Abstract
Cardiovascular magnetic resonance (CMR) imaging in adults is considered the gold standard for assessment of left ventricular mass (LVM) and left ventricular hypertrophy (LVH). The authors aimed to evaluate agreement of LVM measurements and LVH determination between echocardiography (ECHO) and CMR imaging in children with hypertension (HTN) confirmed by 24‐hour ambulatory blood pressure monitoring (ABPM). The children (n=22) underwent contemporaneous ECHO, CMR imaging, and ABPM. Patients had a mean body mass index of 30.9±7.5 (kg/m2), and 81.8% had severe HTN. LVM measured by ECHO was 189.6±62.1 g and by CMR imaging was 164.6±44.7 g (P<.0001). Bland‐Altman analysis revealed significant variability between ECHO and CMR imaging in the measurement of LVM. Interobserver error was higher with ECHO than with CMR imaging. ECHO had high sensitivity and low specificity in LVH determination. In conclusion, ECHO overestimates LVM and is less accurate in measuring LVM as compared with CMR imaging in children with HTN. Further prospective study using CMR imaging to assess LVM in children is warranted.
Increased left ventricular mass (LVM) and left ventricular hypertrophy (LVH) are potent independent predictors of cardiovascular morbidity and mortality in the adult population.1 Adult patients with hypertension and LVH have two to five times the risk and up to nine times the number of cardiac events compared with patients with hypertension alone.2 In hypertensive children and adolescents, the relationship between LVH and increased cardiovascular morbidity has not been established, as longitudinal studies are lacking.3 The presence of LVH is the most commonly used clinical marker of hypertensive end‐organ damage in children.4, 5 Thus, the accurate detection of LVH in hypertensive pediatric patients is clinically important.
Although echocardiography (ECHO) is the most frequently used test to assess LVM, ECHO has technical, observer, and patient‐dependent variables that may adversely affect its accuracy in measuring LVM. LVM by ECHO is computed utilizing myocardial dimensions obtained from specific short‐axis images, resulting in a limited amount of the left ventricle being sampled.
Conversely, cardiovascular magnetic resonance (CMR) imaging is a tomographic technique that allows the computation of total myocardial volume by addition of individual slice volumes, and is thus not limited by the shape of the ventricle.6 Magnetic resonance imaging estimates of LVM have been shown to be closely correlated to actual heart weight determined at autopsy in both animal and human models.7, 8 CMR imaging has been considered the gold standard for assessing ventricular dimensions in hypertensive adult patients.9 Unlike acoustic imaging methods, CMR imaging is not impeded by extensive thoracic fat deposits and chest wall expansion. For these reasons, CMR imaging is the gold standard for assessing ventricular dimensions in hypertensive adult patients9 and was used in a cohort of extremely obese adolescents undergoing weight loss surgery.10 To date, there are no reports directly comparing CMR imaging with ECHO for the quantification of LVM in pediatric hypertensive patients who are at risk for LVH. The aim of this study was to evaluate the agreement of LVM calculation by CMR imaging and ECHO and to assess the accuracy of LVM calculation by ECHO using CMR imaging.
Methods
This retrospective observational study was approved by the internal review board at Stony Brook University. We collected data on children evaluated at the Pediatric Hypertension Center at Stony Brook Children's Hospital from January 2011 through November 2014 who had hypertension confirmed by 24‐hour ambulatory blood pressure monitoring (ABPM). Each patient underwent one ABPM, ECHO, and CMR. At our institution, CMR examinations were performed in the following patients: those with severe ambulatory hypertension who had an LVM index (LVMI) by ECHO >95th percentile (see below), or hypertensive patients in whom ECHO images were poor or if ECHO findings were unable to rule out coarctation. Exclusion criteria comprised generally accepted contraindications to CMR imaging (ie, pacemakers), congenital heart disease, and patients with a glomerular filtration rate ≤60 mL/min/1.73m2.11
ABPM was performed using Spacelabs 90217 ultralight oscillometric devices (Spacelabs Inc, Snoqualmie, WA), using the same protocol for all patients published by Urbina and colleagues.12 Ambulatory and severe ambulatory hypertension was defined by the suggested schema for staging of ambulatory blood pressure (BP) levels in children.13
CMR imaging was performed with a Panorama High Field Open 1.0 Tesla scanner (Philips, Best, The Netherlands). Full details of our CMR protocol have been previously published.14 Briefly, this includes standard retrospectively electrocardiographic‐gated breath‐held cine segmented k‐space balanced steady‐state with free precession sequence in the short‐axis plane of the left ventricle. The images were processed using Philips Medical Systems extended MR workspace (software version 7.1.5.1). Endocardial and epicardial diastolic contours were traced manually with the evaluation software automatically calculating LVM, which include the papillary muscles. LVH by CMR imaging was defined as a z score greater than +2.0 utilizing a recent published review that calculated and tabulated pooled weighted mean values that are specific for age and sex.15 CMR imaging was performed by two trained expert cardiologists. The cardiologists were blinded to the ECHO and ABPM results.
