Abstract
Patients receiving thiazide diuretics have a higher risk of impaired glucose tolerance or even incident diabetes, but the change of blood glucose level varies across different trials. The aim of this study was to investigate the glycemic changes in hypertensive patients with thiazide‐type diuretics. Twenty‐six randomized trials involving 16,162 participants were included. Thiazide‐type diuretics were found to increase fasting plasma glucose (FPG) compared with nonthiazide agents or placebo or nontreatment (mean difference [MD], 0.27 mmol/L [4.86 mg/dL]; 95% confidence interval [CI], 0.15–0.39). Patients receiving lower doses of thiazides (hydrochlorothiazide or chlorthalidone ≤25 mg daily) had less change in FPG (MD, 0.15 mmol/L [2.7 mg/dL]; 95% CI, 0.03–0.27) than those receiving higher doses (MD, 0.60 mmol/L [10.8 mg/dL]; 95% CI, 0.39–0.82), revealed by the subgroup analysis of thiazides vs calcium channel blockers. Thiazide‐type diuretics are associated with significant but small adverse glycemic effects in hypertensive patients. Treatment with a lower dose might reduce or avoid glycemic changes.
Thiazide‐type diuretics, which include thiazide diuretics such as hydrochlorothiazide (HCTZ) and thiazide‐like diuretics such as chlorthalidone (CTD) and indapamide, are a classic class of antihypertensive medications. Studies over decades have demonstrated a reduction in morbidity and mortality of cardiovascular events in patients who receive thiazide‐type diuretics.1, 2 This class has been widely used for more than 40 years and is still recommended as one of the first‐line treatments in the latest guidelines for the management of hypertension.3
However, despite the strong evidence of benefits, there are some adverse effects of thiazide diuretics that have led to debates over their wide use. Randomized trials and observational studies have demonstrated multiple metabolic abnormalities such as dysglycemia, new‐onset diabetes, hypokalemia, hyponatremia, hyperuricemia, and hyperlipidemia.4, 5, 6, 7 Among these adverse metabolic effects, glycemic dysregulation is the greatest concern. Although there has been agreement that patients receiving thiazide diuretics have a higher risk of impaired glucose tolerance or even incident diabetes, the change of blood glucose level in hypertensive patients varies across different trials. We propose that it is essential to quantify the glycemic effect of thiazide diuretics by reviewing trials that used diverse doses and types of thiazides. Therefore, we conducted a systematic review and meta‐analysis of randomized controlled trials to assess the effects of thiazide‐type diuretics on glycemic metabolism in hypertensive patients.
Methods
Search Strategy
We searched PubMed and Web of Science for relevant articles until November 2014, using the following search items: (“thiazide” OR “HCTZ”) AND (“diabetes” OR “glucose”). Species were limited to humans. We also manually checked the reference lists of eligible studies and relevant reviews for further information. The design and conduction of this review followed the recommendations of the Cochrane Collaboration8 and the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) guidelines.9
Study Selection and Data Extraction
Studies were considered eligible if they met the following inclusion criteria: (1) randomized controlled clinical trial in hypertensive patients; (2) comparing thiazide or thiazide‐like diuretics with other hypertensive agents or placebo or nontreatment; (3) assessing one or more glycemic parameters, including fasting plasma glucose (FPG), postprandial plasma glucose (PPG), and glycated hemoglobin (HbA1c); and (4) reporting data both before and after intervention or values changed with intervention, expressed as mean±standard deviation (SD). Trials in normotensive patients were excluded since they might have different metabolic characteristics from hypertensive patients. In studies with multiple doses or duration of treatment, only data on the largest dose and longest duration were extracted. Trials with a crossover design were also excluded to avoid overestimation of their effects. Trials using thiazides in combination with other types of antihypertensive agents were excluded to prevent possible bias caused by the interaction or synergistic effects. Information including type and dose of thiazide‐type diuretics and treatment in the control group, duration of intervention, sample size, percentage of diabetes, data of glycemic and other metabolic parameters, and baseline characteristics of participants were extracted independently by two reviewers. Consensus was reached through discussion and repetitive review of the details in cases of discrepancies.
Assessment of Risk of Bias
The risk of bias of included studies was assessed according to the recommendations of the Cochrane Handbook of Systematic Reviews of Interventions8 in the following domains: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other bias.
Statistical Analysis
For outcome comparison between two groups, the mean differences (MDs) with SDs measuring the changes from baseline, were pooled across studies using the random‐effects model. In case of a missing SD for the changes, we followed the Cochrane Handbook8 and the recommendations of Musini and colleagues.10 Heterogeneity was examined by Cochran Q test (considered significant when P<.10) and quantified by I 2 statistic (considered substantial when I 2 ≥50%). Sensitivity analyses were performed by successively excluding studies. To explore the reasons for heterogeneity, we performed subgroup analyses based on age of patients, type and dose of medication, and duration of treatment. Publication bias was assessed by Begg's funnel plot and Egger's regression test. A P value <.05 was considered statistically significant. Statistical analyses were conducted by Review Manager (RevMan) version 5.2 (The Cochrane Collaboration, Copenhagen, Denmark) and STATA 12.0 (STATA, College Station, TX).
Results
Characteristics of Included Studies
The initial literature search retrieved 1369 relevant articles. Of these, 26 studies met the inclusion criteria and were selected in the meta‐analysis, which included a total of 16,162 participants (Figure S1). Seven trials contained more than one study group except for thiazide and one trial contained two study groups receiving different kinds of thiazides. We included these outcomes and regarded them as different comparisons in the pooled analysis. The basic characteristics of included studies are shown in the Table. The number of participants ranged from 19 to 7703 across the trials,11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 of which only three enrolled obese participants.12, 14, 31 The longest duration of treatment was 6 years,35 but most trials lasted less than half a year. Intervention with HCTZ was the most commonly reported. CTD, bendrofluazide, and indapamide were also included. The dose of treatment varied across different trials (HCTZ 12.5–100 mg/d, CTD 6.25–50 mg/d, bendrofluazide 2.5–10 mg/d, and indapamide 1.25–2.5 mg/d).
Table 1.
