Abstract
The aim of this retrospective study was to evaluate the main independent prognostic factors of negative maternal and fetal outcomes in a relatively large sample of pregnant outpatients (N=906) who were normotensive or affected by chronic hypertension, gestational hypertension, or preeclampsia. Among the studied parameters, the ones significantly associated with negative maternal outcomes were a diagnosis of preeclampsia (vs other forms of hypertension or normotension) and higher serum uric acid level, while antihypertensive treatment, number of previous deliveries, and blood pressure (BP) control at deliveries seemed to be protective. Regarding negative fetal outcomes, the parameters significantly associated with a negative maternal outcome were a diagnosis of preeclampsia (vs other forms of hypertension or normotension) and mother pre‐pregnancy body mass index, while antihypertensive treatment and BP control at delivery seemed to be protective. Specific patient characteristics should help to predict the risk of negative maternal and fetal outcomes.
Hypertension in pregnancy is defined as a diastolic blood pressure (DBP) ≥90 mm Hg and/or a systolic blood pressure (SBP) ≥140 mm Hg measured at least twice separately.1, 2 Severe hypertension is variably defined as a blood pressure (BP) ≥160 mm Hg to 170/110 mm Hg and nonsevere (or “mild‐moderate”) hypertension as a BP between 140/90 mm Hg and 159/109 mm Hg.1, 2
Hypertensive disorders are the leading cause of complications in pregnancy and, together with hemorrhage, are among the major contributors to maternal death in developed and developing countries.3 They affect between 10% and 15% of pregnant women.4 Preeclampsia/eclampsia is the most studied and most severe form of hypertensive disorder in pregnancy and is associated with the highest rate of maternal and fetal deaths.5
The main recommendation is to prevent BP increase during pregnancy through an improvement in dietary and lifestyle habits (also aimed at body weight control), while pharmacologic treatment is considered to prevent progression towards more severe disease and to improve maternal and fetal outcomes.6 Indeed, while there are consistent data regarding the effect of severe hypertension on maternal and fetal morbidity and mortality as well as the effects of antihypertensive treatment in these hypertensive patients,7, 8, 9 less is known about the risk predictors and the effect of pharmacologic treatment in patients with mild to moderate hypertension and the relative effect of antihypertensive treatment.10
In this context, the main aim of our study was to evaluate the independent prognostic factors of maternal and fetal negative outcomes in a relatively large sample of normotensive pregnant women affected by chronic hypertension, gestational hypertension, or preeclampsia. The secondary aim of our study was to evaluate whether pharmacologic treatment in these patients was associated with a different maternal or fetal outcome.
Materials and Methods
This retrospective study was carried out in a relatively large group of pregnant women who consecutively visited the outpatient clinic of the Hypertensive Disorders Research Unit of the Department of Medical and Surgical Sciences of Bologna University from January 2009 to June 2014. We excluded from the survey patients without available complete clinical and laboratory data, those who were bearing twins, those who did not give live birth or experienced very early delivery, those with diabetes and thyroid diseases (systematically screened in our patients) and other endocrinologic diseases (if already known), those becoming pregnant with the support of assisted reproductive technologies, those taking long‐term drugs for any cause, and those with severe nonpreeclamptic hypertension (Figure 1).
Figure 1.
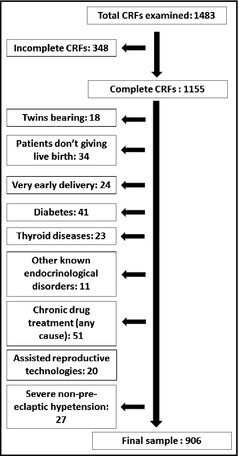
Flow chart showing patient selection. Distribution of number of pregnancies by hypertension group. CRF indicates clinical report form.