Comprehensive two‐dimensional Doppler and M‐mode ECHO was performed by a technician to assess cardiac structure and function. ECHO calculations were performed by two expert pediatric cardiologists. Left ventricular dimensions were measured from a parasternal short‐axis view. LVM was estimated by the Devereux equation.16 LVH was defined as LVMI, calculated as LVM/height2.7 in meters greater than 95th percentile for sex and age, according to recently published age‐specific reference intervals.17 Pediatric LVH was also subjectively described by each cardiologist, eg, results read: “qualitatively the left ventricle appears mildly concentrically hypertrophied” or “borderline elevated LVM” and were assigned as LVH. Cardiologists were blinded to results of ABPM and CMR imaging.
The following laboratory values were collected within 48 hours of ECHO: serum creatinine to calculate estimated GFR by modified Schwartz formula (eGFRmL/min/1.73m 2 = 0.41 × heightcm)/serum creatininemg/dL),11, 18 hemoglobin (g/dL), and microalbumin (µg/mg). The subsequent anthropometric data were collected at the time of ECHO: body surface area (BSA; m2 ) (Dubois Dubois formula) and body mass index (BMI; kg/m2).
Obesity was defined as a BMI at or above the 95th percentile for children of the same age and sex (www.cdc.gov/obesity/childhood/defining.html).
Statistical Analysis
Except where otherwise noted, demographic data were expressed as mean±standard deviation or as percentages. STATA software (version 13.1, Stata Corporation, College Station, TX) and Microsoft Office Excel 2010 (Microsoft Corporation, Redmond, WA) were used for our analysis. Normality tests were performed on continuous variables using Kolmogorov‐Smirnov and Shapiro‐Wilk methods. The performance of ECHO in LVM estimation was assessed with respect to discrimination (receiver operating characteristic curves [ROCs] and area under the ROC curve), and standard accuracy criteria of binary diagnostic tests (sensitivity, specificity, negative and positive predictive values). Pearson's correlation coefficient and the Bland‐Altman method of agreement were used to compare LVM with CMR‐ and ECHO‐derived data. Limits of agreement, intraobserver and interobserver Pearson's correlation coefficient, and repeatability coefficients were calculated. The differences between the measurements of the two observers were plotted against the means of the measurements obtained by both observers to assess the relationship between the difference of the measured values and the magnitude of the measured values. Bias between the two observers was assessed by calculating the 95% confidence interval (CI) for the mean difference between the two observers; if zero lay inside this interval, no bias was assumed to exist.
Results
Patient characteristics, laboratory, ABPM, CMR imaging, and ECHO‐determined LVM values are summarized in Table 1. Patients had ECHO and CMR imaging performed at an average time interval of 3 months apart (median 1 month). The majority of our patients were adolescents, male, white, and without microalbuminuria, and 81.8% had the diagnosis of severe ambulatory hypertension. Fourteen of 22 (64%) patients were obese, and 5 of 22 (23%) had poor acoustic windows on ECHO evaluation. Average LVM measured by ECHO was 189.6±62.1 g and 164.6±44.7 g by CMR imaging (P<.0001).
Table 1.
