessed during the whole research process. Nonphysiologic gynecomastia was diagnosed from detailed history and physical examination and by excluding other secondary causes, such as drugs (digoxin, verapamil, amlodipine), tumor (prostate cancer), and other severe chronic disease (liver failure and cirrhosis) according to previous clinical practice.19 The diagnosis time was each month or anytime if a patient complained about a breast change.
Statistical Analysis
The results were expressed as mean±standard deviation and P<.05 was considered significant (α=0.05 two‐tailed). Differences in means were assessed using independent t‐test or Mann‐Whitney U‐test. Quartiles were compared with chi‐square test. Non‐normally distributed data were compared with nonparametric tests. Changes in LVMI, LVEF, HRA, VEF, and number of AHT drugs from baseline to the end of the 2‐year follow‐up were analyzed with a linear mixed model and the use of a spatial‐power covariance structure among the patients who completed the 2‐year follow‐up study. Kaplan‐Meier curves were plotted to visualize the cumulative proportion of patients with primary or secondary end outcome. Both survival curves were compared via a log‐rank test. The hazard ratios (HRs) after adjustment for age, sex, dialysis modalities, and dialysis vintage were determined by a multivariable cox proportional hazard regression model. In addition, 95% confidence intervals (CIs) and P values were calculated. All statistical analyses were evaluated with SPSS 18.00 (IBM Corporation, Armonk, NY).
Results
Characteristics of Study Patients
Finally, 235 of the 253 screened dialysis patients were evaluated in terms of eligibility. Thirteen patients from the spironolactone group discontinued the study because of transfer to other facilities for all reasons (n=6), switch to renal transplantation (2), or adverse events (5, eg, nonphysiologic gynecomastia, breast tenderness, and nausea). Five patients from the control group quit the study because of transfer to other facilities for all reasons (n=2), switch to renal transplantation (1), or withdrew consent for nonmedical reasons (2). Basic laboratory data and sociodemographic characteristics of the entire study population are depicted in Table 1. There was no significant difference in any baseline characteristic between groups.
Table 1.
Sociodemographic and Clinical Values in Different Groups
| Characteristics | Spironolactone (n=125) | Placebo (n=128) | P Valuea |
|---|---|---|---|
| Age, %, y | 70.3±10.9 | 70.6±8.4 | .59 |
| Male sex, % | 58.4 | 62.5 | .50 |
| Dialysis modality, % | .62 | ||
| Dialysis, month | 42.3±18.7 | 43.1±17.9 | .35 |
| Hemodialysis | 62.4 | 59.4 | |
| Peritoneal dialysis | 37.6 | 40.6 | |
| Primary kidney disease, % | |||
| Glomerulonephritis | 62.4 | 66.4 | .78 |
| Hyptertensive nephrosclerosis | 4.8 | 4.7 | |
| Diabetic nephrology | 20.8 | 18.8 | |
| Polycystic kidney disease | 4.8 | 6.3 | |
| Other | 7.2 | 3.8 | |
| Body mass index, kg/m2 | 69.1±10.9 | 68.3±9.7 | .12 |
| Drinking, % | 33.6 | 31.3 | .69 |
| Smoking, % | 16.8 | 18.8 | .69 |
| Urine volume | .08 | ||
| <500, mL/24 h | 0.68 | 64.1 | .51 |