Baseline Characteristics of Included Trials
| Study | Group | Patients | Number | Duration | Age, ya | Women, % | Baseline SBP/DBP, mm Hga | Diabetic Patients, % | BMI, kg/m2b | Changed FPG, mmol/Lb | Changed PPG, mmol/Lb | Changed HbA1c, %b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thiazide vs placebo or empty control | ||||||||||||
| Chrysant 1994 A11 | HCTZ 25 mg/d | Patients with hypertension | 84 | 12 weeks | 53 | 71.8 | 155/104 | 0 | – | 0.5±2.3 | – | – |
| Placebo | 81 | 53 | 155/103 | – | 0.1±2.2 | – | – | |||||
| Carlsen 199018 | Bendrofluazide 10 mg/d | Patients with mild to moderate hypertension | 51 | 10 weeks | 59 | 45.1 | 166.9±2.7/103.7±0.8 | – | – | 0.27±0.15 | – | – |
| Placebo | 52 | 57 | 55.8 | 161.9±1.9/101.8±0.5 | – | – | −0.08±0.09 | – | – | |||
| Fiddes 199722 | Indapamide 1.25 mg/d | Patients 65 y or older with mild to moderate hypertension | 103 | 8 weeks | 69.4 | 48 | −/98.8 | – | – | 0.194±1.51 | – | – |
| Placebo | 100 | 69.7 | 42 | −/99.8 | – | – | −0.0556±1.15 | – | – | |||
| Fuenmayor 1997 A14 | HCTZ 100 mg/d | Obese patients with mild to moderate hypertension | 14 | 1 week | 50±2 | – | 150±2/103±2 | – | 29±1 | 0.3±0.2 | – | – |
| Placebo | 14 | 50±2 | – | 148±3/103±2 | – | 30±1 | 0.1±0.2 | – | – | |||
| Hall 199423 | Indapamide 1.25 mg/d | Patients with mild to moderate hypertension | 82 | 8 weeks | – | – | −/100.1 | – | – | 0.2±1.5 | – | – |
| Placebo | 90 | – | – | −/99.6 | – | – | 0.2±1.2 | – | – | |||
| Jounela 199413 | HCTZ 25 mg/d | Patients with mild to moderate hypertension | 23 | 6 weeks | 46.4 | 60.9 | 147.0/97.6 | – | – | −0.14±0.82 | – | – |
| Placebo | 22 | 48.5 | 59.1 | 152.5/99.8 | – | – | −0.03±0.50 | – | – | |||
| Mersey 1993 A21 | HCTZ 12.5 mg/d | Patients with mild to moderate hypertension | 69 | 8 weeks | 48.2 | 36.9 | 143.5/97.3 | – | – | −0.12±0.26 | – | – |
| Placebo | 70 | 50.7 | 40.9 | 142.8/97.6 | – | – | −0.02±0.27 | – | – | |||
| Oslo 198415 | HCTZ 50 mg/d | Male patients with mild hypertension | 125 | 5 y | 40–49 | 0 | – | – | – | 0.18±0.59 | – | – |
| Nontreatment | 277 | 57 | 55.8 | – | – | – | 0.13±0.68 | – | – | |||
| Pool 1993 A19 | HCTZ 12.5 mg/d | Patients with hypertension | 67 | 6 weeks | 52.7±10.6 | 27 | 153.4±2.2/100.2±0.5 | – | – | 0.35±0.80 | – | – |
| Placebo | 57 | 53.5±10.5 | 40 | 152.9±1.9/99.9±0.5 | – | – | 0.028±0.66 | – | – | |||
| Reisin 1997 A12 | HCTZ 12.5–50 mg/d | Obese hypertensive patients | 76 | 12 weeks | 51±10 | 46.1 | 148±14/98±5 | 0 | 32.5±3.8 | 0.31±0.99 | – | – |
| Placebo | 79 | 49±10 | 40.5 | 146±13/96±4 | – | 32.2±3.8 | −0.16±0.90 | – | – | |||
| SHEP 199816 | CTD 12.5–25 mg/d | Patients older than 60 y with isolated systolic hypertension | 860 | 3 y | 71.6±6.7 | 56.3 | 170.5±9.5/76.7±9.6 | 10 | 27.5±4.9 | 0.51±1.69 | – | – |
| Placebo | 803 | 71.5±6.7 | 57.4 | 170.1±9.2/76.5±9.8 | 10.2 | 27.5±5.1 | 0.31±1.42 | – | – | |||
| Siegel 1994 A20 | HCTZ 50 mg/d | Hypertensive men aged 35–79 y | 147 | 2 months | 60.8±7.7 | 0 | – | – | 27.8±3.9 | 0.1±0.13 | – | – |
| Placebo | 27 | 60.8±8.5 | 40.9 | – | – | 28.0±3.6 | 0.3±0.14 | – | – | |||
| Siegel 1994 B20 | CTD 50 mg/d | Hypertensive men 35–79 y | 28 | 2 months | 61.4±7.8 | 0 | – | – | 28.0±4.7 | 0.7±0.28 | – | – |
| Placebo | 27 | 60.8±8.5 | 42 | – | – | 28.0±3.6 | 0.3±0.14 | – | – | |||
| Vardan 198717 | CTD 25 mg/d | Patients with mild hypertension | 60 | 12 weeks | 21–69 | 34.2 | 143.4±1.6/93.7±0.7 | 0 | – | 0.61±0.15 | – | – |
| placebo | 59 | 145.7±1.8/93.6±0.7 | – | – | −0.1±0.13 | – | – | |||||
| Thiazide vs CCB | ||||||||||||
| ALLHAT 2006 A26 | CTD 12.5–25 mg/d | Patients with hypertension | 4972 | 4 y | 66.9±7.7 | 47.0 | 146±16/84±10 | 36.2 | 29.7±6.2 | 0.16±3.09 | – | – |
| Amlodipine 2.5–10 mg/d | 2954 | 66.9±7.7 | 47.3 | 146±16/84±10 | 36.7 | 29.8±6.3 | 0.03±3.09 | – | – | |||
| Calvo 200024 | HCTZ 50–100 mg/d | Patients older than 60 y with hypertension | 100 | 8 weeks | 67.6±5.9 | 64 | 177.8±1.2/87.1±0.7 | – | 27.4±3.9 | 0.28±1.22 | – | – |
| Amlodipine 5–10 mg/d | 97 | 69.0±6.4 | 69 | 178.4±1.3/87.1±0.7 | – | 27.4±4.3 | −0.60±1.22 | – | – | |||
| Fuenmayor 1997 B14 | HCTZ 100 mg/d | Obese patients with mild to moderate hypertension | 14 | 1 week | 50±2 | – | 150±2/103±2 | – | 29±1 | 0.3±0.2 | – | – |
| Verapamil 160 mg/d | 14 | 49±2 | – | 149±2/100±1 | – | 30±1 | −0.3±0.2 | – | – | |||
| Jansen 198928 | HCTZ 50 mg/d | Hypertensive patients older than 70 y | 16 | 12 weeks | 74.9±4.0 | 75 | – | – | – | 0.3±0.2 | – | – |
| Nitrendipine 20 mg/d | 15 | 72.3±2.4 | 73 | – | – | – | −0.1±0.5 | – | – | |||