The final database included 906 patients for whom a full set of data was available from the outpatient and delivery case history: age; pre‐pregnancy body mass index (BMI); office BP and heart rate; laboratory data; number of previous pregnancies and parity; time of delivery; maternal complications such as eclampsia and intensive therapy care recovery; fetal complications such as being small for gestational age, distress respiratory syndrome, miscarriage, and intensive therapy care recovery; antihypertensive drug prescribed; and use of acetyl salicylic acid. We also recorded BP values at each trimester and at delivery and classified them as controlled or uncontrolled on the basis of current guidelines.6
BP was measured following the standard measurement guidelines of the European Hypertension Society using a sphygmomanometer with an appropriately sized cuff after a 5‐minute rest.6, 11 The values were double‐checked with another measure recorded 20 minutes after the first one according to an internal protocol.12
All patients were then divided into the following groups on the basis of the diagnosis: normotension (NT) (327 patients who had normal BP level at baseline and during entire pregnancy [occasionally visited in the course of routine screenings or after a single episode of BP increase[; gestational hypertension (G‐HT) (145 patients with hypertension without significant proteinuria [300 mg/24 h] or other clinical anomalies); preeclampsia (PE) (213 patients with SBP ≥160 mm Hg or DBP ≥110 mm Hg on two measurements, carried out at least 6 hours apart and with high proteinuria [>300 mg/24 h]; chronic hypertension (C‐HT) (221 patients with a previous state of high BP or a hypertensive disorder that persisted after delivery).5, 13, 14
Antihypertensive treatments were assigned as per clinical judgment.
The study protocol was approved by the local ethical board and was carried out in agreement with the Declaration of Helsinki.
All data were encoded in a specific database and a full descriptive analysis of the sampled parameters was performed. Analysis of variance by Bonferroni test for multiple comparisons or chi‐square test by Fisher exact test, when appropriate, were used for statistical comparison among the four groups and within each group. Logistic regression analysis was carried out in order to identify independent prognostic factors for the above‐described outcomes, including as potential predictors mother's age, BMI at beginning of pregnancy, number of pregnancies, DBP and SBP at first trimester and at delivery, BP control at delivery (defined as BP <140/90 mm Hg), fasting plasma glucose, estimated glomerular filtration rate, serum uric acid, albuminuria at delivery, and antihypertensive drug and anti‐aggregant drug use. All analyses were carried out with SPSS version 21.0 (SPSS Inc, IBM, Armonk, NY). P values <.05 were considered significant for all tests.
Results
Personal, anthropometric, and hemodynamic data at the beginning of the pregnancy are summarized in Table 1. Table 2 The main laboratory data of the evaluated patients at the first visit in our outpatient clinic are shown in Table 2. Active smokers and ex‐smokers were equally distributed among groups (12% and 16%, respectively). Overall, the groups were balanced for a large part of the considered parameters.
Table 1.
Demographic and Anthropometric Data (Sampled at the Beginning of the Pregnancy) and Hemodynamic and Laboratory Data (Sampled at the First Visit in the Outpatient Clinic) of the Studied Pregnant Patients
| PE (n=213) | G‐HT (n=145) | C‐HT (n=221) | NT (n=327) | P Value (ANOVA) | |
|---|---|---|---|---|---|
| Age, y | 32.1±5.3a | 29.7±5.4 | 32.0±6.4a | 31.1±4.3 | .032 |
| Weight, kg | 76.0±14.5a | 78.7±12.8a | 73.8±15.4a | 70.2±11.2 | .042 |
| Height, cm | 160.7±6.4 | 167.7±5.6 | 164.6±6.4 | 161.7±7.5 | .391 |
| Body mass index, kg/m2 | 23.4±2.1a | 22.8±2.9 | 22.5±2.8 | 21.7±2.5 | .049 |
| Parity, No. | 1.4±0.6a , b , c | 2.0±0.9 | 1.9±0.9 | 1.8±0.7 | .027 |
| SBP, mm Hg | 141.0±12.3a , b | 138.8±8.8a | 147.3±16.6a , b | 108.5±13.5 | .029 |
| DBP, mm Hg | 91.1±9.4a , b | 85.2±6.6a | 89.0±11.7a | 61.9±.9.3 | .018 |
| Heart rate, beats per min | 86.9±15.6b | 94.0±15.5 | 82.5±11.7b | 85.4±11.5b | .011 |
| Fasting plasma glucose, mg/dL | 84.4±13.6 | 83.5±11.2 | 82.9±12.8 | 81.9±14.3 | .679 |
| Total cholesterol, mg/dL | 176.8±14.9 | 181.5±13.8 | 173.3±16.4 | 170.9±14.7 | .543 |
| LDL cholesterol, mg/dL | 90.7±8.6 | 97.7±9.5 | 94.6±7.6 | 91.7±8.7 | .181 |
| Triglycerides, mg/dL | 150.7±26.5 | 157.7±25.8 | 154.6±26.5 | 141.7±27.8 | .386 |
| HDL cholesterol, mg/dL | 50.2±5.3 | 51.2±4.8 | 50.6±5.5 | 53.5±4.7 | .655 |
| Serum uric acid, mg/dL | 5.0±1.3 | 5.8±1.9 | 5.3±1.4 | 4.9±1.8 | .197 |
| Creatinine, mg/dL | 0.7±0.2 | 0.8±0.3 | 0.7±0.3 | 0.6±0.4 | .622 |
| Estimated glomerular filtration rate, mL/min | 97.7±4.9 | 97.5±4.7 | 96.7±5.1 | 98.8±6.2 | .418 |
Abbreviations: ANOVA, analysis of variance; C‐HT, chronic hypertension; DBP, diastolic blood pressure; G‐HT, gestational hypertension; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MBP, mean blood pressure; NT, normotensive; PE, preeclampsia; SBP, systolic blood pressure. a P<.05 vs NT. b P<.05 vs G‐HT. c P<.05 vs C‐HT.