| ≥500, mL/24 h | 0.32 | 35.9 | |
| Blood pressure, mm Hg | |||
| Systolic pressure | 144.7±18.9 | 141.9±16.1 | .21 |
| Diastolic pressure | 76.9±12.0 | 77.4±11.7 | .17 |
| Antihypertensive drugs, % | |||
| ACE inhibitors | 18.4 | 16.4 | .68 |
| ARBs | 86.4 | 82.8 | .43 |
| Calcium channel blockers | 93.6 | 93.8 | .96 |
| Selective β‐blocker | 20.0 | 21.9 | .71 |
| Nonselective β‐blocker | 8.0 | 10.2 | .55 |
| Mean number of antihypertensive drugs | 4.87±2.23 | 5.31±3.39 | .41 |
| Total Kt/vurea | 1.58±0.42 | 1.61±0.59 | .31 |
| Mean hemoglobin, g/L | 12.02±5.23 | 12.33±4.51 | .29 |
| Mean plasm calcium, mmol/L | 1.64±0.24 | 1.70±0.37 | .09 |
| Mean plasm phosphorus, mmol/L | 1.44±0.25 | 1.46±0.32 | .22 |
| Mean plasm intact parathyroid hormone, pg/L | 406.52±78.58 | 389.74±82.36 | .47 |
| Mean plasma potassium, mmol/L | 4.12±0.42 | 3.96±0.51 | .33 |
| Mean plasma hs‐CRP, mg/L | 8.37±3.71 | 7.82±2.49 | .18 |
| Mean plasma albumin, g/L | 35.22±5.83 | 34.96±4.14 | .09 |
| Mean plasma prealbumin, mg/L | 335.65±82.45 | 342.17±52.92 | .23 |
| Mean plasma aldosterone, pg/mL | 57.42±22.61 | 60.37±18.72 | .61 |
| Mean LVMI, g/m2.7 | |||
| Male | 51.74±18.16 | 52.33±20.47 | .79 |
| Female | 48.22±15.18 | 50.49±14.61 | .67 |
| Mean LVEF, % | 57.12±8.17 | 58.36±10.24 | .53 |
| Mean plasma BNP, pg/mL | 77.32±21.72 | 74.85±19.27 | .44 |
| Mean plasma NT‐proBNP, pg/mL | 338.49±121.31 | 357.65±89.52 | .59 |
| Mean FMD, % | 6.72±1.77 | 6.59±2.01 | .31 |
| Comorbidity index | 5.3±1.2 | 5.7±1.3 | .20 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; BNP, brain natriuretic peptide; FMD, flow‐mediated dilation; hs‐CRP, high‐sensitivity C‐reactive protein; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide. aDifferences in proportions were tested using Pearson chi‐square test; differences in means were tested using independent t test or Mann‐Whitney test.
Primary Outcome: Death From CCV Events, ACA, or SCD
During the study, nine patients (7.2%) in the spironolactone group and 23 patients (18.0%) in the control group reached the primary outcome (HR by log‐rank test, 0.40; 95% CI, 0.21–0.84; P=.014, Figure 2 and adjusted HR by Cox proportional hazard model, 0.42; 95% CI, 0.26–0.78; P=.017). Five patients in the spironolactone group (4.0%) and 15 patients in the control group (11.7%) died from CCV events (adjusted HR, 0.33; 95% CI, 0.13–0.85; P=.026). ACA occurred in no patients in the spironolactone group and one patient in the control group (0.78%) (P=.32). There were four cases of SCD in the spironolactone group, and seven cases in the control group (P=.38).
Figure 2.
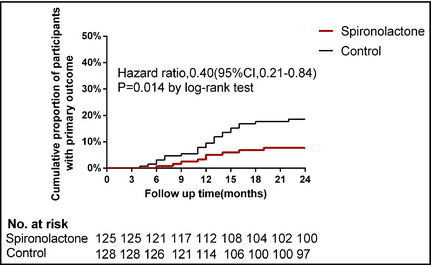
Kaplan‐Meier plot of time to the primary outcome. Death from cardiocerebrovascular events, aborted cardiac arrest, or sudden cardiac death.
Secondary Outcome: Death From All Causes
There were 12 deaths from all causes (9.6%) in the spironolactone group and 25 (19.5%) in the control group, with an HR by log‐rank test of 0.49 (95% CI, 0.26–0.95; P=.036, Figure 3) and an adjusted HR of 0.52 (95% CI, 0.29–0.94; P=.038).
Figure 3.
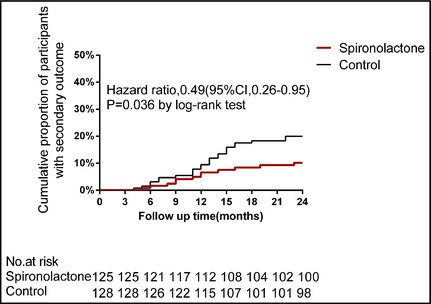
Kaplan‐Meier plot of time to secondary outcome. Death from any cause.