| Pareek 2008 A25 | CTD 6.25 mg/d | Patients with stage I hypertension | 100 | 4 weeks | 46.44±11.79 | 40.0 | 149.43±6.99/93.81±4.33 | 17 | – | 0.24±2.14 | −0.11±3.21 | – |
| Amlodipine 2.5 mg/d | 102 | 48.98±10.83 | 40.2 | 149.66±7.20/93.50±4.48 | 16.7 | – | −0.26±1.52 | −0.71±2.82 | – | |||
| Piecha 2007 A27 | Indapamide 2.5 mg/d | Patients with mild to moderate hypertension | 9 | 6 months | 44.8±12.2 | 67 | 154±5/137±10 | 0 | 30.8±6.0 | 0.4±0.9 | – | – |
| Amlodipine 5–10 mg/d | 10 | 49.6±11.4 | 20 | 161±10/145±13 | – | 30.0±3.8 | 0.6±0.5 | – | – | |||
| Pool 1993 B19 | HCTZ 12.5 mg/d | Patients with hypertension | 67 | 6 weeks | 52.7±10.6 | 27 | 153.4±2.2/100.2±0.5 | – | – | 0.35±0.80 | – | – |
| Diltiazem 120 mg/d | 63 | 55.4±9.2 | 36 | 152.7±1.6/99.4±0.3 | – | – | 0.18±0.69 | – | – | |||
| Thiazide vs ACE inhibitor/ARB | ||||||||||||
| ALLHAT 2006 B26 | CTD 12.5–25 mg/d | Patients with hypertension | 4972 | 4 y | 66.9±7.7 | 47.0 | 146±16/84±10 | 36.2 | 29.7±6.2 | 0.16±3.09 | – | – |
| Lisinopril 10–40 mg/d | 2731 | 66.9±7.7 | 46.2 | 146±16/84±10 | 35.5 | 29.8±6.2 | −0.08±2.85 | – | – | |||
| Chrysant 1994 B11 | HCTZ 25 mg/d | Patients with hypertension | 84 | 12 weeks | 53 | 71.8 | 155/104 | 0 | – | 0.5±2.3 | – | – |
| Lisinopril 10 mg/d | 85 | 54 | 154/104 | – | 0.1±0.8 | – | – | |||||
| Fuenmayor 1997 C14 | HCTZ 100 mg/d | Obese patients with mild to moderate hypertension | 14 | 1 week | 50±2 | – | 150±2/103±2 | – | 29±1 | 0.3±0.2 | – | – |
| Captopril 100 mg/d | 13 | 49±2 | – | 144±4/100±1 | – | 30±1 | 0.4±0.1 | – | – | |||
| Grassi 200331 | HCTZ 25 mg/d | Obese hypertensive patients | 59 | 12 weeks | 50.2±11.2 | 62.7 | 146.2±12.6 | – | 35.1±3.2 | 0.15±0.82 | – | 0.13±0.6 |
| Candesartan cilexetil 8 mg/d | 68 | 51.2±9.5 | 58.8 | 98.8±3.7 | – | 33.7±2.6 | 0.13±0.92 | – | 0.07±0.5 | |||
| Mersey 1993 B21 | HCTZ 12.5 mg/d | Patients with mild to moderate hypertension | 69 | 8 weeks | 48.2 | 36.9 | 143.5/97.3 | – | – | −0.120±0.256 | – | – |
| Captopril 25 mg/d | 68 | 52.0 | 49.2 | 146.6/96.7 | – | – | 0.0367±0.264 | – | – | |||
| Piecha 2007 B27 | Indapamide 2.5 mg/d | Patients with mild to moderate hypertension | 9 | 6 months | 44.8±12.2 | 67 | 154±5/137±10 | 0 | 30.8±6.0 | 0.4±0.9 | – | – |
| Enalapril 10–20 mg/d | 10 | 50.0±9.9 | 60 | 163±10/98±9 | 27.9±2.1 | 0.7±0.6 | – | – | ||||
| Pollare 198929 | HCTZ 40±12 mg/d | Patients with hypertension | 50 | 18 weeks | 58±10 | 34.6 | 166±16/101±4 | 2.0 | 27±4 | 0.6±1.4 | – | 0.7±1.5 |
| Captopril 81±24 mg/d | 48 | 58±12 | 29.2 | 165±14/101±4 | 28±4 | −0.2±1.1 | – | 0.1±0.7 | ||||
| Reisin 1997 B12 | HCTZ 12.5–50 mg/d | Obese hypertensive patients | 76 | 12 weeks | 51±10 | 46.1 | 148±14/98±5 | 0 | 32.5±3.8 | 0.31±0.99 | – | – |
| Lisinopril 10–40 mg/d | 77 | 51±11 | 48.1 | 147±25/98±6 | – | 32.3±3.7 | −0.21±0.71 | – | – | |||
| Stimpel 199833 | HCTZ 25 mg/d | Postmenopausal women with mild to moderate hypertension | 41 | 12 weeks | 62±5 | 100 | 158.8±12.7/100.5±3.9 | – | – | 0.61±0.23 | – | – |
| Moexipril 15 mg/d | 43 | 61±8 | 100 | 159.0±13.7/100.5±4.7 | – | – | −0.13±0.11‐ | – | – | |||
| Weinberger 198530 | HCTZ 37.5 mg/d | Patients with hypertension | 67 | 6 weeks | – | – | 146.1±2.6/97.7±0.9 | – | – | 0.55±0.17 | – | – |
| Captopril 75 mg/d | 69 | – | – | 149.5±2.7/99.6±0.9 | – | – | −0.06±0.17 | – | – | |||
| Zappe 200832 | HCTZ 12.5–25 mg/d | Hypertensive patients with cardiometabolic syndrome | 158 | 16 weeks | 48.8±11 | 58 | 141.7±9/91.4±5 | – | 37.4±7 | 0.22±0.9 | – | 0.2±0.6 |
| Valsartan 320 mg/d | 164 | 50.0±11 | 62 | 143.5±9/91.5±5 | – | 36.2±7 | 0.17±0.9 | – | 0.1±0.5 | |||
| Thiazide vs β‐blocker | ||||||||||||
| Berglund 198135 | Bendrofluazide 2.5–5 mg/d | Middle‐aged men with mild to moderate hypertension | 38 | 6 y | 47–54 | 0 | – | – | – | 0.0±1.0 | – | – |
| Propanolol 160 mg–320 mg/d | 37 | 48.49±12.98 | 43.9 | – | – | – | 0.4±1.6 | – | – | |||
| Fuenmayor 1997 D14 | HCTZ 100 mg/d | Obese patients with mild to moderate hypertension | 14 | 1 week | 50±2 | – | 150±2/103±2 | 29±1 | 0.3±0.2 | – | – | |
| Atenolol 100 mg/d | 14 | 50±2 | – | 140±2/99±1 | 30±1 | −0.2±0.2 | – | – | ||||
| Pareek 2008 B25 | CTD 6.25 mg/d | Patients with stage I hypertension | 100 | 4 weeks | 46.44±11.79 | 40.0 | 149.43±6.99/93.81±4.33 | 17.0 | – | 0.24±2.14 | −0.11±3.21 | – |
| Atenolol 25 mg/d | 98 | 48.49±12.98 | 43.9 | 149.47±7.69/93.23±3.62 | 14.3 | – | −0.26±1.05 | −0.48±1.80 | – | |||
| Piecha 2007 C27 | Indapamide 2.5 mg/d | Patients with mild to moderate hypertension | 9 | 6 months | 44.8±12.2 | 67 | 154±5/137±10 | 0 | 30.8±6.0 | 0.4±0.9 | – | – |
