Abstract
Arterial hypertension is a major risk factor for cardiovascular disease after solid organ transplantation, emphasizing the need for blood pressure (BP) monitoring. The authors studied 24‐hour ambulatory BP monitoring (ABPM) parameters (index, load, dipping) and their predictive value with regard to hypertension as well as correlations with graft function and metabolic parameters such as obesity and dyslipidemias. The ABPM profiles of 111 renal, 29 heart, and 13 liver transplant recipients were retrospectively analyzed 5 to 10 years after transplant (median 5.1 years). The BP profiles among the different transplant groups were similar. The BP index and load were abnormal especially at nighttime and the nocturnal BP dipping was often blunted (in 49% to 83% of the patients). The BP variables were found to be equally valued when assessing hypertension. BP load of 50% instead of 25% seems to be a more adequate cutoff value. The BP variables correlated poorly with the metabolic parameters and kidney function. Antihypertensive medication did not notably change the ABPM profile in renal transplant recipients. Hypertension, including nocturnal hypertension, is present in children receiving solid organ transplant, underlining the importance of use of ABPM in the follow‐up of these patients.
Solid organ transplantation is a successful treatment modality for children with end‐stage organ failure. The long‐term patient and graft survival rates are currently 70% to 95%1 and minimization of treatment‐related adverse effects has become increasingly significant. Arterial hypertension is common in patients after transplant (Tx),2, 3, 4, 5, 6, 7 and cardiovascular disease (CVD) is known to be the leading cause of mortality among adult Tx patients.8 The etiology of both hypertension and CVD is multifactorial, including calcineurin inhibitor (CNI) toxicity, use of glucocorticoids, obesity, and impaired kidney function.9, 10, 11
Blood pressure (BP) monitoring is crucial in the follow‐up of Tx patients, and 24‐hour ambulatory BP monitoring (ABPM) has advantages compared with office BP measurements.12, 13 The relative importance of the three major ABPM variables, namely BP index, load, and dipping, was discussed for years14, 15, 16, 17 until the American Heart Association (AHA) provided guidelines for ABPM in 2008, which were recently refined.18, 19 This classification uses a combination of office BP measurement, mean ambulatory BP values (systolic or diastolic), and loads (systolic or diastolic) in the staging of ABPM.
In this retrospective, cross‐sectional study, we analyzed the ABPM profiles of children and adolescents with renal (RTx), liver (LTx), or heart (HTx) transplants at the median of 5.1 years after the operation using AHA classification. We were especially interested in how the ABPM variables behaved in the three Tx groups that received relatively similar immunosuppressive medication (calcineurin inhibitor, antimetabolite, and low‐dose methylprednisolone). We hypothesized that the RTx patients would have the worst BP profiles. We also studied how the variables correlated with other metabolic factors (relative weight and lipids) and the concurrent and long‐term graft function in RTx patients.
Methods
Patients
All pediatric transplantations in Finland are performed at the study center. The patients are followed‐up annually at the study center until referral to adult care at the age of 18 to 20 years. All RTx, LTx, and HTx patients with an ABPM 5 years after the first Tx were eligible for the study (n=88). If the ABPM data at 5 years post‐Tx were missing, the nearest subsequent monitoring up to 10 years post‐Tx was used (in patients with several ABPMs). Thus, data on 27 (18%), 14 (9%), 9 (6%), 8 (5%), and 7 (5%) patients were included at 6, 7, 8, 9, and 10 years post‐Tx, respectively. The study did not include patients with an acute rejection <3 months prior to the study in order to avoid the confounding effect of high‐dose corticosteroid anti‐rejection therapy on BP. A total of 153 (95 male) recipients fulfilled the criteria and were enrolled into the study. Four patients had undergone a combined kidney and liver Tx and their data were analyzed in the RTx subgroup. This study was approved by the ethics committee for Pediatrics, Gynecology and Obstetrics, and Psychiatry of the Hospital District of Helsinki.
Immunosuppressive and Antihypertensive Medication
The study center's default immunosuppressive protocol for all Tx patients consisted of triple medication including cyclosporine A (CsA), azathioprine, and methylprednisolone (MP). The CsA dose was adjusted to obtain trough blood concentration of 60 μg/L to 100 μg/L during maintenance therapy (at 1 year post‐Tx and thereafter). The MP was tapered to a low‐dose alternate‐day dosing at 3 to 6 months post‐Tx, and the dose was not increased alongside growth after the first year post‐Tx. Azathioprine dose during the maintenance phase was 1.0 mg/kg/day to 1.4 mg/kg/day. In case of recurrent rejections, gradually increasing creatinine, or major cosmetic problems (hypertrichosis, gum hyperplasia), CsA was switched to tacrolimus. Mycophenolate was used instead of azathioprine in patients with recurrent rejections or gradually increasing creatinine. Also, if CNI toxicity was suspected, CsA or tacrolimus dosing was reduced and azathioprine was replaced with mycophenolate. At the time of ABPM analysis, one fifth of the patients were taking tacrolimus and the others were taking CsA‐based immunosuppression.