Other Outcomes
A total of 198 patients (100 in the spironolactone group and 98 in the control group) were involved in analysis of other parameters, including LVMI, LVEF, HRV, FMD, and number of AHT drugs. There was no significant difference in any baseline characteristic between groups (not shown). Several CCV‐related parameters, including LVMI, LVEF, FMD, and number of AHT drugs, were significantly improved from baseline in the spironolactone group, but not in the control group (Table 2). However, low‐dose spironolactone supplementation did not significantly improve HRV parameters compared with the control group.
Table 2.
Change in Studied Variables After Intervention According to Study Group
| Spironolactone (n=100) | Placebo (n=98) | |
|---|---|---|
| Studied efficacy endpoints | ||
| Mean LVMI, g/m2.7 (men) | ||
| Baseline | 52.31±17.59 | 52.87±21.43 |
| 2 years | 45.52±14.26 | 56.18±18.02 |
| Mean change from baseline vs placebo (95% CI) | −10.1 (−11.0 to −9.2) | – |
| P value | .006 | – |
| Mean LVMI, g/m2.7 (women) | ||
| Baseline | 46.37±14.66 | 48.29±14.25 |
| 2 years | 42.05±13.19 | 52.68±17.42 |
| Mean change from baseline vs placebo (95% CI) | −8.7 (−9.4 to −8.0) | – |
| P value | .008 | – |
| Mean LVEF, % | ||
| Baseline | 61.78±10.33 | 61.29±11.51 |
| 2 years | 64.29±11.02 | 58.73±9.67 |
| Mean change from baseline vs placebo (95% CI) | 5.1 (4.7 to 5.5) | – |
| P value | .027 | – |
| Mean FMD, % | ||
| Baseline | 7.05±1.49 | 6.88±1.72 |
| 2 years | 9.88±3.02 | 6.31±1.89 |
| Mean change from baseline vs placebo (95% CI) | 3.4 (3.1 to 3.7) | – |
| P value | .033 | – |
| Mean number of antihypertensive drugs | ||
| Baseline | 4.52±1.88 | 4.97±2.85 |
| 2 years | 3.29±1.51 | 5.36±2.44 |
| Mean change from baseline vs placebo (95% CI) | −1.6 (−1.7 to −1.5) | – |
| P value | .041 | – |
| Laboratory variables | ||
| Mean plasma potassium | ||
| Baseline | 4.12±0.42 | 3.96±0.51 |
| 2 years | 5.32±0.68 | 4.68±0.32 |
| Mean change from baseline vs placebo (95% CI) | 0.48 (0.42 to 0.54) | – |
| P value | .13 | – |
| Mean BNP, pg/mL | ||
| Baseline | 77.32±21.72 | 74.85±19.27 |
| 2 years | 75.38±19.47 | 81.69±17.41 |
| Mean change from baseline vs placebo (95% CI) | −8.8 (−9.4 to −8.2) | – |
| P value | .07 | – |
| Mean plasma NT‐proBNP, pg/mL | ||
| Baseline | 338.49±121.31 | 357.65±89.52 |
| 2 years | 337.42±117.58 | 360.31±107.25 |
| Mean change from baseline vs placebo (95% CI) | −3.73 (−7.4 to −0.1) | – |
| P value | .69 | – |
| Mean plasma aldosterone, pg/mL | ||
| Baseline | 56.42±21.33 | 60.35±19.05 |
| 2 years | 58.25±20.47 | 67.21±23.59 |
| Mean change from baseline vs placebo (95% CI) | −5.0 (−6.0 to −4.1) | – |
| P value | .71 | – |
Abbreviations: BNP, brain natriuretic peptide; CI, confidence interval; FMD, flow‐mediated dilation; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide.