| Metoprolol 50–200 mg/d | 11 | 50.3±10.5 | 54 | 157±9/140±13 | 0 | 28.1±3.0 | −0.2±0.6 | – | – | |||
| Veterans 198534 | HCTZ 50–200 mg/d | Hypertensive men | 174 | 1 y | 49.8±9.9 | 0 | 146.5±15.8/101.3±4.5 | – | – | 0.26±1.61 | 1.44±4.12 | – |
| Propranolol 80–640 mg/d | 119 | 49.6±9.8 | 146.0±14.4/101.6±4.6 | – | – | 0.36±1.02 | 1.01±2.27 | – | ||||
| Thiazide vs α‐blocker | ||||||||||||
| Alderman 198636 | HCTZ 25–50 mg/d | Patients with hypertension | 9 | 1 y | 53.4±8.8 | 23.3 | 150.8±14.2/107.6±8.5 | – | – | 0.49±0.82 | – | – |
| Prazosin 1–2 mg/d | 10 | 51.4±9.8 | 15.6 | 152.2±13.8/105.8±5.2 | – | – | −0.17±0.64 | – | – | |||
| Fuenmayor 1997 E14 | HCTZ 100 mg/d | Obese patients with mild to moderate hypertension | 14 | 1 week | 50±2 | – | 150±2/103±2 | – | 29±1 | 0.3±0.2 | – | – |
| Prazosin 6 mg/d | 13 | 49±2 | – | 142±2/100±1 | – | 30±0.4 | 0.0±0.1 | – | – | |||
Abbreviations: ACE, angiotensin‐converting enzyme; ALLHAT, Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CCB, calcium channel blocker; CTD, chlorthalidone; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HCTZ, hydrochlorothiazide; PPG, postprandial plasma glucose; SBP, systolic blood pressure; SHEP, Systolic Hypertension in the Elderly Program; – data unavailable. aMean or mean±standard deviation or range. bMean±standard deviation.
The Risk of Bias and Publication Bias
We determined the risk of bias in seven domains using the criteria of the Cochrane handbook.8 Most studies did not report the process of randomization or allocation concealment and we therefore judged them as having an unclear risk of bias (Figure S2 and Figure S3). Funnel plots for FPG suggested asymmetry visually (Figures S4–S6) while Egger's tests did not show sufficient evidence of publication bias (thiazide vs nonthiazide: P=.421; thiazide vs placebo or nontreatment: P=.643; thiazide vs angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker: P=.395).
Outcomes
Fasting Plasma Glucose
All trials reported the changes of FPG. Thiazide or thiazide‐like diuretics were found to increase FPG level compared with nonthiazide agents or placebo or nontreatment (MD, 0.27 mmol/L [4.86 mg/dL]; 95% confidence interval [CI], 0.15–0.39 [P<.0001]) (Figure 1). Heterogeneity was substantial in the pooled analysis (I 2=97%, P<.00001). Since different kinds of hypertensive agents with different pharmacology were included, we performed subgroup analysis based on types of medication in control groups. In addition, after classifying the studies into five categories including placebo or nontreatment, calcium channel blocker (CCB), angiotensin‐converting enzyme inhibitor (ACE) inhibitor or angiotensin receptor blocker (ARB), β‐blocker, and α‐blocker, we performed further analysis in these groups, respectively. Thiazide‐type diuretics did not significantly increase FPG level when compared with placebo or nontreatment or β‐blockers (thiazide vs placebo or nontreatment: MD, 0.21 mmol/L [3.78 mg/dL]; 95% CI, 0.00–0.41 [P=.05] and thiazide vs β‐blocker: MD, 0.23 mmol/L [4.14 mg/dL]; 95% CI, −0.14 to 0.59 [P=.22]). However, subgroup analysis did not show a significant difference between different kinds of control groups (Figure S7). Subgroup analysis of the CCB group based on dose of medication significantly reduced heterogeneity and indicated that patients receiving lower doses of thiazides (HCTZ or CTD ≤25 mg daily, n=5148) had less change in FPG (MD, 0.15 mmol/L [2.7 mg/dL]; 95% CI, 0.03–0.27) compared with patients receiving higher doses (n=130) (MD, 0.60 mmol/L [10.8 mg/dL]; 95% CI, 0.39–0.82) (P=.0003) (Figure 2). Patients with a longer duration of treatment (≥6 months, n=221) also had less glycemic change (MD, −0.01 mmol/L [−0.18 mg/dL]; 95% CI, −0.47 to 0.45) than patients with short treatment duration (n=114) (MD, 0.50 mmol/L [9.0 mg/dL]; 95% CI, 0.36–0.64), revealed by the subgroup analysis of thiazide vs β‐blocker (P=.04) (Figure 3). Sensitivity analyses did not significantly reduce the heterogeneity.
Figure 1.
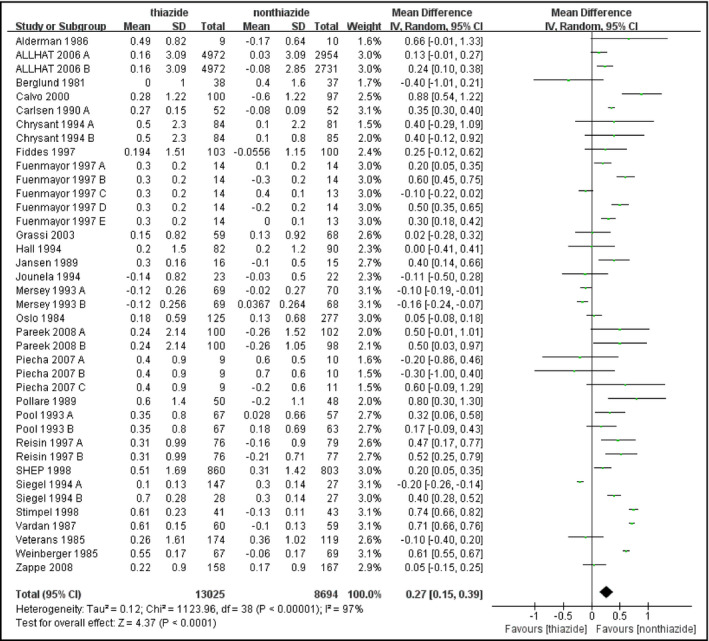
Forest plot for the mean difference (MD) of the change in fasting plasma glucose (FPG) comparing thiazide‐type diuretics with nonthiazide medications or placebo or nontreatment. MDs from the individual studies are presented by squares and the size of the square represents the statistical weight of the study in the summary estimate. The horizontal line indicates the 95% confidence interval (CI) of the study.
