Abstract
Objective
To understand patient burden of treatment of repeated intravitreal injections (IVI) in the management of exudative retinal diseases.
Methods and analysis
Participants were sampled from a large urban retina specialty practice in Houston, Texas, USA, based on history of ongoing receipt of IVI. The 50-item Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections questionnaire was developed to evaluate the patient experience including discomfort, anxiety, inconvenience and satisfaction. Categorial principal components analysis (CATPCA) was performed to assess construct validity and internal consistency. A subset of these items was used to establish a measure of total treatment burden, referred to as the IVI Treatment Burden Score (TBS).
Results
142 patients participated in this study. CATPCA analysis revealed five dimensions of patient burden: disruption of normal routine or capacity, anxiety, frequency of visits, chronicity of disease and perceived treatment value or satisfaction. Together, these dimensions accounted for 67% of variance explained. Cronbach’s alpha was 0.97. The most frequently cited cause of discomfort was the feeling after anaesthetic wore off. The most common source of anxiety was fear of injection and associated discomfort or pain. Regarding inconvenience, patients reported temporary postinjection debilitation, requiring an average of 8 hours for recovery per treatment. The most frequently identified sources of satisfaction were confidence in the provider or treatment and interactions with staff.
Conclusions
Understanding and quantifying the patient burden associated with repeated IVI for exudative retinal diseases can reveal opportunities to improve delivery methods. The TBS could serve to inform strategies to maximise treatment adherence and optimise patient experiences.
Keywords: retina, treatment medical, neovascularisation
Key messages.
What is already known about this subject?
Repeat intravitreal injections (IVIs) pose a substantial burden on patients, which may influence treatment adherence and thereby visual outcomes. To date, there are no validated tools that specifically emphasise the patient burden of treatment.
What are the new findings?
We report the first phase of Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections, a survey to evaluate the patient experience of receiving IVI. We present the Treatment Burden Score, a single score based on patient-reported data that serves as a validated measure of patient burden.
How might these results change the focus of research or clinical practice?
Understanding the nature and magnitude of patient burden for repeat IVI could reveal opportunities to change practice patterns to optimise patient experiences and outcomes.
Introduction
Over the last two decades, intravitreal injections (IVIs) have become one of the most common procedures performed in medicine.1 2 This is in large part due to the advent of antivascular endothelial growth factor (VEGF) agents and the large number of exudative retinal pathophysiologies now recognised to be driven by upregulation of VEGF. Injection of these anti-VEGF drugs has become the cornerstone of therapy for a number of exudative retinal disorders, including neovascular age-related macular degeneration (nAMD),3 diabetic macular oedema (DME),4 diabetic retinopathy (DR)5 and retinal venous occlusive disease (RVO).6 Worldwide, the prevalence of each of these conditions ranges in the tens of millions.3 5 6 Accordingly, it is estimated that over 20 million IVIs are administered on an annual basis.1
Several randomised studies have demonstrated the efficacy of anti-VEGF agents for nAMD,7 8 DME,4 DR9–12 and RVO.13 To manage pathological neovascularisation and exudation in these disorders, treatment typically involves repeated anti-VEGF injections over an indefinite time course. Outcomes can thereby depend greatly on patient adherence, which can in turn be influenced by patient experiences and perceptions of satisfaction versus treatment burden. There is, therefore, great interest in optimising patient experiences, towards the goal of maximising adherence.
Studies of the experiences of patients undergoing repeated IVI14 have used long-standing tools such as the macular disease Treatment Satisfaction Questionnaire (MacTSQ),15 designed to examine satisfaction with IVI in the treatment of nAMD. Interestingly, others have reported that patients with high levels of satisfaction measured by MacTSQ continued to identify several burdensome aspects of treatment.14 In one single-centre retrospective study, the most frequent reason patients discontinued treatment was fear of injection.16 Thus, careful examination of the factors underlying patient burden could yield further insight into areas of improvement in the management of exudative retinal disease.
While multiple authors have highlighted the challenging aspects of patient experiences with IVI,17–22 no single quantitative model of patient treatment burden has emerged. In an effort to balance vision benefits against associated burdens of repeated IVI for exudative retinal diseases, the exact frequency of injections needed to maintain optimal clinical outcomes has been the subject of several investigations.4 5 11–13 18 19 23 24 To facilitate this effort, it would be useful for practitioners who manage exudative retinal diseases to understand the magnitude of patient treatment burden associated with repeated IVI.