Patient Characteristics
| Total (N=22) | Nonobese (n=8) | Obese (n=14) | P Valuea | |
|---|---|---|---|---|
| BMI, kg/m2 | 31.1±7.2 | 23.8±2.6 | 34.9±6.2 | N/A |
| BSA, m2 | 2.0±0.36 | 1.7±0.2 | 2.2±0.3 | |
| Men | 63.6 (14) | 62.5 (5) | 64.3 (9) | .99f |
| African American | 18.2 (4) | 25 (2) | 14.3 (2) | .93f |
| Caucasian | 59.1(13) | 50 (4) | 64.3 (9) | |
| Age, y | 16±3.1 (range 8–20) | 16.1±3.9 (range 8–20) | 16±2.7 (range 9–20) | .93t |
| eGFR, mL/min/1.72m2 | 84.3±19.4 | 75.3±20 | 89.4±17.6 | .10t |
| Hemoglobin, g/dL | 14.0±1.9 | 14.2±2.3 | 13.9±1.7 | .73t |
| Microalbuminuria (>30 µg/mg) | 22.7 (5) | 37.5 (3) | 14.3 (2) | .31f |
| Severe ambulatory HTN | 81.8 (18) | 100 (8) | 71.4 (10) | .25f |
| Ambulatory HTN | 18.2 (4) | 28.6 (4) | ||
| LVMI by ECHO, g/m2.7 | 56.3±14.1 | 53.0±20.0 | 58.1±9.8 | .43t |
| LVM by ECHO, g | 189.6±62.1 | 157.1±54.9 | 208.1±59.9 | .06t |
| LVM by CMR, g | 164.6±44.7 | 130.4±32.8 | 184.1±39.0 | .004t |
Abbreviations: BMI, body mass index; BSA, body surface area; ECHO, echocardiography; eGFR, estimated glomerular filtration rate (mL/min/1.73m2 using modified Schwartz formula11); f, Fisher exact test; HTN, hypertension; LVM, left ventricular mass; LVMI, left ventricular mass index; N/A, not applicable; t, Student t test. Values are expressed as mean±standard deviation or percentage (number). aStatistical differences between nonobese and obese patients.
ECHO Diagnostic Accuracy
Ten of 22 (45.4%) patients (7 of 14 obese patients) had true LVH by CMR imaging (gold standard) and 18 of 22 (81.8%) of all patients and 13 of 14 (92.9%) obese patients were characterized as having LVH by ECHO (Table 2A). Determination of LVH by ECHO had high sensitivity but low specificity. This was especially pronounced in obese patients. It should be worth noting that when evaluating LVH as a dichotomous variable (“either/or”) based on whether the pediatric cardiologist qualitatively assessed the left ventricle as having LVH or not, the sensitivity, specificity, and AUC were poor (Table 2B). This is consistent with recommendations to avoid subjective assessment of LVH.17
Table 2.
Diagnosis of LVH Based on ECHO vs CMR z Score
| ECHO | CMR z Score (Gold Standard) | |||||
|---|---|---|---|---|---|---|
| LVH– | LVH+ | Total | ||||
| All Patients | Obese | All Patients | Obese | All Patients | Obese | |
| (A) Method g/ht2.7 | ||||||
| LVH– | 3 | 0 | 1 | 1 | 4 | 1 |
| LVH+ | 9 | 7 | 9 | 6 | 18 | 13 |
| Total | 12 | 7 | 10 | 7 | 22 | 14 |
| All patients | Obese patients | |||||
| Sensitivity, 90% (95% CI, 77.5%–100%) | Sensitivity, 85.7% (95% CI, 67.4%–100%) | |||||
| Specificity, 25% (95% CI, 6.9%–43.1%) | Specificity, 0% (95% CI, 0%–0%) | |||||
| PPV, 50% (95% CI, 29.1%–70.9%) | PPV, 46.15% (95% CI, 20.4%–72.3%) | |||||
| NPV, 75% (95% CI, 56.9%–93.1%) | NPV, 0% (95% CI, 0%–0%) | |||||
| AUC, 0.62 (95% CI, 0.35–0.90) | AUC, 0.23 (95% CI, 0–1) | |||||
| (B) Subjective method | ||||||
| LVH– | 5 | 7 | 2 | 5 | 12 | 7 |
| LVH+ | 7 | 3 | 5 | 2 | 10 | 7 |
| Total | 12 | 10 | 7 | 7 | 22 | 14 |
| All patients | Obese patients | |||||
| Sensitivity, 30.0% (CI, 10.8%–49.2%) | Sensitivity, 28.6% (95% CI, 4.9%–52.2%) | |||||
| Specificity, 41.67% (CI, 21.1%–62.3%) | Specificity, 28.6% (95% CI, 4.9%–52.2%) | |||||
| PPV, 30% (CI, 10.8%–49.1%) | PPV, 28.6% (95% CI, 4.9%–52.2%) | |||||
| NPV, 41.67% (CI, 21.1%–62.3%) | NPV, 28.6% (95% CI, 4.9%–52.2%) | |||||
| AUC, 0.36 (CI, 0.15–0.57) | AUC, 0.28 (95% CI, 0.03–0.54) | |||||
Abbreviations: AUC, area under the receiver operating characteristic curve; CMR, cardiovascular magnetic resonance imaging; ECHO, echocardiography; LVH–, left ventricular hypertrophy not present; LVH+, left ventricular hypertrophy present; NPV, negative predictive value; PPV, positive predictive value.