Figure 2.
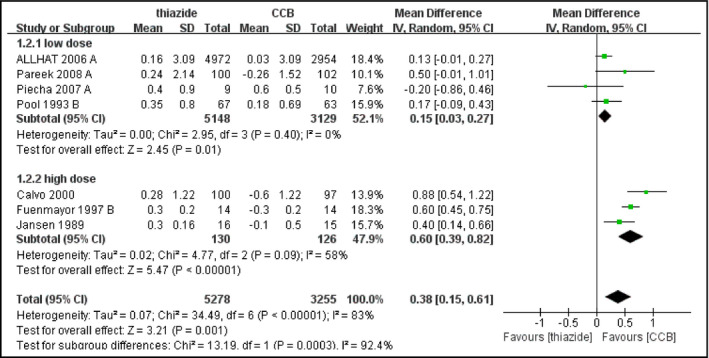
Forest plot for subgroup analysis of change in fasting plasma glucose (FPG) comparing thiazide‐type diuretics with calcium channel blockers (CCBs) based on dose of treatment. Mean differences (MDs) from the individual studies are presented by squares and the size of the square represents the statistical weight of the study in the summary estimate. The horizontal line indicates the 95% confidence interval (CI) of the study.
Figure 3.
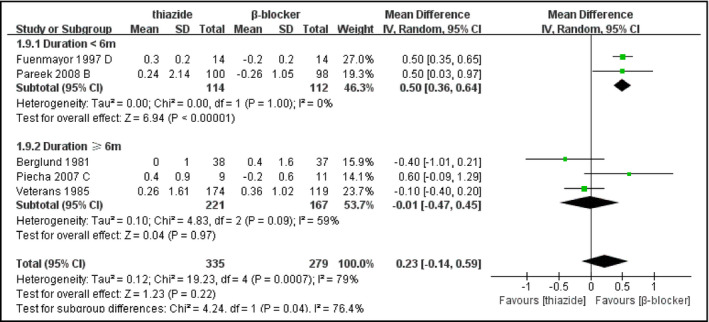
Forest plot for subgroup analysis of change in fasting plasma glucose (FPG) comparing thiazide‐type diuretics with β‐blockers based on duration of treatment. Mean differences (MDs) from the individual studies are presented by squares and the size of the square represents the statistical weight of the study in the summary estimate. The horizontal line indicates the 95% confidence interval (CI) of the study.
Postprandial Plasma Glucose
Only three studies reported PPG changes, thus the sample size was relatively small (thiazide: n=245, nonthiazide: n=234). Change in PPG was not significantly different between patients receiving thiazides and patients receiving other treatments (MD, 0.46 mmol/L [8.28 mg/dL]; 95% CI, −0.05 to 0.97 [P=.07]) (Figure 4). There was no evidence of heterogeneity (I 2=0%, P=.92).
Figure 4.
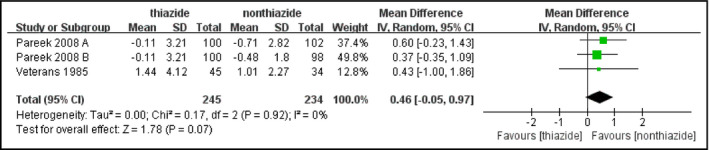
Forest plot for the mean difference (MD) of the change in postprandial plasma glucose (PPG) comparing thiazide‐type diuretics with other treatments. MDs from the individual studies are presented by squares and the size of the square represents the statistical weight of the study in the summary estimate. The horizontal line indicates the 95% confidence interval (CI) of the study.
Glycated Hemoglobin
The comparison only included three studies, involving 547 hypertensive patients (thiazide: n=267, nonthiazide: n=280). No significant increase in HbA1c level was observed in patients receiving thiazides compared with other patients (MD, 0.15%; 95% CI, −0.04 to 0.34 [P=.12]) (Figure 5). Heterogeneity was substantial among the studies (I 2=57%, P=.10). Exclusion of the study by Pollare and colleagues30 reduced I 2 to 0, which might be explained by the largest dose of HCTZ in this study.
Figure 5.
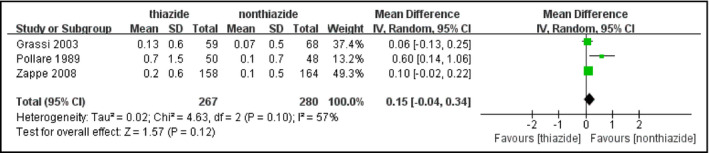
Forest plot for the mean difference (MD) of the change in glycated hemoglobin (HbA1c) comparing thiazide‐type diuretics with other treatments. MDs from the individual studies are presented by squares and the size of the square represents the statistical weight of the study in the summary estimate. The horizontal line indicates the 95% confidence interval (CI) of the study.
Moreover, we found decreasing trends in metabolic parameters including high‐density lipoprotein cholesterol, potassium, and sodium. On the other hand, uric acid and major lipid parameters except high‐density lipoprotein cholesterol showed a tendency to increase. We generated pooled estimates for the aforementioned metabolic changes, but the level of heterogeneity was statistically significant and could not be explained through subgroup or sensitivity analyses. Therefore, we did not present these outcomes.