Roughly one third (n=55) of the Tx recipients were taking antihypertensive medication at the time of the study. Of the treated patients, calcium channel blockers and β‐blockers were most often used (Table 1). Thirteen Tx patients were taking two antihypertensive drugs and one RTx recipient was taking triple antihypertensive medication.
Table 1.
Clinical Characteristics of 153 Solid Organ Tx Recipients
| Variable | Renal Tx (n=111) | Heart Tx (n=29) | Liver Tx (n=13) |
|---|---|---|---|
| Male sex, No. (%) | 73 (66) | 15 (52) | 7 (54) |
| Age at Tx, y | 4.0 (0.7–15.9) | 7.5 (1.0–14.5) | 3.2 (0.7–14.2) |
| Follow‐up after Tx, y | 5.0 (4.9–10.1) | 5.0 (5.0–9.0) | 6.0 (5.0–10.1) |
| Measured GFR at study, mL/min/1.73 m2 | 49.3 (16.4–105.0) | 76.8 (38.9–115.0) | 83.0 (44.1–130.0) |
| Calcineurin inhibitor: CsA/tacrolimus, No. (%) | 90/21 (81/19) | 26/3 (90/10) | 11/2 (85/15) |
| Methylprednisolone daily dose, mg/kg | 0.06 (0.02–0.15) | 0.05 (0.03–0.15) | 0.03 (0.00–0.06) |
| Age at study, y | 11.2 (5.9–20.8) | 13.0 (6.1–19.5) | 10.2 (6.3–20.4) |
| Weight, kg | 33.8 (15.7–106.2) | 42.7 (17.0–90.3) | 31.2 (19.8–96.0) |
| Height, cm | 137.4 (101.5–185.8) | 148.0 (109.5–173.6) | 132.1 (110.3–179.7) |
| Body mass index, kg/m2 | 18.0 (14.2–34.6) | 19.1 (12.5–33.3) | 17.8 (15.6–29.7) |
| Antihypertensive medication, No. (%) | 45 (41) | 8 (28) | 2 (15) |
| Calcium channel–blocking agents | 25 | 5 | 2 |
| β‐Blockers | 17 | 4 | 1 |
| Angiotensin‐converting enzyme inhibitors | 8 | 1 | 0 |
| Angiotensin II receptor blockers | 4 | 0 | 0 |
| Loop diuretic | 1 | 0 | 0 |
| α‐Adrenergic–blocking agent | 1 | 0 | 0 |
Abbreviations: CsA, cyclosporine A; GFR, glomerular filtration rate; NA, not applicable; Tx, transplant. Values are number of patients (percentages) or median (range).
Clinical Data Collection
We performed a retrospective analysis of medical data on underlying disease, age at Tx and ABPM analysis, height, weight, biochemical metabolic parameters (total cholesterol, high‐density lipoprotein [HDL], low‐density lipoprotein [LDL], and triglycerides), and medication, as appropriate. Overweight was defined, according to the Finnish growth references, by exceeding the body mass index (BMI) percentile >25 kg/m2 at the age of 18 years, as recommended by the International Obesity Task Force Criteria.20, 21 Kidney function was assessed by measuring the 51Cr‐EDTA clearance as mL/min/1.73 m2.
BP Measurements
Office BP was measured in the beginning of the annual follow‐up visit during which the ABPM was performed or at a preceding outpatient visit within 3 months. We measured BP three times using an automated oscillometric device with a size‐appropriate cuff after the patient had remained seated for 5 minutes and used the average as office BP. Ambulatory BP was monitored by an automated device that was used one at a time over consecutive periods: ABPM 5100 or ABPM 6100 (Welch Allyn Inc, Skaneateles Falls, NY) or Schiller BR‐102 Plus (Schiller AG, Baar, Switzerland), which uses oscillometric measurements as backup to ensure the accuracy of auscultatory measurements. The devices have previously been tested to meet the Association for the Advancement of Medical Instrumentation US National Standard or the British Hypertension Society Standard.22, 23 The ABPM was accepted if no continuous interruption exceeding 2 hours occurred and at least 70% of the total measurement count was approved. The device was programmed to measure BP every 30 minutes from 7 am to 10 pm and hourly from 10 pm to 7 am. Daytime and nighttime periods were defined according to the ABPM diary held by the patients, or, in case the information was not provided by the patient (30%), daytime was defined as time between 8 am and 8 pm and nighttime from 12 am to 6 am in order to rule out bias by individual bedtime habits as suggested by Jones and Sinha.24
Systolic and diastolic and daytime and nighttime BP indices were calculated by dividing the average BP by the corresponding 95th percentile cutoff value for healthy European Caucasian children.25 BP load was calculated by dividing the count of measurements exceeding the 95th percentile cutoff value by the count of measurements during the study period. The difference between the average daytime and nighttime BP was denoted as nocturnal dipping.