Agreement Between ECHO and CMR Imaging
We used Bland‐Altman analysis to evaluate the agreement between two different measurement techniques (Figure). The plot of the difference between LVM (in g) (ECHO‐CMR imaging) against the mean LVM estimate of the two techniques [(ECHO+CMR)/2] for each patient demonstrated that there was poor agreement between LVM by ECHO vs CMR values. The mean difference was 24.7 g (standard deviation±39.5). The 95% confidence limits of agreement between the two methods were 103.7 g and –54.3 g. As seen in the Figure, there is large variability between ECHO and CMR results.
Figure 1.
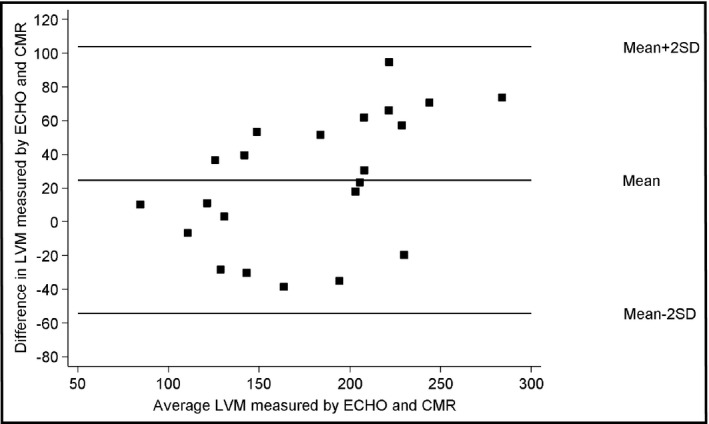
Bland‐Altman plot of left ventricular mass (LVM). ECHO indicates echocardiography; CMR, cardiovascular resonance imaging; SD, standard deviation.
Test–Retest Reliability
Test‐retest reliability was examined in all 22 patients by reanalyzing each ECHO and CMR from the same patient and by the same cardiologist. Table 3A presents intraobserver differences for ECHO and CMR imaging. The repeated calculation of LVM showed good correlation that was consistently high for CMR measurements, but more variable series of results and generally less precise correlation for ECHO. The coefficient of repeatability was lower by CMR than by ECHO.
Table 3.
(A) Intraobserver Difference for ECHO and CMR Imaging and (B) Interobserver Difference for ECHO and CMR Imaging
| LVM by ECHO, g | LVM by CMR Imaging, g | |
|---|---|---|
| (A) | ||
| Mean difference | –12.4 | –0.01 |
| SD | 25.3 | 7.5 |
| Pearson's correlation coefficient (r) | 0.95 | 0.99 |
| Coefficient of repeatability | 49.6 | 14.7 |
| Upper limit of agreement (CI) | 38.3 (18.8–57.7) | 15 (9.2–20.8) |
| Lower limit of agreement (CI) | –63.1 (–82.6 to –43.7) | –15 (–20.8 to –9.3) |
| (B) | ||
| Mean difference | 20.5 | −5.1 |
| SD | 34.7 | 13.2 |
| Pearson's correlation coefficient (r) | 0.9 | 0.97 |
| Coefficient of repeatability | 68 | 25.9 |
| Upper limit of agreement (CI) | 89.9 (63.2–116.5) | 21.4 (11.2–31.6) |
| Lower limit of agreement (CI) | –48.9 (–75.5 to –22.2) | –31.7 (–41.9 to –21.5) |
Abbreviations: CI, confidence interval; CMR, cardiovascular magnetic resonance; ECHO, echocardiography; LVM, left ventricular mass; SD, standard deviation.
Interobserver differences were examined in all 22 patients by remeasuring each ECHO and CMR LVM in the same patient by a different cardiologist (Table 3B). The coefficient of repeatability was lower for CMR imaging than for ECHO. The 95% CI for precision of LVM estimates was determined to be –48.9 g to 89.9 g for ECHO and –31.7 g to 21.4 g for CMR. Therefore, the precision of the CMR estimates was better than those provided by ECHO.
Discussion
Analysis of LVM measurements by ECHO and CMR imaging in our cohort of hypertensive children demonstrated that ECHO overestimates LVM. In addition, large variability between ECHO and CMR measurement results were seen. CMR imaging precision appears to be better than ECHO. These findings are consistent with those found in the adult literature.