Table 2.
Baseline Laboratory Characteristics of the Studied Pregnant Patients
| PE (n=213) | G‐HT (n=145) | C‐HT (n=221) | NT (n=327) | |
|---|---|---|---|---|
| Fasting plasma glucose, mg/dL | 84.4±13.6 | 83.5±11.2 | 82.9±12.8 | 81.9±14.3 |
| Total cholesterol, mg/dL | 176.8±14.9 | 181.5±13.8 | 173.3±16.4 | 170.9±14.7 |
| LDL cholesterol, mg/dL | 90.7±8.6 | 97.7±9.5 | 94.6±7.6 | 91.7±8.7 |
| Triglycerides, mg/dL | 150.7±26.5 | 157.7±25.8 | 154.6±26.5 | 141.7±27.8 |
| HDL cholesterol, mg/dL | 50.2±5.3 | 51.2±4.8 | 50.6±5.5 | 53.5±4.7 |
| Serum uric acid, mg/dL | 5.0±1.3 | 5.8±1.9 | 5.3±1.4 | 4.9±1.8 |
| Creatinine, mg/dL | 0.7±0.2 | 0.8±0.3 | 0.7±0.3 | 0.6±0.4 |
| Estimated glomerular filtration rate, mL/min | 97.7±4.9 | 97.5±4.7 | 96.7±5.1 | 98.8±6.2 |
Abbreviations: C‐HT, chronic hypertension; G‐HT, gestational hypertension; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; NT, normotensive; PE, preeclampsia.
Primiparous, secondiparous, and multiparous women had a similar prevalence of hypertension diagnoses beyond preeclamptic women who were more often primiparous (P<.05).
The distribution of antihypertensive drug intake was not significantly different among the considered hypertensive patient groups (P>.05), with a large prevalence of patients treated with alpha‐methyldopa or nifedipine. Acceptable BP control (<140/90 mm Hg) was achieved in 51.1% of C‐HT patients, in 77.4% of G‐HT patients (P<.001 vs other groups), and in 42% of PE patients, independent from the treatment employed.
Anti‐aggregant prophylaxis was taken by 23.1% of the C‐HT patients, 18.6% of the G‐HT patients, and 17.8% of the PE ones (P>.05).
During pregnancy, BP level tended to increase in all hypertensive groups, but only significantly in the PE group (P<.05). On the contrary, BP tended to decrease in normotensive women (Figure 2).
Figure 2.