Discussion
The present study reveals an increase in FPG level in patients receiving thiazide‐type diuretics, which is consistent with findings from previous studies.4 Adverse glycemic effects of thiazides have been reported since the application of these medications. Significantly greater concern was generated when thiazides were found to be associated with significantly higher risk of incident diabetes as compared with other antihypertensive medications.5, 37 However, since the glycemic change was small in most included studies and in the pooled outcome (FPG 0.27 mmol/L [4.86 mg/dL]) and no significant changes in PPG or HbA1c were found in the meta‐analysis, we wonder how these small changes are able to translate into a higher incidence of new‐onset diabetes. In fact, it remains undefined whether the small glycemic changes or the incident diabetes during thiazide therapy has a strong impact on cardiovascular or other major outcomes. Some researchers compared the 4‐year cumulative incidence of new‐onset diabetes in patients assigned to CTD and those assigned to amlodipine in the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) and argued that 85% of cases of diabetes associated with a thiazide diuretic was not induced by the diuretic itself.38 Moreover, diabetes diagnosed during thiazide therapy was not found to be related to an increase in cardiovascular outcomes or mortality.39
The mechanism of the adverse glycemic effect of thiazide diuretics has not yet been fully elucidated. Several hypotheses exist. Hypokalemia is a classic hypothesis most frequently mentioned by researchers. The inverse relationship between potassium and glucose was confirmed and potassium supplementation was correlated with a smaller increase in serum glucose.40 Abdominal obesity and impaired insulin release were found to be involved in this process. Thiazide‐induced hyperuricemia may also be responsible for some adverse metabolic effects and may be associated with increased cardiovascular risk and renal injury. Other possible mechanisms related to dysglycemia include visceral fat accumulation, changes in renin‐angiotensin‐aldosterone system (RAAS) activity, hepatic insulin resistance, and genetic variations.41
It seems that thiazide diuretics influence glucose metabolism in a dose‐dependent manner. Lower doses of thiazides have therefore been recommended by international guidelines to reduce adverse effects.42 In this meta‐analysis, we found that serum glucose levels were significantly lower in patients receiving low doses of thiazides (HCTZ or CTD ≤25 mg daily) than in those receiving high doses. Similar results were presented in previous studies.43 Apart from the relatively favorable metabolic outcomes, low‐dose thiazides are also effective in controlling blood pressure and preventing cardiovascular events.10, 44 A recent systematic review demonstrated that low‐dose HCTZ could effectively lower blood pressure. For thiazide diuretics other than HCTZ, the lowest doses could lower blood pressure maximally and higher doses did not exhibit increased effects on blood pressure.10 Low‐dose thiazide treatment has been proven to be well‐tolerated and could significantly reduce cardiovascular outcomes for hypertensive patients.44 Another finding of our meta‐analysis is that patients with longer duration of treatment had less glycemic change compared with patients with short‐term treatment. However, this result should be cautiously interpreted given the specific comparative agent (β‐blocker) and the few number of studies and participants included. For patients with hypertension, blood glucose levels tend to increase over time. According to the results of randomized trials, changes in blood glucose were most evident in the first 1 or 2 years in patients receiving thiazides. This disparity in blood glucose between patients receiving thiazides and patients receiving other treatments appeared to wane over time.16, 26 Nevertheless, an observational study published recently indicated that prolonged duration of thiazide treatment was associated with increased fasting glucose.45 Long‐term randomized clinical trials are needed for more accurate comparisons regarding treatment duration.
Diabetic patients receiving thiazides were shown to have a moderate increase in serum glucose. A recent meta‐analysis46 reported a higher increase in FPG (MD, 1.69 mmol/L [30.42 mg/dL]; 95% CI, 0.69–2.69) in diabetic patients taking thiazide diuretics compared with that in our study. Considering the impaired glucose metabolism of existing diabetes, it can be assumed that the use of thiazides in diabetic patients needs to be more cautiously monitored.
Similar to thiazide diuretics, ACE inhibitors and ARBs are medications widely used and also recommended as first‐line antihypertensive medications. These classes showed beneficial effects on glucose metabolism in contrast to thiazide diuretics,5 probably because of their inhibition of RAAS. The combinations of low‐dose thiazides and ACE inhibitors or ARBs were demonstrated to be effective and well‐tolerated in hypertensive patients without apparent adverse effects on glucose and lipid profiles,47 suggesting complementary impacts of these drugs. Based on the neutral or less‐adverse glycemic effects, these combinations might be considered for patients with impaired glucose metabolism. On the contrary, β‐blockers were not recommended to be used in combination with thiazide‐type diuretics. Because of the negative impacts of both classes on glucose metabolism, patients at increased risk for diabetes should avoid using these combination regimens.
A meta‐analysis by Mukete and Rosendorff48 investigated the glycemic effects of thiazide diuretics and showed that low‐dose thiazide diuretics were associated with significantly increased FPG.48 However, only 10 studies were included and no comparison based on doses of thiazides was shown in this study. Although the total sample size was larger than our study, some nonrandomized trials and some trials using combination therapies containing thiazides were included.
Study Limitations
There are some limitations to our study. First, except for seven trials,15, 16, 22, 23, 26, 32, 34 the other included studies were small, with fewer than 200 participants. Second, most studies measured only the changes of FPG without referring to other glycemic parameters, which led to a small sample size in the pooled analysis of PPG and HbA1c. In addition, despite the positive findings in subgroup analyses, the sample sizes of the high‐dose thiazide subgroup and both subgroups in the analysis based on duration of treatment are relatively small. Third, most studies did not clearly report the process of randomization, which might increase the risk of bias. Finally, since glycemic outcome was not the major endpoint in some trials and most trials lasted less than a year, further randomized controlled trials with longer duration and larger populations are needed for more conclusive results. In fact, some recommendations have been released by a national working group of the United States to encourage more research on thiazide‐induced dysglycemia.49 Previous evidence suggests that there may be no increased cardiovascular risk in patients with dysglycemia or incident diabetes during thiazide therapy.39 However, a study with a 15‐year follow‐up reported that diabetes associated with diuretic use was linked to significant cardiovascular risk. In addition, patients with incident diabetes receiving antihypertensive treatment that included diuretics were shown to have higher cardiac morbidity rates than those without diabetes.50 Since antihypertensive treatment is usually a persistent or even lifelong process, further studies are needed to evaluate long‐term consequences of the glycemic effects of thiazides, especially their impact on major cardiovascular outcomes.
Conclusions
This meta‐analysis demonstrated a significant but small magnitude of glycemic abnormalities associated with thiazide‐type diuretics in hypertensive patients. Treatment with lower doses might reduce or avoid adverse effects. Further investigations are needed to clarify whether these metabolic changes are of clinical significance.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1. Flowchart of literature search and selection.
Figure S2. Risk of bias of included studies.
Figure S3. Risk of bias summary.
Figure S4. Begg's funnel plot for the mean difference of the change in fasting plasma glucose (FPG) comparing thiazide‐type diuretics with nonthiazide medications or placebo or nontreatment.
Figure S5. Begg's funnel plot for the mean difference of the change in fasting plasma glucose (FPG) comparing thiazide‐type diuretics with calcium channel blockers (CCBs).
Figure S6. Begg's funnel plot for the mean difference of the change in fasting plasma glucose (FPG) comparing thiazide‐type diuretics with angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers.