The current study uses a patient survey to characterise burdensome aspects of repeated IVI and to develop a quantitative measure of patient burden. Herein, we describe the development and psychometric analysis of the Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII). We establish a single score of treatment burden, the IVI Treatment Burden Score (TBS) from this survey. Measurements such as the TBS could inform approaches to optimise patient experiences and adherence for a broad range of retinopathies and/or for comparative analysis of retinal therapeutics.
Materials and methods
Participants
Participants from a large urban retinal specialty clinic (Retina Consultants of Houston, Houston, Texas, USA) were invited by posted signage to self-administer the electronic survey on the day of presentation to the clinic. No prior medical record review was performed for this study, and no patient-specific identifying information was monitored or recorded.
Patient and public involvement
Patients were involved in the design our research via informal interviews, during which patient feedback was obtained on the ease of participation and clarity of survey questions.
Study design
An initial version of the QUALITII survey was conceptualised based on literature review, expert input and informal interviews of patients receiving repeated IVI. Multiple phases of content review were performed by several readers, including retina specialists, technicians and office staff to assess content, scope, and readability. The final survey was a 50-item questionnaire (online supplemental appendix 1), which was delivered electronically on Apple iPads located inside the clinic.
bmjophth-2020-000669supp001.pdf (68.2KB, pdf)
The QUALITII survey includes questions on demographics, disease and treatment history, and patient perceptions of discomfort, anxiety, inconvenience and satisfaction associated with treatment. Response scales were a combination of multiple-choice items ranked on a 7-point Likert scale with graded continuum, multiple-choice items without ranked scales, and free response. The variety of response styles was selected to account for the heterogeneous nature of question items. The wide scope of subject matter further lent itself to a relatively long list of question items. Because of this length, we attempted to control for the tendency of respondents to select answers reflexively, by intermittently reversing the direction of graded response scales, where relevant.
Psychometric analysis
From the original 50-item questionnaire, psychometric analysis was performed on a subset of 20 items, estimated to address the most salient aspects of burden (online supplemental appendix 2A). These items (hereafter referred to as 20 ‘major’ items) assessed aspects of the patient experience that were hypothesised to influence perceived patient burden, including discomfort, anxiety, the frequency and cumulative number of treatments, and satisfaction with treatment. Given the use of multiple measurement levels (nominal and ordinal) across question items, we performed CATPCA with Varimax rotation with Kaiser normalisation (IBM Statistics SPSS V.24). To evaluate the internal consistency of these 20 question items, Cronbach’s alpha analyses were performed. The threshold value for loading and cross loading was 0.4. Three items with loading below 0.4 or cross loading over 0.4 were individually reviewed and ultimately retained in the 20-item construct due to their clinical significance.
bmjophth-2020-000669supp002.pdf (64.2KB, pdf)
IVI TBS
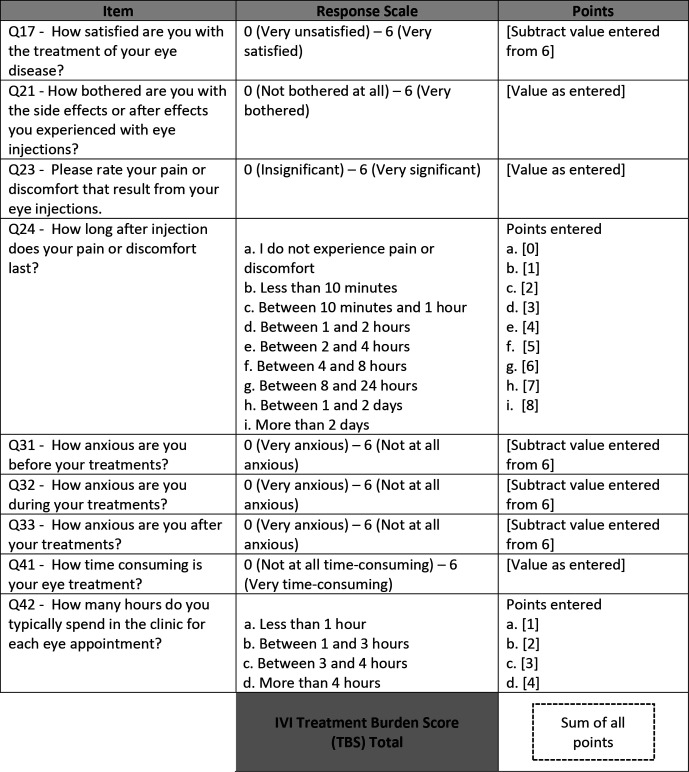
Following psychometric analysis as described above, a refined subset of 9 items (Q17, Q21, Q23, Q24, Q31, Q32, Q33, Q41, Q42) was used to construct the TBS, a numeric score of the perceived burden of treatment. The response scale of five of these items (Q21, Q23, Q24, Q41, Q42) directly correlated with burden, and the response scale of the remaining four items (Q17, Q31, Q32, Q33) inversely correlated with burden. Calculation of the TBS required arithmetic addition of the five subscores from the former group and four subscores from the latter, which were obtained by subtracting the patient-reported score from the maximum possible score on the scale provided figure 1).