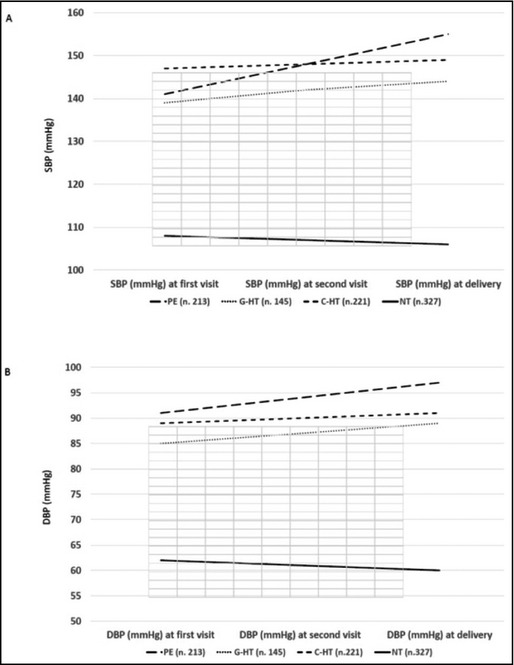
Systolic blood pressure (SBP) (A) and diastolic blood pressure (DBP) (B) trend in the different considered groups of women during pregnancy and at delivery. Distribution of antihypertensive drug prescription among different hypertensive patient groups. PE indicates preeclampsia; G‐HT, gestational hypertension; C‐HT, chronic hypertension; NT, normotensive.
A total of 225 patients experienced hypertension‐related complications (24.8%) and most were preeclamptic (89%).
Among children, 94 of them had hypertension‐related complications (10.4%) and most of them had a preeclamptic mother (66%).
Among women with complications, 29.4% were treated with nifedipine, 23.8% with both alpha‐methyldopa and nifedipine, 22.4% with alpha‐methyldopa, 14.1% with a different therapy, and 10.3% were not pharmacologically treated.
Among children with complications, 28.2% of mothers were treated with nifedipine, 22.0% with both alpha‐methyldopa and nifedipine, 20.1% with alpha‐methyldopa, 14.9% were treated with a different therapy, and 14.8% were not pharmacologically treated.
Among the studied parameters, those significantly associated with negative maternal outcomes were a diagnosis of preeclampsia (vs other forms of hypertension or normotension) and higher serum uric acid level, while antihypertensive treatment, number of previous deliveries, and BP control at deliveries seemed to be protective (Table 3).
Table 3.
Parameters Independently Associated With Negative Maternal and Fetal Outcomes in the Sample of Studied Pregnant Women
| Parameter | Maternal Outcomes | Parameter | Fetal Outcomes | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | ||
| Preeclampsia vs nonpreeclampsia | 1.99 | 1.32–3.08 | <.001 | Preeclampsia vs nonpreeclampsia | 1.87 | 1.18–2.85 | .003 |
| Serum uric acid | 1.21 | 1.02–1.84 | .003 | Mother pre‐pregnancy BMI | 1.13 | 1.01–1.41 | .039 |
| Antihypertensive treatment | 0.74 | 0.51–0.98 | .009 | Antihypertensive treatment | 0.81 | 0.55–0.99 | .045 |
| Number of previous deliveries | 0.63 | 0.39–0.85 | .008 | BP control at delivery | 0.79 | 0.35–0.99 | .049 |
| BP control at delivery | 0.54 | 0.08–0.97 | .011 | ||||
Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval; OR, odds ratio.
Regarding fetal negative outcomes, the parameters significantly associated with negative maternal outcomes were a diagnosis of preeclampsia (vs other forms of hypertension or normotension) and mother pre‐pregnancy BMI, while antihypertensive treatment and BP control at delivery seemed to be protective (Table 3).
Discussion
The aim of antihypertensive therapy in the management of pregnancy‐related high BP is to prevent complications caused by hypertension while prolonging the course of pregnancy and allowing the physiologic maturation of the fetus.15 The most commonly used antihypertensive drugs in pregnancy are nifedipine, methyldopa, labetalol, and hydralazine.15 In our study, methyldopa and nifedipine were the most prescribed, according to internal protocols.
Methyldopa is a centrally acting drug that inhibits sympathetic outflow16 and whose safety profile is well‐known in clinical practice.17, 18 Its use is currently suggested in patients with C‐HT and P‐HT, despite causing drowsiness in a relatively large number of patients.16, 19
Nifedipine is the other most common antihypertensive drug prescribed during pregnancy, and is a calcium channel blocker that reduces high BP more rapidly than methyldopa.20
Of note, there is a lack of randomized placebo‐controlled trials of antihypertensive agents in pregnancy that study the important maternal and fetal/neonatal outcomes. As a result, many data on antihypertensive use in pregnancy are derived from reviews and meta‐analyses of small retrospective studies. The majority of studies compare one antihypertensive with no treatment, thus comparative efficacy and safety data to guide choices between alternative antihypertensive agents are generally lacking. Even among those studies that compare agents head‐to‐head, allocation was generally not blinded, raising concerns for possible biases and confounding of data.21
In our study, both controlled BP and antihypertensive drug use seemed to be important predictors of positive maternal and fetal outcomes.