Interpretation of the ABPM
The ABPMs were classified according to the updated AHA recommendations.19 Office BP was categorized as normal if below the 90th percentile and hypertensive if equal to or above the 95th percentile based on the sex‐ and height‐adjusted normative data provided by the National High Blood Pressure Education Program (NHBPEP).26 Office BP equal to or above the 90th percentile or more than 120/80 mm Hg but below the 95th percentile was regarded as prehypertensive. Mean ambulatory BP was defined as hypertensive, regardless of antihypertensive medication, if any of the daytime or nighttime, systolic or diastolic BP indices were equal to or more than one. Similarly, BP load was regarded as hypertensive if at least one BP load was 25% to 50% or as severely hypertensive if more than 50%. Nondipping was defined as <10% decline of the mean nighttime BP with respect to daytime. Patients with two related patterns on ABPM were categorized as unclassified: (1) office BP equal to or above the 95th percentile, normal BP indices, and elevated BP loads; and (2) office BP below the 90th percentile, normal BP indices, but elevated loads.
Statistics
Numerical results are reported as mean or median and SD or range. Student t test or Mann‐Whitney U test was used, as appropriate, when comparing continuous variables of two groups and, similarly, one‐way analysis of variance or Kruskall‐Wallis analysis of variance was used when comparing three or more groups. Chi‐square test or Fisher exact test was used when comparing categorical variables of two groups. Pearson correlation coefficient was used for assessment of linear dependence. Among the RTx patients, logistic regression analyses were performed to assess the impact of a number of factors on the likelihood for hypertensive result in each ambulatory BP variable. The model contained five dichotomous factors: office hypertension, decreased kidney function, overweight, increased total cholesterol, and increased triglycerides. SPSS Statistics 19.0 (SPSS Inc, Chicago, IL) was used for data analyses. P value <.05 was considered statistically significant.
Results
We analyzed the ABPM profiles of 153 pediatric Tx patients (111 RTx, 29 HTx, and 13 LTx patients) at the median of 5.1 years (5–10 years) post‐Tx. The clinical characteristics are presented in Table 1. The anthropometric variables among the Tx groups were very similar.
ABPM Profiles in Tx Recipients
The BP profiles and AHA classification in Tx subgroups are presented in Table 2. Interestingly, white‐coat hypertension was rare (0%–2%) but masked hypertension was common (26%–46%) in all Tx groups. Severe ambulatory hypertension was found in 28% to 38% of the patients. Normal BP was seldom found, indicating poor BP control. The proportions of patients in the categories were statistically similar (P=.280).
Table 2.
Ambulatory BP Monitoring Classification According to American Heart Association Criteria19
| Classification, % | Renal Tx (n=111) | Heart Tx (n=29) | Liver Tx (n=13) |
|---|---|---|---|
| Normal BP | 13 (14) | 21 (6) | 0 (0) |
| White‐coat hypertension | 2 (2) | 3 (1) | 0 (0) |
| Prehypertension | 5 (6) | 3 (1) | 8 (1) |
| Masked hypertension | 26 (29) | 45 (13) | 46 (6) |
| Ambulatory hypertension | 1 (1) | 0 (0) | 0 (0) |
| Severe ambulatory hypertension a | 38 (42) | 28 (8) | 23 (3) |
| Unclassified ambulatory BP monitoring b | 15 (17) | 0 (0) | 23 (3) |
Abbreviation: Tx, transplant. aIn severe ambulatory hypertension, blood pressure (BP) load is >50%, whereas in ambulatory hypertension it is 25% to 50%. bThe definition for unclassified ambulatory BP monitoring: (1) office BP ≥95th percentile, normal BP indices, and elevated BP loads; or (2) office BP <90th percentile and normal BP indices but elevated BP loads.
BP index, load, and dipping were compared in the Tx patients as shown in Figure 1. Overall, the parameters were similar among the groups. The major finding among the three Tx groups was that the RTx and HTx patients had higher BP indices and loads at nighttime as compared with daytime (P<.010 in all). In HTx patients, this resulted in clearly blunted systolic and diastolic dippings, which were on average only 3.9% and 5.3%, respectively. The values were significantly lower than in the RTx and LTx groups (Figure 1).
Figure 1.