Figure S7. Forest plot for subgroup analysis of change in fasting plasma glucose (FPG) comparing thiazide‐type diuretics with nonthiazide medications or placebo or nontreatment based on types of medication in control groups. Mean differences (MDs) from the individual studies are presented by squares and the size of the square represents the statistical weight of the study in the summary estimate. The horizontal line indicates the 95% confidence interval of the study.
Acknowledgments
This research received no specific financial support.
J Clin Hypertens (Greenwich). 2016;18:342–351. DOI: 10.1111/jch.12679 © 2015 Wiley Periodicals, Inc.
References
- 1. Multiple Risk Factor Intervention Trial Research Group . Mortality after 10 1/2 years for hypertensive participants in the Multiple Risk Factor Intervention Trial. Circulation. 1990;82:1616–1628. [DOI] [PubMed] [Google Scholar]
- 2. Brown MJ, Palmer CR, Castaigne A, et al. Morbidity and mortality in patients randomised to double‐blind treatment with a long‐acting calcium‐channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet. 2000;356:366–372. [DOI] [PubMed] [Google Scholar]
- 3. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 4. Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta‐analysis. Lancet. 2007;369:201–207. [DOI] [PubMed] [Google Scholar]
- 5. Rastogi D, Pelter MA, Deamer RL. Evaluations of hospitalizations associated with thiazide‐associated hyponatremia. J Clin Hypertens (Greenwich). 2012;14:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vandell AG, McDonough CW, Gong Y, et al. Hydrochlorothiazide‐induced hyperuricaemia in the pharmacogenomic evaluation of antihypertensive responses study. J Intern Med. 2014;276:486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deshmukh M, Lee HW, McFarlane SI, et al. Antihypertensive medications and their effects on lipid metabolism. Curr Diab Rep. 2008;8:214–220. [DOI] [PubMed] [Google Scholar]
- 8. Higgins J, Green S. Cochrane handbook for systematic reviews of intervention 5.1.0 (2011) Cochrane Collaboration. http://handbook.cochrane.org. Accessed October 16, 2014.
- 9. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Musini VM, Nazer M, Bassett K, et al. Blood pressure‐lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database Syst Rev 2014;5:CD003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chrysant SG. Antihypertensive effectiveness of low‐dose lisinopril–hydrochlorothiazide combination. A large multicenter study. Lisinopril–Hydrochlorothiazide Group. Arch Intern Med. 1994;154:737–743. [PubMed] [Google Scholar]
- 12. Reisin E, Weir MR, Falkner B, et al. Lisinopril versus hydrochlorothiazide in obese hypertensive patients: a multicenter placebo‐controlled trial. Treatment in Obese Patients With Hypertension (TROPHY) Study Group. Hypertension. 1997;30:140–145. [DOI] [PubMed] [Google Scholar]
- 13. Jounela AJ, Lilja M, Lumme J, et al. Relation between low dose of hydrochlorothiazide, antihypertensive effect and adverse effects. Blood Press. 1994;3:231–235. [DOI] [PubMed] [Google Scholar]
- 14. Fuenmayor NT, Moreira E, de los Rios V, et al. Relations between fasting serum insulin, glucose, and dihydroepiandrosterone‐sulfate concentrations in obese patients with hypertension: short‐term effects of antihypertensive drugs. J Cardiovasc Pharmacol 1997;30:523–527. [DOI] [PubMed] [Google Scholar]
- 15. Helgeland A, Leren P, Foss OP, et al. Serum glucose levels during long‐term observation of treated and untreated men with mild hypertension. The Oslo study. Am J Med. 1984;76:802–805. [DOI] [PubMed] [Google Scholar]
- 16. Savage PJ, Pressel SL, Curb JD, et al. Influence of long‐term, low‐dose, diuretic‐based, antihypertensive therapy on glucose, lipid, uric acid, and potassium levels in older men and women with isolated systolic hypertension: the Systolic Hypertension in the Elderly Program. SHEP Cooperative Research Group. Arch Intern Med. 1998;158:741–751. [DOI] [PubMed] [Google Scholar]
- 17. Vardan S, Mehrotra KG, Mookherjee S, et al. Efficacy and reduced metabolic side effects of a 15‐mg chlorthalidone formulation in the treatment of mild hypertension. A multicenter study. JAMA. 1987;258:484–488. [DOI] [PubMed] [Google Scholar]
- 18. Carlsen JE, Køber L, Torp‐Pedersen C, et al. Relation between dose of bendrofluazide, antihypertensive effect, and adverse biochemical effects. BMJ. 1990;300:975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pool PE, Applegate WB, Woehler T, et al. A randomized, controlled trial comparing diltiazem, hydrochlorothiazide, and their combination in the therapy of essential hypertension. Pharmacotherapy. 1993;13:487–493. [PubMed] [Google Scholar]
- 20. Siegel D, Saliba P, Haffner S. Glucose and insulin levels during diuretic therapy in hypertensive men. Hypertension. 1994;23:688–694. [DOI] [PubMed] [Google Scholar]
- 21. Mersey J, D'Hemecourt P, Blaze K. Once‐daily fixed combination of captopril and hydrochlorothiazide as first line therapy for mild to moderate hypertension. Curr Ther Res Clin Exp. 1993;53:502–512. [Google Scholar]
- 22. Fiddes R, Blumenthal J, Dawson JE, et al. Evaluation of indapamide 1.25 mg once daily in elderly patients with mild to moderate hypertension. J Hum Hypertens. 1997;11:239–244. [DOI] [PubMed] [Google Scholar]
- 23. Hall WD, Weber MA, Ferdinand K, et al. Lower dose diuretic therapy in the treatment of patients with mild to moderate hypertension. J Hum Hypertens. 1994;8:571–575. [PubMed] [Google Scholar]
- 24. Calvo C, Gude F, Abellan J, et al. A comparative evaluation of amlodipine and hydrochlorothiazide as monotherapy in the treatment of isolated systolic hypertension in the elderly. Clin Drug Invest. 2000;19:317–326. [Google Scholar]
- 25. Pareek A, Karnik N, Salagre SB, et al. Clinical effectiveness of low‐dose chlorthalidone (6.25 mg) + atenolol combination in stage I hypertensive patients: a multicenter, randomized, controlled study. Curr Med Res Opin. 2008;24:1771–1779. [DOI] [PubMed] [Google Scholar]
- 26. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . The antihypertensive and lipid‐lowering treatment to prevent heart attack trial. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 27. Piecha G, Adamczak M, Chudek J, et al. Indapamide decreases plasma adiponectin concentration in patients with essential hypertension. Kidney Blood Press Res. 2007;30:187–194. [DOI] [PubMed] [Google Scholar]