On the other hand, Ray and colleagues22 reported that the use of antihypertensive agents during pregnancy was strongly associated with negative fetal outcomes such as prematurity, admission to neonatal intensive care unit, small for gestational age, decreased birth weight, and preterm birth. However, the authors did not differentiate between types of medications, stating only that β‐blockers were the most common drugs prescribed in their study. β‐Blockers are not indicated as first‐line therapy by current guidelines for chronic treatment of hypertension in pregnancy5 and, consequently, they were rarely administered to our patients.
Beyond drug treatment, among the other predictors of outcomes, it is important to stress the observation of the main role of baseline serum uric acid, a marker of systemic oxidative stress that is acquiring increased relevance in the assessment of cardiovascular disease risk during the last years, being associated with hypertension incidence and its clinical consequences.23, 24 More specifically, in pregnant women, it has been observed that serum uric acid could be an independent predictor of incident eclampsia25 and its severity,26 but also an early predictor of gestational hypertension.27
Study Limitations and Strengths
Our study has several limitations. First, it is not a randomized clinical trial; therefore, we cannot definitively conclude that one of the assumed antihypertensive drugs is more effective than others in preventing maternal and/or fetal complications. Moreover, the number of events in nonpreeclamptic women was relatively low, as is seen in other studies,22, 28 and negative outcomes were mostly recorded among preeclamptic patients. This, of course, has reduced the representativity of negative outcomes in the other considered patient groups. In addition, uteroplacental parameters were not systematically recorded; therefore, we did not systematically investigate the actions of the antihypertensive therapy on uteroplacental flow.
To the best of our knowledge, these are innovative data on the effect of antihypertensive therapy on maternal and fetal outcomes in patients not affected by severe hypertension. In fact, it is well‐known that severe hypertension in pregnancy is associated with worse mother and child outcome and require adequate treatment, while no conclusive data are available regarding moderate BP increase. In fact, a recent meta‐analysis of randomized clinical trials concluded that the use of antihypertensive treatment in women affected by mild to moderate hypertension during pregnancy could reduce the risk of developing severe hypertension by half, but its effect on maternal and children outcomes is not yet clear.29 Thus, our study could add some useful information to this field, being one of the largest studies carried out on a European patient sample in the setting of clinical practice and reflect a realistic picture of the current management of hypertension in pregnancy.
Conclusions
In a large cohort of pregnant women with nonsevere hypertension, antihypertensive drug therapy and delivery BP control had a very important role in defining maternal and fetal outcomes. Other predictors of outcomes were mother diagnosis of preeclampsia, pre‐pregnancy BMI, and serum uric acid.
Disclosures
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
J Clin Hypertens (Greenwich). 2015:777–782. DOI: 10.1111/jch.12614. © 2015 Wiley Periodicals, Inc.