Changes in Laboratory Parameters
There was no difference in plasma hs‐CRP, BNP, NT‐proBNP, or aldosterone levels before and after dialysis or between the two groups (Table 2).
Adverse Effects
The potassium level rose in the spironolactone group during 2‐year follow‐up, but not significantly compared with the control group (P>.05). Plasma potassium levels increased to 6.0 mmol/L to 6.5 mmol/L in only three patients and to 5.5 mmol/L to 6.0 mmol/L in seven patients in the spironolactone group. Adverse events including nonphysiologic gynecomastia, breast tenderness, and nausea were observed after the use of spironolactone. Adverse events led to discontinuation of medication in the spironolactone group (five because of nonphysiologic gynecomastia, one because of breast tenderness, and two because of nausea).
Discussion
The current study focused on the long‐term efficacy of spironolactone among non–heart failure dialysis patients. We found that over the 2‐year follow‐up, the number of deaths from CCV events or any causes was significantly smaller in the spironolactone group. Other relevant parameters such as LVMI, LVEF, VEF, and number of AHT drugs were improved more obviously in the spironolactone group vs the control group. No participant needed to stop the study because of hyperkalemia. The favorable efficacy could be attributed mainly to the use of low‐dose spironolactone, since there was no between‐group difference in any baseline characteristic.
In previous studies, different doses of spironolactone (50 6 or 25 mg 3 times a week,20 and 25 mg dialy21, 22) were used for dialysis patients with ESRD. These doses were all well‐tolerated and safe. The dose of spironolactone used in the present study was similar to other studies.4, 18 These two studies both reported the effects of cardiovascular and cerebrovascular protection.4, 18 As is well‐known, the pathogenesis of CCV diseases in ESRD patients involves several causes (eg, activation of renin‐angiotensin system, oxidative stress, elevated asymmetric dimethyl arginine, low‐grade inflammation with increased circulating cytokines, and dyslipidemia involved). Persistent hyperaldosteronemia was mostly seen among dialysis patients with ESRD,23 and hyperaldosteronemia/or activation of MRs can promote cardiac fibrosis, possibly through generation of signals promoting profibrotic transforming growth factor β production and collagen formation.24, 25 Our study demonstrates the improvement in survival rate and CCV parameters in the spironolactone group vs the control group. Spironolactone is more than a diuretic in the sense that it has specific vascular effects independent of any modification of the electrolyte or water balance. The body weights before or after 2‐year use of spironolactone did not change and interdialytic weight gain was well controlled, suggesting that volume status was unchanged. Volume overload was excluded as a contributor to hypertension or heart failure.
There were some reasons for the CCV protective effect of spironolactone. First, spironolactone reduces blood pressure, independent of changes in intravascular volume in chronic kidney disease patients.26, 27 Second, it reduces LV remodeling and myocardial collagen deposition by attenuating formation of myocardial fibrosis and blocking aldosterone effect on collagen formation.28 Third, spironolactone improves VEF by the AHT effect or improvement in bioactivity of endothelial nitric oxide29 or reducing the progression of carotid intima‐media thickness.30 Fourth, it improves HRV parameters by alleviating the symptoms of congestive heart failure (BNP or NT‐proBNP as a parameter) or inhibiting ion channel remodeling.31 Fifth, spironolactone as the aldosterone receptor antagonist maintains peritoneal function by preventing peritoneal inflammation and fibrosis.32 The fourth effect was not seen in the present study probably because the patients with heart failure were excluded, while these patients mostly experienced varied arrhythmias. Meanwhile, the levels of ions such as potassium and calcium did not change significantly. The overall mortality rate was higher in the present study compared with a previous study with a longer follow‐up period.4 The reasons might be that more elderly patients and PD patients were included (70.3±10.9 spironolactone/70.7±8.4 placebo vs 67.4±12.3/67.7±11.2). It was well‐known that P mortality is typically improved following PD compared with HD. Meanwhile, dialysis adequacy was also unsatisfactory in the present study (no depiction in the other study).