In children, CMR imaging is routinely used in the evaluation of cardiac anatomy and function in patients with congenital heart disease, and additional uses for CMR imaging in pediatrics have been recently described. Schaefer and colleagues19 reported that kidney transplant in children is associated with significant improvement in cardiac structure and function by CMR imaging. CMR imaging could also play a potentially useful role in the early detection and monitoring of cardiac dysfunction in children and young adults on maintenance dialysis.20 This report suggests that possible future use of CMR imaging may be used to measure LVM in pediatric hypertensive patients.
This investigation shows that CMR imaging is more reliable in assessing LVM. This is most likely because CMR imaging has improved visualization of the blood‐endocardial border and better‐quality images are maintained across the entire left ventricle. In addition, CMR imaging yields high‐quality pictures that are not impeded by thoracic fat deposits and chest wall expansion, which can cause poor acoustic windows in ECHO. However, only a minority of patients in our cohort had poor acoustic windows. Our finding that LVM is overestimated by ECHO may have important implications in the assessment of LVH, especially in obese hypertensive patients.
The reproducibility of LVM measurements is important for assessing changes over time, both for individuals and research studies. This encompasses interstudy (ie, test–retest reliability) and interobserver and intraobserver variability values. Our analysis conforms to the findings in adults of superior intraobserver and interobserver reproducibility of CMR LVM measurements as compared with ECHO.
It should be noted that CMR imaging has potential disadvantages.19 It is not widely available and is more expensive and the duration of a cardiac assessment can be longer compared with ECHO. Claustrophobia and the need for sedation in small children presents an extra burden with potentially increased risks (none of our patients underwent sedation). Although LVM determined with CMR imaging is more accurate, ECHO has lower cost and is a more accessible technique compared with CMR. These practical issues have supported the use of M‐mode ECHO for the assessment of LVM and diagnosis of LV hypertrophy in the routine clinical setting.
Study Limitations
This study has limitations inherent to its retrospective design and small sample size. In addition, there was population bias since all of our patients were hypertensive, had elevated LVMI on ECHO, and were mostly obese. Another limitation is that each patient had only one ECHO and one CMR at a median of 1 month apart. Ideally, to measure precision and reproducibility, each patient would undergo two CMR and two ECHO imaging studies in random order with a minimal time interval between each study and between each technique. This would avoid physiologic day‐to‐day changes in ventricular filling and size and ensure comparable results between techniques. However, this should not have any bearing on interobserver/intraobserver variability, and it is unlikely that LVH would progress/regress in this relatively short time. LVH and remodeling are frequently seen in hypertensive patients and results from a complex interaction of several hemodynamic and nonhemodynamic variables.21 Although increased BP is considered a major determinant of left ventricular alterations, ethnicity, sex, environmental factors (such as salt intake, obesity, and diabetes mellitus), and neurohormonal and genetic factors might influence LV mass and geometry.21 In addition, data from recent studies suggest that transition from LV concentric hypertrophy to dilation and systolic dysfunction is not a common finding, especially in the absence of coronary artery disease.22, 23, 24
Conclusions
When CMR imaging became available at our institution, clinicians began utilizing it for LVM measurements in patients with abnormal ECHO findings. This report shows that LVM measured by CMR imaging in pediatric hypertensive patients has less error and is therefore more reproducible than ECHO. CMR imaging has promise as an alternative to ECHO, especially in patients with poor acoustic windows or in those who are obese. Our data should be confirmed in larger prospective studies of obese, nonobese, hypertensive, and normotensive patients. We suggest that the diagnosis of LVH determined by ECHO in hypertensive patients be confirmed by CMR imaging.
Conflict of interest
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
Authors' contributions
Muzammil Musani: data collection, data analysis, and critical revision of the article. James C. Nielsen: concept, data analysis, critical revision of the article, and approval of the article. Laurie E. Panesar: data collection, data analysis, and critical revision of the article. Katarina Supe‐Markovina: concept, design, data collection, data analysis, drafting the article, statistics, critical revision of the article, and approval of the article. Robert P. Woroniecki: concept, design, data analysis, statistics, critical revision of the article, and approval of the article.
J Clin Hypertens (Greenwich). 2016;18:976–981. Doi: 10.1111/jch.12808. © 2016 Wiley Periodicals, Inc.