Figure 1.
Method of calculation of Treatment Burden Score (TBS).
Statistical analysis and reporting
Averages reported are arithmetic means, unless otherwise described. Free response answers, along with answers involving a binned response range, were individually reviewed and converted to numerical values for statistical analysis, where relevant. Questions with specific answer choices that could not be immediately converted to a single numeric value were evaluated case by case, to determine how and whether to use these answer choices in estimating the average value of response. We tested normality of distribution of the TBS by K-S test. Other statistical tests are described in the Results section.
Results
Participant demographics
A total of 142 patients completed this initial, single-centre phase of the QUALITII survey. The mean age of participants was 65.6 years, with 45% identifying as male and 55% female. Retinopathies represented among patients included AMD (31%), DME or DR (38%), RVO 9.2% or another condition (11.3%), with 10.6% reporting uncertain diagnosis. The demographic profile of participants is summarised in table 1.
Table 1.
Demographic profile of survey participants
| Q1: Language (n=142) | Proportions |
| English | 95% |
| Spanish | 5% |
| Q2: Age (n=142) | Proportions |
| 20–37 years | 3% |
| 38–55 years | 23% |
| 56–73 years | 46% |
| 74–91 years | 27% |
| 92–109 years | 2% |
| Q3: Gender (n=142) | Proportions |
| Male | 45% |
| Female | 55% |
| Q4: Ethnicity (n=142) | Proportions |
| Caucasian (white) | 51% |
| African American (black) | 25% |
| Latino (Hispanic) | 20% |
| Asian | 4% |
| American Indian | 0% |
| Pacific Islander | 0% |
| Other | 1% |
| Q5: Insurance (n=142) | Proportions |
| Private health insurance policy | 44% |
| Marketplace insurance policy (Obamacare) | 2% |
| Military healthcare (like TRICARE) | 1% |
| Public health insurance policy (Medicare or Medicaid) | 50% |
| No insurance/self-pay | 2% |
| Q6: Condition (n=142) | Proportions |
| Age-related macular degeneration | 31% |
| Diabetic macular oedema and/or diabetic retinopathy | 38% |
| Retinal vein occlusion | 9% |
| Other | 11% |
| Uncertain | 11% |
Patient-reported details of IVI treatment protocols
The median number of years since diagnosis was 2.5. Most received anti-VEGF (71%), while 3.4% received dexamethasone or another medication, and 25.7% were unaware of which medication they receive. The median frequency of injection was once every 4.5 weeks, with a range of every 4–5 weeks (64.8%) up to every 13–16 weeks (1.4%), and 4.2% reporting injections on an as needed or PRN (pro re nata) basis. Treatment protocols experienced by respondents included a variety of numbing approaches, most frequently numbing gel or drops, and lasted a median of 2 hours. Table 2 summarises the characteristics of treatment protocols reported.
Table 2.