Hyperkalemia is usually caused by dietary indiscretions or the effects of residual renal function and some drugs, such as angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, and nonselective β‐blockers. In order to reduce the impacts of confounding factors on plasm a K contents, daily potassium intake <1.5 g was advised for both groups. There was no significant difference in residual renal function (assessed by urine volume) or number of antihypertensive drugs (angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, and nonselective β‐blockers) between groups (all P>.05, shown in Table 1). Hyperkalemia is also a well‐recognized complication of spironolactone. The fear of hyperkalemia, a fatal complication of dialysis, might prevent a physician from using spironolactone. The present study confirms previous observations that spironolactone does not significantly increase plasma K level in HD or PD patients. However, a previous study also showed an uptrend of plasma potassium concentration by only 0.012 mEq/L per month during 24‐month follow‐up,30 while the follow‐up time in the present study was only 2 years. Five patients dropped out of the study because of hyperkalemia in HD patients other than PD patients. Spironolactone was safer among dialysis patients than nondialysis patients, since potassium metabolism occurs slightly in the kidney, but mainly through the excretion by dialysis. This may be a great advantage when spironolactone is selected for PD patients who are constantly prone to hyporkalemic.
Study Limitations
Several limitations in the present randomized controlled study must be pointed out. First, the small sample size in addition to limited racial diversity prevents subanalyses of the racial effect of spironolactone in dialysis patients with ESRD. Second, the follow‐up period was relatively short. Further studies with larger sample size, multiple races, long‐term effects of aldosterone, and evaluation of clinical outcomes will help to improve the findings of this study.
Conclusions
By the effect of CCV protection, spironolactone (25 mg/d) may safely and effectively reduce the incidence of death from CCV or any cause in dialysis patients with ESRD. More prospective and large‐sample clinical trials are necessary to reveal the actual long‐term efficacy of spironolactone on clinical prognosis in dialysis patients.
Disclosure
There are no competing financial interests in relation to the current work.
J Clin Hypertens (Greenwich). 2016;18:121–128. DOI: 10.1111/jch.12628. © 2015 Wiley Periodicals, Inc.
References
- 1. Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014;13:823–833. [DOI] [PubMed] [Google Scholar]
- 2. Power A. Stroke in dialysis and chronic kidney disease. Blood Purif. 2013;36:179–183. [DOI] [PubMed] [Google Scholar]
- 3. Noris M, Remuzzi G. Cardiovascular complications in atypical haemolytic uraemic syndrome. Nat Rev Nephrol. 2014;10:174–180. [DOI] [PubMed] [Google Scholar]
- 4. Matsumoto Y, Mori Y, Kageyama S, et al. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol. 2014;63:528–536. [DOI] [PubMed] [Google Scholar]
- 5. Pelliccia F, Rosano G, Patti G, et al. Efficacy and safety of mineralocorticoid receptors in mild to moderate arterial hypertension. Int J Cardiol 2015;200:8–11. [DOI] [PubMed] [Google Scholar]
- 6. Flevari P, Kalogeropoulou S, Drakou A, et al. Spironolactone improves endothelial and cardiac autonomic function in non heart failure hemodialysis patients. J Hypertens. 2013;31:1239–1244. [DOI] [PubMed] [Google Scholar]
- 7. Ni X, Zhang J, Zhang P, et al. Effects of spironolactone on dialysis patients with refractory hypertension: a randomized controlled study. J Clin Hypertens (Greenwich). 2014;16:658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalantar‐Zadeh K, Regidor DL, Kovesdy CP, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long‐term hemodialysis. Circulation. 2009;119:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee MJ, Doh FM, Kim CH, et al. Interdialytic weight gain and cardiovascular outcome in incident hemodialysis patients. Am J Nephrol. 2014;39:427–435. [DOI] [PubMed] [Google Scholar]