References
- 1. Magee LA, Abalos E, von Dadelszen P, et al. How to manage Hypertension in pregnancy effectively. Br J Clin Pharmacol. 2011;72:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Collaborating Centre for Women's and Children's Health (UK) . Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. National Institute for Health and Clinical Excellence: Guidance. RCOG Press: London, England; 2010:46. [PubMed] [Google Scholar]
- 3. Regitz‐Zagrosek V, BlomstromLundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:3147–3197. [DOI] [PubMed] [Google Scholar]
- 4. Khan KS, Wojdyla D, Say L, et al. WHO Analysis of cause of maternal death: a systematic review. Lancet. 2006;367:1066–1074. [DOI] [PubMed] [Google Scholar]
- 5. Chandiramani M, Shennan A. Hypertensive disorders of pregnancy: a UK‐based perspective. Curr Opin Obstet Gynecol. 2008;20:96–101. [DOI] [PubMed] [Google Scholar]
- 6. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 7. Buchbinder ASibai BM, Caritis S, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66–71. [DOI] [PubMed] [Google Scholar]
- 8. Vigil‐De Gracia P, Montufar‐Rueda C, Smith A. Pregnancy and severe chronic hypertension: maternal outcome. Hypertens Pregnancy. 2004;23:285–293. [DOI] [PubMed] [Google Scholar]
- 9. Yücesoy G, Ozkan S, Bodur H, et al. A maternal and perinatal outcome in pregnancies complicated with hypertensive disorder of pregnancy: a seven year experience of a tertiary care centre. Arch Gynecol Obstet. 2005;273:43–49. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization . WHO recommendations for prevention and treatment of pre‐eclampsia and eclampsia. 2011. http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/9789241548335/en/. Accessed April 3, 2015. [PubMed]
- 11. O'Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821–848. [DOI] [PubMed] [Google Scholar]
- 12. Mustafa R, Ahmed S, Gupta A, Venuto RC. A comprehensive review of hypertension in pregnancy. J Pregnancy. 2012;2012:105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roccella EJ. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 14. Helewa ME, Burrows RF, Smith J, et al. Report of the Canadian Hypertension Society Consensus Conference: 1. Definitions, evaluation and classification of hypertensive disorders in pregnancy. Can Med Assoc J. 1997;157:715–725. [PMC free article] [PubMed] [Google Scholar]
- 15. Naden RP, Redman CW. Antihypertensive drugs in pregnancy. Clin Perinatol. 1985;12:521–538. [PubMed] [Google Scholar]
- 16. Podimow T, August P. Antihypertensive drugs in pregnancy, 2011. Semin Nephrol. 2011;31:70–85. [DOI] [PubMed] [Google Scholar]
- 17. Redman CW, Beilin LJ, Bonnar JBL. Treatment of hypertension in pregnancy with methyldopa: blood pressure control. Br J Obstet Gynaecol. 1977;84:419–426. [DOI] [PubMed] [Google Scholar]
- 18. Cockburn J, Moar VA, Ounsted M, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet. 1982;1:647–649. [DOI] [PubMed] [Google Scholar]
- 19. Ghanem FA, Movahed A. Use of antihypertensive drugs during pregnancy and lactation. Cardiovasc Ther. 2008;26:38–49. [DOI] [PubMed] [Google Scholar]
- 20. Fenakel K, Fenakel G, Appelman Z, et al. Nifedipine in the treatment of severe preeclampsia. Obstet Gynecol. 1991;77:331–337. [PubMed] [Google Scholar]
- 21. Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129:1254–1261. [DOI] [PubMed] [Google Scholar]
- 22. Ray JG, Burrows RF, Burrows EA, Vermeulen MJ. MOS HIP: McMaster outcome study of hypertension in pregnancy. Early Human Dev. 2001;64:129–143. [DOI] [PubMed] [Google Scholar]
- 23. Cicero AFG, Salvi P, D'Addato S, et al; for the Brisighella Heart Study group . Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella Heart Study. J Hypertens. 2014;32:57–64. [DOI] [PubMed] [Google Scholar]
- 24. Borghi C, Verardi FM, Pareo I, et al. Hyperuricemia and cardiovascular disease risk. Expert Rev Cardiovasc Ther 2014;12:1219–1225. [DOI] [PubMed] [Google Scholar]
- 25. Livingston JR, Payne B, Brown M, et al. Uric acid as a predictor of adverse maternal and perinatal outcomes in women hospitalized with preeclampsia. J Obstet Gynaecol Can. 2014;36:870–877. [DOI] [PubMed] [Google Scholar]
- 26. Pereira KN, Knoppka CK, da Silva JE. Association between uric acid and severity of pre‐eclampsia. Clin Lab. 2014;60:309–314. [DOI] [PubMed] [Google Scholar]
- 27. Martell‐Claros N, Blanco‐Kelly F, Abad‐Cardiel M, et al. Early predictors of gestational hypertension in a low‐risk cohort. Results of a pilot study. J Hypertens. 2013;31:2380–2385. [DOI] [PubMed] [Google Scholar]
- 28. Gaugler‐Senden IPM, Huijssoon AG, Visser W, et al. Maternal and perinatal outcome of preeclampsia with an onset before 24 weeks gestation: audit in a tertiary referral centre. Eur J Obstet Gynecol Reprod Biol. 2006;128:216–221. [DOI] [PubMed] [Google Scholar]
- 29. Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2014;(2):CD002252. [DOI] [PubMed] [Google Scholar]