- 28. Jansen RW, Van Lier HJ, Hoefnagels WH. Nitrendipine versus hydrochlorothiazide in hypertensive patients over 70 years of age. Clin Pharmacol Ther. 1989;45:291–298. [DOI] [PubMed] [Google Scholar]
- 29. Pollare T, Lithell H, Berne C. A comparison of the effects of hydrochlorothiazide and captopril on glucose and lipid metabolism in patients with hypertension. N Engl J Med. 1989;321:868–873. [DOI] [PubMed] [Google Scholar]
- 30. Weinberger MH. Blood pressure and metabolic responses to hydrochlorothiazide, captopril, and the combination in black and white mild‐to‐moderate hypertensive patients. J Cardiovasc Pharmacol. 1985;7(Suppl 1):S52–S55. [DOI] [PubMed] [Google Scholar]
- 31. Grassi G, Seravalle G, Dell'Oro R, et al. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. J Hypertens. 2003;21:1761–1769. [DOI] [PubMed] [Google Scholar]
- 32. Zappe DH, Sowers JR, Hsueh WA, et al. Metabolic and antihypertensive effects of combined angiotensin receptor blocker and diuretic therapy in prediabetic hypertensive patients with the cardiometabolic syndrome. J Clin Hypertens (Greenwich). 2008;10:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stimpel M, Koch B, Oparil S. Antihypertensive treatment in postmenopausal women: results from a prospective, randomized, double‐blind, controlled study comparing an ACE inhibitor (moexipril) with a diuretic (hydrochlorothiazide). Cardiology. 1998;89:271–276. [DOI] [PubMed] [Google Scholar]
- 34. Veterans Administration Cooperative Study Group on Antihypertensive Agents . Propranolol or hydrochlorothiazide alone for the initial treatment of hypertension. IV. Effect on plasma glucose and glucose tolerance. Hypertension. 1985;7:1008–1016. [DOI] [PubMed] [Google Scholar]
- 35. Berglund G, Andersson O. Beta‐blockers or diuretics in hypertension? A 6 year follow‐up of blood pressure and metabolic side effects. Lancet. 1981;1:744–747. [DOI] [PubMed] [Google Scholar]
- 36. Alderman MH, Davis TK, Carroll L. Initial antihypertensive therapy. Comparison of prazosin and hydrochlorothiazide. Am J Med. 1986;80:120–125. [DOI] [PubMed] [Google Scholar]
- 37. Verdecchia P, Angeli F, Reboldi GP, et al. New‐onset diabetes in treated hypertensive patients. Curr Hypertens Rep. 2005;7:174–179. [DOI] [PubMed] [Google Scholar]
- 38. Barzilay JI, Davis BR, Whelton PK. The glycemic effects of antihypertensive medications. Curr Hypertens Rep. 2014;16:410. [DOI] [PubMed] [Google Scholar]
- 39. Kostis JB, Wilson AC, Freudenberger RS, et al. Long‐term effect of diuretic‐based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol. 2005;95:29–35. [DOI] [PubMed] [Google Scholar]
- 40. Zillich AJ, Garg J, Basu S, et al. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006;48:219–224. [DOI] [PubMed] [Google Scholar]
- 41. Eriksson JW, Jansson PA, Carlberg B, et al. Hydrochlorothiazide, but not candesartan, aggravates insulin resistance and causes visceral and hepatic fat accumulation: the mechanisms for the diabetes preventing effect of candesartan (MEDICA) study. Hypertension. 2008;52:1030–1037. [DOI] [PubMed] [Google Scholar]
- 42. Guidelines Subcommittee . 1999 World Health Organization‐International Society of Hypertension guidelines for the management of hypertension. J Hypertens. 1999;17:151–183. [PubMed] [Google Scholar]
- 43. Harper R, Ennis CN, Sheridan B, et al. Effects of low dose versus conventional dose thiazide diuretic on insulin action in essential hypertension. BMJ. 1994;309:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weir MR, Flack JM, Applegate WB. Tolerability, safety, and quality of life and hypertensive therapy: the case for low‐dose diuretics. Am J Med. 1996;101:83S–92S. [DOI] [PubMed] [Google Scholar]
- 45. Karnes JH, Gong Y, Arwood MJ, et al. Alteration in fasting glucose after prolong treatment with a thiazide diuretic. Diabetes Res Clin Pract. 2014;104:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hirst JA, Farmer AJ, Feakins BG, et al. Quantifying the effects of diuretics and beta‐blockers on glycaemic control in diabetes mellitus – a systematic review and meta‐analysis. Br J Clin Pharmacol. 2015;79:733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fujiwara W, Izawa H, Ukai G, et al. Low dose of hydrochlorothiazide, in combination with angiotensin receptor blocker, reduces blood pressure effectively without adverse effect on glucose and lipid profiles. Heart Vessels. 2013;28:316–322. [DOI] [PubMed] [Google Scholar]
- 48. Mukete BN, Rosendorff C. Effects of low‐dose thiazide diuretics on fasting plasma glucose and serum potassium – a meta‐analysis. J Am Soc Hypertens. 2013;7:454–466. [DOI] [PubMed] [Google Scholar]
- 49. Carter BL, Einhorn PT, Brands M, et al. Thiazide‐induced dysglycemia: call for research from a working group from the National Heart, Lung, and Blood Institute. Hypertension. 2008;52:30–36. [DOI] [PubMed] [Google Scholar]
- 50. Aksnes TA, Kjeldsen SE, Rostrup M, et al. Impact of new‐onset diabetes mellitus on cardiac outcomes in the Valsartan Antihypertensive Long‐term Use Evaluation (VALUE) trial population. Hypertension. 2007;50:467–473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flowchart of literature search and selection.
Figure S2. Risk of bias of included studies.
Figure S3. Risk of bias summary.
Figure S4. Begg's funnel plot for the mean difference of the change in fasting plasma glucose (FPG) comparing thiazide‐type diuretics with nonthiazide medications or placebo or nontreatment.
Figure S5. Begg's funnel plot for the mean difference of the change in fasting plasma glucose (FPG) comparing thiazide‐type diuretics with calcium channel blockers (CCBs).
Figure S6. Begg's funnel plot for the mean difference of the change in fasting plasma glucose (FPG) comparing thiazide‐type diuretics with angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers.
Figure S7. Forest plot for subgroup analysis of change in fasting plasma glucose (FPG) comparing thiazide‐type diuretics with nonthiazide medications or placebo or nontreatment based on types of medication in control groups. Mean differences (MDs) from the individual studies are presented by squares and the size of the square represents the statistical weight of the study in the summary estimate. The horizontal line indicates the 95% confidence interval of the study.