- 10. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 11. Schumm J, Greulich S, Wagner A, et al. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson. 2014;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. [DOI] [PubMed] [Google Scholar]
- 13. Takasaki K, Miyata M, Imamura M, et al. Left ventricular dysfunction assessed by cardiac time interval analysis among different geometric patterns in untreated hypertension. Circ J. 2012;76:1409–1414. [DOI] [PubMed] [Google Scholar]
- 14. Hwang MH, Yoo JK, Luttrell M, et al. Mineralocorticoid receptors modulate vascular endothelial function in human obesity. Clin Sci (Lond). 2013;125:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inrig JK, Van Buren P, Kim C, et al. Probing the mechanisms of intradialytic hypertension: a pilot study targeting endothelial cell dysfunction. Clin J Am Soc Nephrol. 2012;7:1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Korkmaz H, Onalan O. Evaluation of endothelial dysfunction: flow‐mediated dilation. Endothelium. 2008;15:157–163. [DOI] [PubMed] [Google Scholar]
- 17. Atkinson G, Batterham AM, Thijssen DH, et al. A new approach to improve the specificity of flow‐mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens. 2013;31:287–291. [DOI] [PubMed] [Google Scholar]
- 18. Chen JY, Tsai SH, Chuang PH, et al. A comorbidity index for mortality prediction in Chinese patients with ESRD receiving hemodialysis. Clin J Am Soc Nephrol. 2014;9:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Braunstein GD. Clinical practice. Gynecomastia. N Engl J Med. 2007;357:1229–1237. [DOI] [PubMed] [Google Scholar]
- 20. Saudan P, Mach F, Perneger T, et al. Safety of low‐dose spironolactone administration in chronic haemodialysis patients. Nephrol Dial Transplant. 2003;18:2359–2363. [DOI] [PubMed] [Google Scholar]
- 21. Yongsiri S, Thammakumpee J, Prongnamchai S, et al. Randomized, double‐blind, placebo‐controlled trial of spironolactone for hypokalemia in continuous ambulatory peritoneal dialysis patients. Ther Apher Dial. 2015;19:81–86. [DOI] [PubMed] [Google Scholar]
- 22. Matsumoto Y, Kageyama S, Yakushigawa T, et al. Long‐term low‐dose spironolactone therapy is safe in oligoanuric hemodialysis patients. Cardiology. 2009;114:32–38. [DOI] [PubMed] [Google Scholar]
- 23. Hung SC, Lin YP, Huang HL, et al. Aldosterone and mortality in hemodialysis patients: role of volume overload. PLoS One. 2013;8:e57511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zannad F, Alla F, Dousset B, et al. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation. 2000;102:2700–2706. [DOI] [PubMed] [Google Scholar]
- 25. Ritz E. Left ventricular hypertrophy in renal disease: beyond preload and afterload. Kidney Int. 2009;75:771–773. [DOI] [PubMed] [Google Scholar]
- 26. Bomback AS, Klemmer PJ. Mineralocorticoid receptor blockade in chronic kidney disease. Blood Purif. 2012;33:119–124. [DOI] [PubMed] [Google Scholar]
- 27. Hirsch JS, Drexler Y, Bomback AS. Aldosterone blockade in chronic kidney disease. Semin Nephrol. 2014;34:307–322. [DOI] [PubMed] [Google Scholar]
- 28. Kosmala W, Przewlocka‐Kosmala M, Szczepanik‐Osadnik H, et al. Fibrosis and cardiac function in obesity: a randomised controlled trial of aldosterone blockade. Heart. 2013;99:320–326. [DOI] [PubMed] [Google Scholar]
- 29. Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594–597. [DOI] [PubMed] [Google Scholar]
- 30. Vukusich A, Kunstmann S, Varela C, et al. A randomized, double‐blind, placebo‐controlled trial of spironolactone on carotid intima‐media thickness in nondiabetic hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michea L, Vukusich A, Gonzalez M, et al. Effect of spironolactone on K(+) homeostasis and ENaC expression in lymphocytes from chronic hemodialysis patients. Kidney Int. 2004;66:1647–1653. [DOI] [PubMed] [Google Scholar]
- 32. Zhang L, Hao JB, Ren LS, et al. The aldosterone receptor antagonist spironolactone prevents peritoneal inflammation and fibrosis. Lab Invest. 2014;94:839–850. [DOI] [PubMed] [Google Scholar]