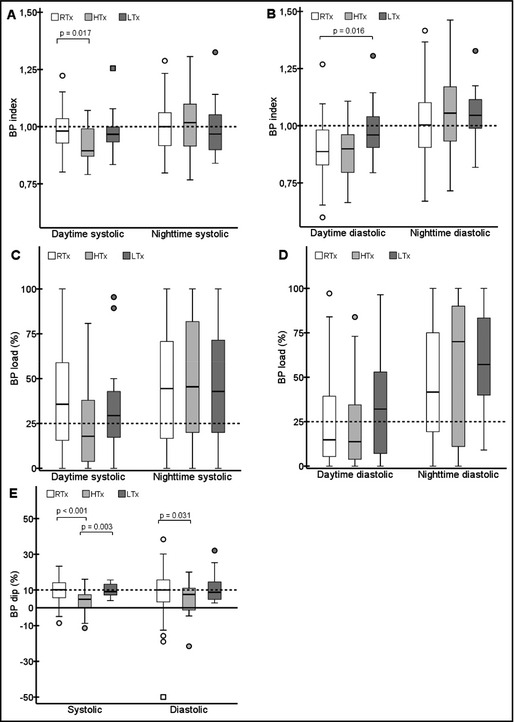
Systolic and diastolic blood pressure (BP) indices (A and B) and loads (C and D) according to time of day and dipping values (E) in renal transplant (RTx), heart transplant (HTx), and liver transplant (LTx) patients. The dashed lines indicate the thresholds of abnormal values (≥1.0 in index, ≥25% in load, and <10% in dipping).
BP profiles in RTx patients with (n=45) or without (n=66) antihypertensive medication are shown in Figure 2. Again, no major differences in the profiles were observed in the two groups. Somewhat surprisingly, the systolic BP indices and loads were higher in patie‐nts taking antihypertensive medication than in those not taking antihypertensive medication. The diastolic daytime measures were constantly lower than the nighttime countermeasures in patients with or without antihypertensive medication (P<.001 in all). In the systolic measures, the data showed similar results only in the group taking antihypertensive medication. No significant differences regarding use of antihypertensive medication were observed in the diastolic BP indices or loads, nor in the dipping values.
Figure 2.
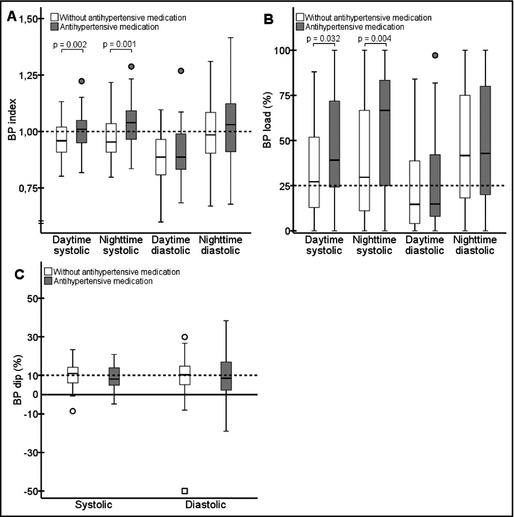
Systolic and diastolic blood pressure (BP) indices (A) and loads (B) according to time of day and dipping (C) values in renal transplant (RTx) patients with respect to antihypertensive medication. The dashed lines indicate the thresholds of abnormal values (≥1.0 in index, ≥25% in load, and <10% in dipping).
Frequency of Hypertensive BP Parameters
The frequency of patients with hypertensive BP index (≥1.0) and BP load (≥25% or ≥50%) was markedly higher during nighttime than daytime in all Tx groups (Table 3). Eighty‐four percent (n=129) of the patients (76%–100% among the subgroups) had at least one BP load measure ≥25%, whereas 64% (n=98; 62%–69% among the subgroups) had at least one BP load measure ≥50%.
Table 3.
Proportions of Study Patients With Abnormal BP Index, BP Load, and BP Dipping in Ambulatory BP Measurement
| Variable | Renal Tx (n=111) | Heart Tx (n=29) | Liver Tx (n=13) |
|---|---|---|---|
| Proportion, % | Proportion, % | Proportion, % | |
| Daytime BP, % | |||
| Systolic index ≥1.0 | 41 a | 17 | 23 |
| Diastolic index ≥1.0 | 19 | 14 | 31 |
| Systolic load ≥25% (≥50%) | 61 (33) | 41 (21) | 54 (23) |
| Diastolic load ≥25% (≥50%) | 37 (14 b ) | 31 (14) | 62 (39) |
| Nighttime BP, % | |||
| Systolic index ≥1.0 | 51 | 55 | 39 |
| Diastolic index ≥1.0 | 51 | 62 | 62 |
| Systolic load ≥25% (≥50%) | 67 (49) | 66 (48) | 69 (46) |
| Diastolic load ≥25% (≥50%) | 73 (48) | 72 (62) | 92 (54) |
| BP dipping, % | |||
| Systolic <10% | 50 c | 83 | 54 |
| Diastolic <10% | 49 | 69 | 54 |
Abbreviations: HT, hypertensive; Tx, transplant. Blood pressure (BP) index is the mean BP divided by and BP load is the percentage of measurements over the 95th percentile of the normal population.25 BP dipping is the relative difference between mean daytime and nighttime BP. P values from chi‐square tests between the Tx groups were not statistically significant except for a P=.017 and c P=.001 between the RTx and HTx patients and b P=.036 between the RTx and LTx patients.