Treatment details of the IVI regimen experienced by participants
| Q7: Years of diagnosis (n=142) | Proportions |
| Within the last year | 18% |
| 1–4 years ago | 44% |
| 5–10 years ago | 26% |
| >10 years ago | 6% |
| Uncertain | 6% |
| Q9: Medication (multiple selections allowed) (n=142) | No of patients |
| Avastin (bevacizumab) | 17 |
| Eylea (aflibercept) | 43 |
| Lucentis (ranibizumab) | 45 |
| Ozurdex (dexamethasone) | 4 |
| Other | 1 |
| Unknown | 38 |
| Patients with multiple (>1) selections | 5 |
| Q10: Tried more than one medication (n=142) | Proportions |
| Yes | 33% |
| No | 37% |
| Uncertain | 30% |
| Q11: Frequency of injection (n=142) | Proportions |
| Every 4–5 weeks | 65% |
| Every 6–8 weeks | 23% |
| Every 10–12 weeks | 6% |
| Every 13–16 weeks | 1% |
| Only as needed (PRN) | 4% |
| Q12: Frequency of visits (n=142) | Proportions |
| Every 4–5 weeks | 65% |
| Every 6–8 weeks | 21% |
| Every 10–12 weeks | 7% |
| Every 13–16 weeks | 1% |
| Every six mos or more | 4% |
| Other | 1% |
| Q13: Total no of injections (n=142) | Proportions |
| 0–5 | 23% |
| 6–10 | 18% |
| 11–20 | 15% |
| 20–50 | 24% |
| >50 | 20% |
| Q14: Numbing medication (multiple selections allowed) (n=142) | No of patients |
| Numbing drops and/or numbing gel only | 80 |
| Injection of numbing medicine | 60 |
| Q-tips soaked with numbing medicine pushed onto your eye | 10 |
| Uncertain | 21 |
| Patients with multiple (>1) selections | 25 |
| Q15: Use of speculum (n=142) | Proportions |
| Yes | 72% |
| No | 28% |
| Q16: Receive injections in both eyes each visit (n=142) | Proportions |
| Yes | 32% |
| No | 60% |
| Sometimes | 8% |
IVI, intravitreal injection.
Patient-reported treatment burdens of repeated IVI
The broad categories of burden examined by the QUALITII survey included discomfort, anxiety and burdens on time and functional capacity. A majority of participants (75.8%) experienced at least some discomfort with treatment. Most (52.3%) rated discomfort between 0 and 2 at the lower range of the response scale, but a large proportion (28.1%) rated discomfort between 4 and 6, where 6 represented very significant discomfort.
The three most uncomfortable aspects of treatment identified were use of betadine prior to injection, injection itself, and the feeling after anaesthetic wore off. Corresponding levels of anxiety experienced before, during, and after treatment were each rated at a median value of 4, on a 7-point scale, where a maximum score of 6 represented no anxiety. Therefore, patients reported moderately low but persistent anxiety throughout treatment. Among patients reporting anxiety, the most common reasons were fear of the injection itself (30.8%), fear of discomfort/pain from injection (23.1%) and fear of losing sight (12.8%).
In our study, a substantial component of patient burden arose from a combination of discomfort and the time and functional burdens imposed by treatment. This was conceptualised as disruption to normal activity or capacity. Though the average rating of discomfort was low to moderate (2.41 on a 7-point scale, where a maximum value of 6 represented very significant discomfort), a sizeable proportion (38.3%) of patients responded that injections resulted in restriction of usual activity or capacity, with a median 8-hour recovery period after each treatment.
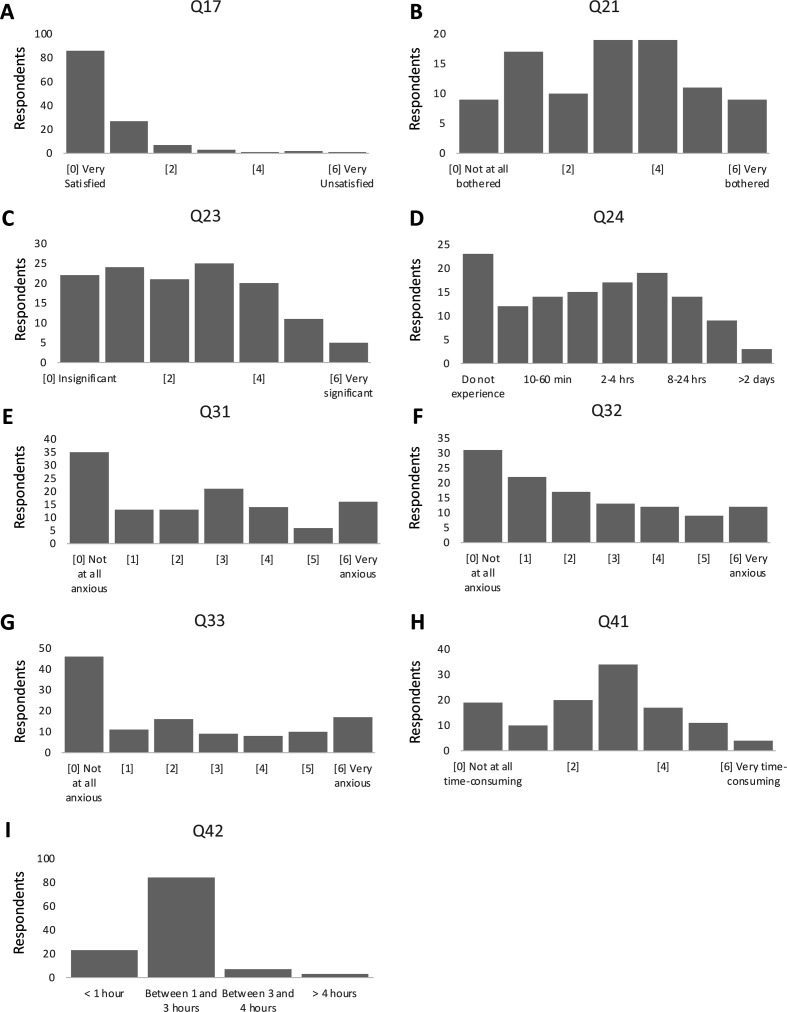
Linear regression analysis showed that perceived burden decreased with increased satisfaction (p=0.0139, R²=0.077). The most frequently cited reasons for satisfaction in our study were confidence in the provider or treatment and pleasant interactions with staff. Interestingly, our data suggest that even patients who reported maximum satisfaction experienced non-negligible levels of distress and considerable time sacrifices. Patient responses to the 20 major question items assessing aspects of burden and satisfaction levels, are summarised in figure 2, with remaining items assessing burden and satisfaction detailed in online supplemental appendix 3.