References
- 1. Levy D, Garrison RJ., Savage DD, et al. Left ventricular mass and incidence of coronary heart disease in an elderly cohort: the Framingham Heart Study. Ann Intern Med 1989;110:101–107. [DOI] [PubMed] [Google Scholar]
- 2. Koren MJ, Devereux RB, Casale PN, et al. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991;114:345–352. [DOI] [PubMed] [Google Scholar]
- 3. Kupferman JC, Paterno K, Mahgerefteh J, et al. Improvement of left ventricular mass with antihypertensive therapy in children with hypertension. Pediatr Nephrol. 2010;25:1513–1518. [DOI] [PubMed] [Google Scholar]
- 4. Sorof J, Cardwell G, Franco K, Portman RJ. Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension. 2002;39:903–908. [DOI] [PubMed] [Google Scholar]
- 5. Litwin M, Niemirska A, Sladowska J, et al. Left ventricular hypertrophy and arterial wall thickening in children with essential hypertension. Pediatr Nephrol. 2006;21:811–819. [DOI] [PubMed] [Google Scholar]
- 6. Shapiro E, Rogers W, Beyar R, et al. Determination of left ventricular mass by magnetic resonance imaging in hearts deformed by acute infarction. Circulation. 1989;79:706–711. [DOI] [PubMed] [Google Scholar]
- 7. Katz J, Milliken MC, Stray‐Gundersen J, et al. Estimation of human myocardial mass with MR imaging. Radiology 1988;169:495–498. [DOI] [PubMed] [Google Scholar]
- 8. Sechtem U, Pflugfelder PW, Gould RG, et al. Measurement of right and left ventricular volumes in healthy individuals with cine MR imaging. Radiology 1987;163:697–702. [DOI] [PubMed] [Google Scholar]
- 9. Bottini PB, Carr AA, Prisant ML, et al. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens 1995;8:221–228. [DOI] [PubMed] [Google Scholar]
- 10. Michalsky MP, Raman SV, Teich S, et al. Cardiovascular recovery following bariatric surgery in extremely obese adolescents: preliminary results using cardiac magnetic resonance (CMR) imaging. J Pediatr Surg 2013;48:170–177. [DOI] [PubMed] [Google Scholar]
- 11. Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009;20:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Urbina E, Alpert B, Flynn J, et al; American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee. Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment. Hypertension. 2008;52:433–451. [DOI] [PubMed] [Google Scholar]
- 13. Flynn JT, Urbina EM. Pediatric ambulatory blood pressure monitoring: indications and Interpretations. J Clin Hypertens (Greenwich) 2012;14:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu JC, Nielsen JC, Morowitz L, et al. Use of a 1.0 Tesla open scanner for evaluation of pediatric and congenital heart disease: a retrospective cohort study. J Cardiovasc Magn Reson. 2015;25:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawel‐Boehm N, Maceira A, Valsangiacomo‐Buechel ER, et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necroscopy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 17. Philip RK, Mitsnefes M, Daniels SR, Kimball TR. Age‐specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 2009;22:709–714. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832–1843. [DOI] [PubMed] [Google Scholar]
- 19. Schaefer B, Rusai K, Toth A, et al. Cardiac magnetic resonance imagining in children with chronic kidney disease and renal transplantation. Pediatr Transplant 2012;16:350–356. [DOI] [PubMed] [Google Scholar]
- 20. Malatesta‐Muncher R, Wansapura J, Taylor M, et al. Early cardiac dysfunction in pediatric patients on maintenance dialysis and post kidney transplant. Pediatr Nephrol 2012;27:1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nadruz W. Myocardial remodeling in hypertension. J Hum Hypertens. 2015;29:1–6. [DOI] [PubMed] [Google Scholar]
- 22. Milani RV, Drazner MH, Lavie CJ, et al. Progression from concentric left ventricular hypertrophy and normal ejection fraction to left ventricular dysfunction. Am J Cardiol 2011;108:997–1001. [DOI] [PubMed] [Google Scholar]
- 23. Krishnamoorthy A, Brown T, Ayers CR, et al. Progression from normal to reduced left ventricular ejection fraction inpatient with concentric left ventricular hypertrophy after long‐term follow‐up. Am J Cardiol 2011;108:997–1001. [DOI] [PubMed] [Google Scholar]
- 24. Velagaleti RS GP, Pencina MJ, Argam J, et al. Left ventricular hypertrophy patterns and incidence of heart failure with preserved versus reduced ejection fraction. Am J Cardiol 2014;113:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]