The BP indices and loads showed a highly significant correlation (r=0.836–0.919; P<.001 in all groups). Importantly, 23% to 49% of the patients with a BP index <1.0 already had the correspondent BP load above 25%, but only 1% to 4% had the correspondent BP load above 50%. Thus, the majority (91%–97%) of patients with a normal BP index had the concomitant BP load between 25% and 50%.
The lack of systolic or diastolic dipping ≥10% was common in Tx patients (49%–83%) (Table 3). The nighttime BP indices and loads in the RTx patients without 10% dipping were significantly higher and the daytime values were comparable to those with at least a 10% dipping, explaining the difference between the two groups (data not shown).
Relationship Between ABPM Parameters and Clinical Variables in RTx Patients
None of the 10 ABPM parameters (daytime or nighttime, systolic or diastolic, indices, loads, or BP dippings) significantly correlated with the glomerular filtration rate (GFR) at the time of the ABPM among the RTx patients. This was confirmed by logistic regression analyses showing that GFR <60 mL/min/1.73 m2 did not predict any BP index above 1 or load above 50%, nor dipping below 10%. Furthermore, GFR levels were comparable between RTx patients divided into two groups according to BP load above or below 50% (P=.088–.542). In diastolic dipping, however, the proportion of patients with GFR <60 mL/min/1.73 m2 at the time of the ABPM was greater in those with abnormal diastolic dipping in comparison to those with normal diastolic BP dipping (85% vs 68%, P=.045).
Triglycerides correlated with nighttime diastolic BP index (r=0.215; P=.026). Among other metabolic factors (total cholesterol, HDL, LDL, and obesity) and age at the time of the study, no significant correlations or odds ratios with any of the ABPM parameters were found (data not shown).
The systolic office BP index showed significant correlations with all daytime and nighttime indices and loads (r=0.252–0.578; P<.001–.008). Office BP did not, however, correlate with systolic or diastolic dipping (P=.117–.805). The usability of the office BP results when assessing likelihood for abnormality in each daytime and nighttime BP parameter was verified by logistic regression analyses showing that hypertensive office BP predicts hypertensive systolic and diastolic BP variables both during the day and at night (data not shown).
Discussion
In this national cohort study, the BP profiles of pediatric patients with kidney, liver, and heart Tx were evaluated retrospectively. The BP profiles were quite alike in all Tx patient groups. In most Tx patients, the hypertensive BP values occurred at night even in RTx patients taking antihypertensive medication. The threshold of 25% for the BP load seems to overstate the prevalence of hypertension as BP load exceeding 25% simultaneously with normal BP index was common but not vice versa. BP dipping was not related to the levels of daytime BP index or load in RTx patients. Clinical metabolic factors and kidney graft function were weakly associated with the BP parameters. The results of our study support the superiority of ABPM over office BP measurements.
The ABPM profiles did not differ significantly between different Tx groups. The reduced dipping and predominance of nocturnal hypertension in all of the Tx groups were evident, as reported previously in RTx patients.27 The cause of reduced nocturnal dipping has been under debate. ABPM recording may interfere with the sleep of a study patient and this, of course, may increase BP levels during the night. In our unit, patients with essential hypertension undergo similar BP monitoring (data not shown) and show hypertensive levels during the daytime and a more prominent circadian variation in BP profile compared with Tx patients, suggesting that the nocturnal predominance in Tx patients was not a technical bias. There is also some evidence about disturbance in the autonomic BP regulation in the diabetic population leading to nighttime hypertension.28 This may also be the case in the Tx population but remains to be confirmed.