Figure 2.
Distributions of patient responses to nine TBS items assessing levels of burden and satisfaction. Response scales for all question items have been standardised to indicate increased burden with increasing scores. (A) Histogram of responses to Q17: How satisfied are you with the treatment of your eye disease?, n=127. (B) Histogram of responses to Q21: How bothered are you with the side effects or after effects you experienced with eye injections?, n=94. (C) Histogram of responses to Q23: Please rate your pain or discomfort that result from your eye injections, n=28. (D) Histogram of responses to Q24: How long after injection does your pain or discomfort last?, n=126. (E) Histogram of responses to Q31: How anxious are you before your treatments?, n=118. (F) Histogram of responses to Q32: How anxious are you during your treatments?, n=116. (G) Histogram of responses to Q33: How anxious are you after your treatments?, n=117. (H) Histogram of responses to Q41: How time consuming is your eye treatment?, n=115. (I) Histogram of responses to Q42: How many hours do you typically spend in the clinic for each eye appointment?, n=117. TBS, Treatment Burden Score.
bmjophth-2020-000669supp003.pdf (91.9KB, pdf)
Psychometric analysis of the QUALITII survey
CATPCA was performed on a selection of 20 major question items from the QUALITII survey, thought to broadly represent components of perceived treatment burdens. Five dimensions of burden emerged: disruption to normal activity or capacity, anxiety, frequency of visits, chronicity of disease, and satisfaction/perceived value of treatment. Cronbach’s alpha was 0.97, suggesting a high level of internal consistency and reliability. Together, these dimensions accounted for 67% of variance explained. Proportions of variance explained by of each dimension are provided in online supplemental appendix 4. In our analysis, three items did not load (Q30, Q39, Q43). A table of loading factors for each item is provided in online supplemental appendix 2B. Exclusion of all three of these items increased Cronbach’s alpha to 0.98, with variance explained rising to 76%. On individual review, these items were retained in our model due to clinically significant content.
bmjophth-2020-000669supp004.pdf (42.7KB, pdf)
IVI TBS
Of the 20 items underlying the five dimensions of patient burden identified by CATPCA, we identified a refined subset of nine items (Q17, Q21, Q23, Q24, Q31, Q32, Q33, Q41, Q42) whose response scales could be used as direct measures of burden. In general, response points derived from each of these items are summed towards the total TBS. The method of calculating the TBS from select QUALITII questionnaire items is provided in figure 1. Cronbach’s alpha for items included in the TBS was 0.75. In this study, the average TBS was 21.6 (actual range of 1–46), with a theoretical range of 1–54 (where 54 represents maximum burden). The SD of TBS scores across patients was 9.3, with a normal distribution. Average TBS did not differ between patients who received fewer than 20 injections vs at least 20 or greater injections (22.5 vs 20.5, student’s t-test, p=0.37). Further, logistic regression analysis showed that a higher TBS was a risk factor for the likelihood that patients would feel bothered enough by treatment to consider discontinuing (n=76, OR 1.26, 95% CI 1.0722 to 1.4885).