ABPM is a feasible method to confirm office BP results in school‐aged children and adolescents with a suspicion of hypertension. A major problem, however, has been that BP indices and BP loads as well as their combinations have been used diversely.14, 15, 16 Also, the significance of nocturnal dipping has been discussed, although it has been regarded as an important parameter especially in secondary hypertension.29 The AHA recommendations from 2008 helped in the classification but raised some concerns. A fresh update to this classification was recently published taking into account issues such as prehypertension and introduced an unclassified patient group with office BP below the 90th percentile or above the 95th percentile, normal mean BP, but elevated BP loads.19 In our data, the BP index strongly correlated with the corresponding BP load. Concurrently, the data suggested that the limit of 25% in BP load may emphasize hypertension, thus our findings support the results by Koshy and colleagues,30 who reported the use of a BP load of 50% as a cutoff would lead to better agreement with BP index. The use of a higher cutoff is also justified by the fact that BP load does not take into account the magnitude of excess of the actual BP measure. In our data, only solitary patients had BP load below 25% with the corresponding BP index exceeding one.
The majority of our RTx patients were hypertensive, in line with previous results in RTx patients.16, 30, 31, 32, 33 Similar to previous studies, hypertension was often also detected in RTx patients already receiving antihypertensive medication. This reflects the fact that ABPM was commonly used as an adjunctive method with no strict criteria and the BP levels in most cases looked “quite normal,” thus not leading to intensified therapy. In addition, nocturnal hypertension was prevalent among the patients treated with antihypertensive drugs most likely because of insufficient duration of effect of the pill taken in the morning.
So far, data on the use of ABPM published in other Tx patients than RTx are relatively scarce.34, 35, 36, 37 Two studies have reported 30% and 50% prevalence rates of hypertension in LTx and HTx patients, respectively.37, 38 To the best of our knowledge, no study to date has compared the BP profiles of different Tx recipient groups with each other. In our study, the frequency of abnormal ABPM in the LTx and HTx patients was 100% and 76%, respectively, resembling the high prevalence of hypertension observed in RTx patients. Our different Tx groups received similar immunosuppressive medication (CNI, azathioprine or a mycophenolate, and low‐dose MP), which makes the comparison of ABPM results among these cohorts interesting.
In the RTx patients, decreased kidney function (measured GFR <60 mL/min/1.73 m2) could be predicted only by a decreased diastolic BP dipping. No direct correlations were found, however, between the BP parameters and the GFR or several clinical variables in Tx patients. Previously, an association between GFR and hypertension has been seen in Tx patients.39, 40, 41, 42 On the other hand, two Swedish studies have reported that hypertension compared with normotension or hypertension during daytime or nighttime did not affect the velocity of decline in graft function.43, 44 The high incidence of hypertension in all three Tx groups, however, suggests that the immunosuppressive medication (CNIs and MP), as such, is largely responsible for the elevated BP levels.
Study Limitations
The major limitations of this study are its retrospective nature and the low number of LTx patients. In our current follow‐up protocol, annual ABPM is routinely performed only in RTx and HTx patients. In LTx patients, ABPM is performed only if there is suspicion of hypertension. This explains the high prevalence of abnormal ABPM results among LTx patients. In addition, the timing of the ABPM between 5 and 10 years after transplant may introduce a time bias as graft function is subject to decrease post‐Tx. The majority of the measurements (84%) were, however, performed at 5 to 7 years after Tx, which is a relatively short period. The main goal of the present study was to compare the ABPM profiles among the different Tx patient groups and to illuminate the possible differences in the information provided by the numerous ABPM parameters.
Conclusions
Hypertension is common in children and adolescents after Tx and the BP profiles are similar among Tx patient groups. The BP variables provided by the ABPM support each other when assessing hypertension. The threshold of 25% in BP load seems to be the most sensitive variable in raising the prevalence of hypertension, but, in the light of its predictive value, a load of 50% would be a superior cutoff value. ABPM is an essential tool as a part of long‐term follow‐up of patients after solid organ Tx.
Funding and disclosure
This study was supported by grants from the Helsinki University Central Hospital Fund, the Foundation for Pediatric Research, the Paivikki and Sakari Sohlberg Foundation, and the Sigrid Juselius Foundation. The authors report no conflicts of interest to disclose.
J Clin Hypertens (Greenwich). 2015;17:154–161. DOI: 10.1111/jch.12465. © 2015 Wiley Periodicals, Inc.