Discussion
This paper reports the development and validation of the QUALITII survey, with the aim of understanding patient treatment burdens of repeated IVI. Principal components analysis of select survey items demonstrated five underlying dimensions of burden: disruption of normal routine or capacity, anxiety, frequency of visits, chronicity of disease and perceived treatment value or satisfaction. These items together demonstrated a high level of internal consistency and accounted for 67% of variance.
The first dimension of burden identified (disruption of normal routine or capacity) consisted of items assessing severity of discomfort, length of time discomfort lasted, and perceptions of time consumed by treatment. Our results suggest that rather than the incidence of discomfort or incapacitation alone, this component of burden arises from the amount of time spent experiencing discomfort or debility. For patients who experienced any incapacitation, an average of 8 hours of time was required to recover normal activity levels. The implied functional time lost over the course of ongoing IVI could amount to substantial cumulative burden for patients. Given that discomfort may last for the remainder of the day following treatment, one way clinicians could intervene to reduce burden is to create a postprocedure pain management plan spanning this time period.
The second dimension of burden that emerged was anxiety. In this study, patients reported moderate but persistent levels of anxiety throughout the procedure, most commonly induced by fear of the injection itself and fear of discomfort/pain, in accordance with other studies.14 16 This poses a challenge for strategising immediate interventions, because current technologies require repeat injections for optimal management. That said, patients reported that factors such as clinic delays can worsen anxiety, and factors such as adequate pain medication during the procedure and having the procedure explained can lessen anxiety. Thus, in addition to maximising pain control intraprocedurally and postprocedurally, two simple interventions to reduce patient anxiety could be to minimise treatment delays and to communicate consistently with patients through the procedure.
The third and fourth dimensions of burden in our study were frequency of visits and chronicity of disease, respectively. Question items that loaded onto these dimensions were determined to represent historical information and/or indirect contributions to burden and were thus excluded from final calculation of the TBS. Interestingly, in preliminary analysis, the burden score appeared to be inversely related to disease chronicity, perhaps representing the finding that patients with lower perceived burdens are more likely to pursue ongoing treatment. Future studies may consider incorporating these measures into estimates of burden.
The final dimension of patient burden identified in our study was perceived treatment value or satisfaction. As expected, satisfaction displayed an inverse relationship to perceived burden, demonstrating a relationship also reported by others.17 25 Existing measures of treatment satisfaction in management of retinal disorders, such as the MacTSQ15 and RetTSQ,26 address burdensome features of treatment, including side effects, apprehension and pain. The TBS is a quantitative measure of patient burdens of repeated IVI that complements the above satisfaction scores, with additional emphasis on understanding the impact of treatment on quality of life. Moreover, the TBS describes the burdens of a shared treatment modality across nAMD, DME, DR and RVO. Thus, its applicability as a single score extends to a wide range of retinal disease. Such measures could be used to understand how commonly provided procedures impact patients and could potentially be used for comparisons of different retinal therapeutics.
The current study is limited by participation of patients from a single clinical practice and assessment of a predominantly English-speaking population. Further, the majority of represented patients in this study reported receiving monthly injections, perhaps introducing selection bias for patients with lower perceived burden, who could tolerate more frequent treatment. The next phases of the QUALITII study expand to multiple treatment centres and aim to diversify patient profile. Future studies may further address how burdens of IVI compare with other types of retinal procedures requiring frequent clinical visits.
Acknowledgments
The authors would like to acknowledge the contributions of research and administrative staff at Retina Consultants of Houston, who assisted with survey logistics and maintenance of signage and equipment for provision of this study.
Footnotes
Contributors: Valeria Lerma, in addition to research and administrative staff at Retina Consultants of Houston, served as survey administration coordinators and as critical liaisons in communicating patient feedback regarding the survey and its contents.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
It was determined by the Houston Methodist Research Institute Institutional Review Board that this study does not meet the definition of Human Subject Research per 45 CFR 46 and does not require prior IRB review or approval at Houston Methodist Hospital.
References
- 1.Martin DF. Evolution of intravitreal therapy for retinal Diseases-From CMV to CNV: the LXXIV Edward Jackson memorial lecture. Am J Ophthalmol 2018;191:xli–lviii. 10.1016/j.ajo.2017.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim LA, D'Amore PA, D’Amore P. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol 2012;181:376–9. 10.1016/j.ajpath.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–16. 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- 4.Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-Week results from the VISTA and vivid studies. Ophthalmology 2015;122:2044–52. 10.1016/j.ophtha.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 5.Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556–64. 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010;117:313–9. 10.1016/j.ophtha.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J Ophthalmol 2008;145:239–48. 10.1016/j.ajo.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Erfurth U, Eldem B, Guymer R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the excite study. Ophthalmology 2011;118:831–9. 10.1016/j.ophtha.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: rise and ride. Ophthalmology 2012;119:789–801. 10.1016/j.ophtha.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 10.Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs Intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 2015;314:2137–46. 10.1001/jama.2015.15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (bolt study). Ophthalmology 2010;117:1078–86. 10.1016/j.ophtha.2010.03.045 [DOI] [PubMed] [Google Scholar]
- 12.Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (bolt) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol 2012;130:972–9. 10.1001/archophthalmol.2012.393 [DOI] [PubMed] [Google Scholar]
- 13.Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117:1102–12. 10.1016/j.ophtha.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 14.Boyle J, Vukicevic M, Koklanis K, et al. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med 2018;23:127–40. 10.1080/13548506.2016.1274040 [DOI] [PubMed] [Google Scholar]
- 15.Mitchell J, Bradley C. Design and development of the MacTSQ measure of satisfaction with treatment for macular conditions used within the IVAN trial. J Patient Rep Outcomes 2017;2:5. 10.1186/s41687-018-0031-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polat O, İnan S, Özcan S, et al. Factors affecting compliance to intravitreal anti-vascular endothelial growth factor therapy in patients with age-related macular degeneration. Turk J Ophthalmol 2017;47:205–10. 10.4274/tjo.28003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye 2013;27:787–94. 10.1038/eye.2013.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle J, Vukicevic M, Koklanis K, et al. Experiences of patients undergoing anti-VEGF treatment for neovascular age-related macular degeneration: a systematic review. Psychol Health Med 2015;20:296–310. 10.1080/13548506.2014.936886 [DOI] [PubMed] [Google Scholar]
- 19.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388–98. 10.1016/j.ophtha.2012.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wykoff CC, Croft DE, Brown DM, et al. Prospective trial of Treat-and-Extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology 2015;122:2514–22. 10.1016/j.ophtha.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 21.Wykoff CC, Ou WC, Brown DM, et al. Randomized trial of Treat-and-Extend versus monthly dosing for neovascular age-related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmol Retina 2017;1:314–21. 10.1016/j.oret.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 22.Rufai SR, Almuhtaseb H, Paul RM, et al. A systematic review to assess the ‘treat-and-extend’ dosing regimen for neovascular age-related macular degeneration using ranibizumab. Eye 2017;31:1337–44. 10.1038/eye.2017.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt-Erfurth U, Kaiser PK, Korobelnik J-F, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the view studies. Ophthalmology 2014;121:193–201. 10.1016/j.ophtha.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 24.Ho AC, Busbee BG, Regillo CD, et al. Twenty-four-month efficacy and safety of 0.5 Mg or 2.0 Mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014;121:2181–92. 10.1016/j.ophtha.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 25.Tailor R. Evaluation of patients’ experiences at different stages of the intravitreal injection procedure – what can be improved? OPTH 2011;5:1499–502. 10.2147/OPTH.S24358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brose LS, Bradley C. Psychometric development of the retinopathy treatment satisfaction questionnaire (RetTSQ). Psychol Health Med 2009;14:740–54. 10.1080/13548500903431485 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjophth-2020-000669supp001.pdf (68.2KB, pdf)
bmjophth-2020-000669supp002.pdf (64.2KB, pdf)
bmjophth-2020-000669supp003.pdf (91.9KB, pdf)
bmjophth-2020-000669supp004.pdf (42.7KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.