References
- 1. Magee JC, Krishnan SM, Benfield MR, et al. Pediatric transplantation in the United States, 1997‐2006. Am J Transplant. 2008;2:935–945. [DOI] [PubMed] [Google Scholar]
- 2. Offner G, Latta K, Hoyer PF, et al. Kidney transplanted children come of age. Kidney Int. 1999;55:1509–1517. [DOI] [PubMed] [Google Scholar]
- 3. Boucek MM, Waltz DA, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: ninth official pediatric heart transplantation report–2006. J Heart Lung Transplant. 2006;25:893–903. [DOI] [PubMed] [Google Scholar]
- 4. O'Sullivan JJ, Derrick G, Gray J. Blood pressure after cardiac transplantation in childhood. J Heart Lung Transplant. 2005;24:891–895. [DOI] [PubMed] [Google Scholar]
- 5. Bucuvalas JC, Ryckman FC. Long‐term outcome after liver transplantation in children. Pediatr Transplant. 2002;6:30–36. [DOI] [PubMed] [Google Scholar]
- 6. Avitzur Y, De Luca E, Cantos M, et al. Health status ten years after pediatric liver transplantation–looking beyond the graft. Transplantation. 2004;78:566–573. [DOI] [PubMed] [Google Scholar]
- 7. McLin VA, Anand R, Daniels SR, et al; SPLIT Research Group . Blood pressure elevation in long‐term survivors of pediatric liver transplantation. Am J Transplant. 2012;12:183–190. [DOI] [PubMed] [Google Scholar]
- 8. Groothoff JW, Gruppen MP, Offringa M, et al. Mortality and causes of death of end‐stage renal disease in children: a Dutch cohort study. Kidney Int. 2002;61:621–629. [DOI] [PubMed] [Google Scholar]
- 9. Gordjani N, Offner G, Hoyer PF, Brodehl J. Hypertension after renal transplantation in patients treated with cyclosporin and azathioprine. Arch Dis Child. 1990;65:275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarwal MM, Vidhun JR, Alexander SR, et al. Continued superior outcomes with modification and lengthened follow‐up of a steroid‐avoidance pilot with extended daclizumab induction in pediatric renal transplantation. Transplantation. 2003;76:1331–1339. [DOI] [PubMed] [Google Scholar]
- 11. Lurbe E, Alvarez V, Liao Y, et al. The impact of obesity and body fat distribution on ambulatory blood pressure in children and adolescents. Am J Hypertens. 1998;1:418–424. [DOI] [PubMed] [Google Scholar]
- 12. Calzolari A, Giordano U, Matteucci MC, et al. Hypertension in young patients after renal transplantation: ambulatory blood pressure monitoring versus casual blood pressure. Am J Hypertens. 1998;1:497–501. [DOI] [PubMed] [Google Scholar]
- 13. Ferraris JR, Ghezzi L, Waisman G, Krmar RT. ABPM vs office blood pressure to define blood pressure control in treated hypertensive paediatric renal transplant recipients. Pediatr Transplant. 2007;11:24–30. [DOI] [PubMed] [Google Scholar]
- 14. Lingens N, Dobos E, Witte K, et al. Twenty‐four‐hour ambulatory blood pressure profiles in pediatric patients after renal transplantation. Pediatr Nephrol. 1997;11:23–26. [DOI] [PubMed] [Google Scholar]
- 15. Seeman T, Simkova E, Kreisinger J, et al. Control of hypertension in children after renal transplantation. Pediatr Transplant. 2006;10:316–322. [DOI] [PubMed] [Google Scholar]
- 16. Sorof JM, Poffenbarger T, Portman R. Abnormal 24‐hour blood pressure patterns in children after renal transplantation. Am J Kidney Dis. 2000;35:681–686. [DOI] [PubMed] [Google Scholar]
- 17. Kennedy SE, Mackie FE, Rosenberg AR, et al. Agreement on reporting of ambulatory blood pressure monitoring in children. Pediatr Nephrol. 2005;20:1766–1768. [DOI] [PubMed] [Google Scholar]
- 18. Urbina E, Alpert B, Flynn J, et al. Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association atherosclerosis, hypertension, and obesity in youth committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52:433–451. [DOI] [PubMed] [Google Scholar]
- 19. Flynn JT, Daniels SR, Hayman LL, et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63:1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saari A, Sankilampi U, Hannila ML, et al. New Finnish growth references for children and adolescents aged 0 to 20 years: length/height‐for‐age, weight‐for‐length/height, and body mass index‐for‐age. Ann Med. 2011;43:235–248. [DOI] [PubMed] [Google Scholar]
- 21. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denchev SV, Simova II, Matveev MG. Evaluation of the SCHILLER BR‐102 plus noninvasive ambulatory blood pressure monitor according to the international protocol introduced by the working group on blood pressure monitoring of the European Society of Hypertension. Blood Press Monit. 2007;12:329–333. [DOI] [PubMed] [Google Scholar]
- 23. Modesti PA, Costoli A, Cecioni II, et al. Clinical evaluation of the QuietTrak blood pressure recorder according to the protocol of the British Hypertension Society. Blood Press Monit. 1996;1:63–68. [PubMed] [Google Scholar]
- 24. Jones HE, Sinha MD. The definition of daytime and nighttime influences the interpretation of ABPM in children. Pediatr Nephrol. 2011;26:775–781. [DOI] [PubMed] [Google Scholar]
- 25. Wuhl E, Witte K, Soergel M, et al; German Working Group on Pediatric Hypertension . Distribution of 24‐h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens. 2002;20:1995–2007. [DOI] [PubMed] [Google Scholar]
- 26. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 27. Morgan H, Khan I, Hashmi A, et al. Ambulatory blood pressure monitoring after renal transplantation in children. Pediatr Nephrol. 2001;16:843–847. [DOI] [PubMed] [Google Scholar]
- 28. Kario K, Motai K, Mitsuhashi T, et al. Autonomic nervous system dysfunction in elderly hypertensive patients with abnormal diurnal blood pressure variation: relation to silent cerebrovascular disease. Hypertension. 1997;30:1504–1510. [DOI] [PubMed] [Google Scholar]
- 29. Seeman T, Palyzova D, Dusek J, Janda J. Reduced nocturnal blood pressure dip and sustained nighttime hypertension are specific markers of secondary hypertension. J Pediatr. 2005;147:366–371. [DOI] [PubMed] [Google Scholar]
- 30. Koshy S, Macarthur C, Luthra S, et al. Ambulatory blood pressure monitoring: mean blood pressure and blood pressure load. Pediatr Nephrol. 2005;20:1484–1486. [DOI] [PubMed] [Google Scholar]
- 31. Giordano U, Matteucci MC, Calzolari A, et al. Ambulatory blood pressure monitoring in children with aortic coarctation and kidney transplantation. J Pediatr. 2000;136:520–523. [DOI] [PubMed] [Google Scholar]
- 32. Seeman T. Hypertension after renal transplantation. Pediatr Nephrol. 2009;24:959–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McGlothan KR, Wyatt RJ, Ault BH, et al. Predominance of nocturnal hypertension in pediatric renal allograft recipients. Pediatr Transplant. 2006;10:558–564. [DOI] [PubMed] [Google Scholar]
- 34. Reeves RA, Shapiro AP, Thompson ME, Johnsen AM. Loss of nocturnal decline in blood pressure after cardiac transplantation. Circulation. 1986;73:401–408. [DOI] [PubMed] [Google Scholar]
- 35. Roche SL, Kaufmann J, Dipchand AI, Kantor PF. Hypertension after pediatric heart transplantation is primarily associated with immunosuppressive regimen. J Heart Lung Transplant. 2008;27:501–507. [DOI] [PubMed] [Google Scholar]
- 36. Del Compare ME, D'Agostino D, Ferraris JR, et al. Twenty‐four‐hour ambulatory blood pressure profiles in liver transplant recipients. Pediatr Transplant. 2004;8:496–501. [DOI] [PubMed] [Google Scholar]
- 37. Bayrakci US, Baskin E, Ozcay F, et al. Abnormal circadian blood pressure regulation in liver transplanted children. Pediatr Transplant. 2012;16:160–164. [DOI] [PubMed] [Google Scholar]
- 38. Walker AH, Locke TJ, Braidley PC, Al‐Mohammed A. The importance of 24 hour ambulatory blood pressure monitoring after thoracic organ transplantation. J Heart Lung Transplant. 2005;24:1770–1773. [DOI] [PubMed] [Google Scholar]
- 39. Mange KC, Cizman B, Joffe M, Feldman HI. Arterial hypertension and renal allograft survival. JAMA. 2000;283:633–638. [DOI] [PubMed] [Google Scholar]
- 40. Mitsnefes MM, Khoury PR, McEnery PT. Early posttransplantation hypertension and poor long‐term renal allograft survival in pediatric patients. J Pediatr. 2003;143:98–103. [DOI] [PubMed] [Google Scholar]
- 41. Jacobi J, Rockstroh J, John S, et al. Prospective analysis of the value of 24‐hour ambulatory blood pressure on renal function after kidney transplantation. Transplantation. 2000;70:819–827. [DOI] [PubMed] [Google Scholar]
- 42. Fernandez‐Fresnedo G, Palomar R, Escallada R, et al. Hypertension and long‐term renal allograft survival: effect of early glomerular filtration rate. Nephrol Dial Transplant. 2001;16(Suppl 1):105–109. [DOI] [PubMed] [Google Scholar]
- 43. Krmar RT, Berg UB. Blood pressure control in hypertensive pediatric renal transplants: role of repeated ABPM following transplantation. Am J Hypertens. 2008;21:1093–1099. [DOI] [PubMed] [Google Scholar]
- 44. Cameron C, Vavilis G, Kowalski J, et al. An observational cohort study of the effect of hypertension on the loss of renal function in pediatric kidney recipients. Am J Hypertens. 2014;27:579–585. [DOI] [PubMed] [Google Scholar]


