To the Editor:
Hypertension is a chronic disease that affects about 25% of the population around the world.1 Treatment by pharmacologic intervention is effective but has side effects and significant costs. Techniques that reduce respiratory rate have been shown to be an effective nonpharmacologic treatment in controlling blood pressure (BP). Evidence has shown that a slow and deep breathing rate, around 10 breaths or less per minute, significantly reduces BP.2
The physiological mechanisms involved in reduction of BP caused by decreased respiratory rate, however, are not yet known. In this report, we show an expressive reduction of sympathetic nervous activity with regular use of device‐guided breathing.
A white 52‐year‐old male nurse presented with elevated BP in casual measurement (systolic BP between 140 mm Hg and 150 mm Hg and diastolic BP between 90 mm Hg and 100 mm Hg) for about 1 year. The patient denied other chronic diseases or use of continuous prescription medications, including antihypertensive agents. He reported having a father and a sister with hypertension. He denied smoking or abusive consumption of alcohol and practices resistance exercise 3 times a week. On physical examination, he presented with body mass index of 28.1 kg/m2 and BP 134/91 mm Hg (mean of 2 measurements). There were no other alterations in the physical examination.
Ambulatory blood pressure monitoring (ABPM) performed with the Mobil‐O‐Graph (I.E.M., Solberg, Germany) device, validated according to the British Hypertension Society3 protocol, showed elevation in the mean values of diastolic BP over 24 hours (131/92 mm Hg), awake (139/100 mm Hg), and at sleep (113/75 mm Hg). Ancillary tests showed normal renal function (creatinine: 0.76 mg/dL), normokalemia (K: 4.4 mEq/L), sinus rhythm on electrocardiography, and no evidence of left ventricular hypertrophy.
With a diagnosis of primary arterial hypertension, the patient was introduced to nonpharmacologic treatment by means of device‐guided breathing, an alternative he readily accepted. Briefly, the equipment initially monitors the individual's breathing and then personalizes a melody composed of two different tones, one for inspiration and the other expiration. The patient synchronizes respiration with the melody proposed by the equipment, which gradually prolongs the tone of expiration, inducing the individual to breathe more slowly. The patient received training on how to use the equipment (Resperate, Intercure, Israel) and was instructed to perform daily 15‐minute sessions with the objective of decreasing breathing rate to <10 respiratory incursions per minute during 8 weeks. At the beginning and after the period of 8 weeks of treatment, blood samples were collected to measure plasma catecholamines by using the high‐performance liquid chromatography technique, and peripheral sympathetic nervous activity was measured by the microneurography technique that enables registration of action potentials of sympathetic fibers A and C in peripheral nerves. The technique consists, initially, of an electrical percutaneous stimulation (40 V–120 V) to map the trajectory of the fibular nerve. Posteriorly, a tungsten microelectrode (30–40 mm in length) is inserted into the nerve, at the site of best response to the percutaneous stimulation, with internal stimulation of 4 V to 5 V until a location is obtained where this stimulus triggers involuntary contraction of the leg muscle, without paresthesia. The nervous signal was recorded during 20 minutes on a microcomputer by the LabChart 7 Pro program (ADInstruments, Dunedin, New Zealand) at a sample frequency of 2 K/s. Sympathetic nervous activity was measured by counting the number of nervous impulses per minute.
The patient showed good compliance with the guided breathing exercise: memory of the equipment showed use of the device on 100% of the days during the time recommended, with a final mean of respiratory incursions of 6.9 breathing movements per minute. There was a discreet reduction in office BP (122/88 mm Hg) and in the mean values of 24‐hour (127/89 mm Hg) and awake (132/97 mm Hg) ABPM, but not during sleep (117/76 mm Hg).
For the measurement of sympathetic nervous activity, there was a significant decrease in the values of noradrenalin before and after the treatment period (903 pg/mL vs 220 pg/mL), as well as of peripheral sympathetic nervous activity measured by microneurography (51 bursts per minute vs 22 bursts per minute) (Figure1).
Figure 1.
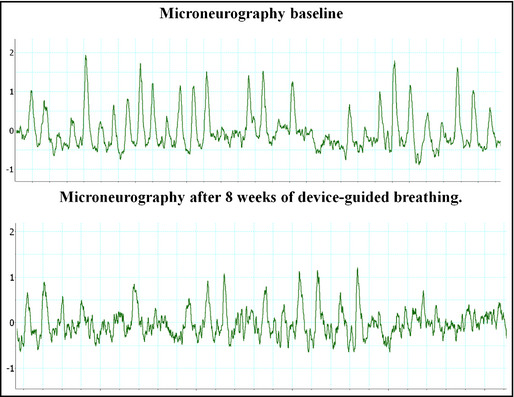
Peripheral sympathetic activity before and after the use of device‐guided breathing.
Reduction of BP with the regular use of device‐guided breathing is well documented in a series of clinical studies,4, 5, 6, 7 and it is also recommended as an alternative to drug treatment in a recent guideline of the American Heart Association.8
Nevertheless, the mechanisms through which the reduction of respiratory frequency brings down BP need to be better understood. One of the hypotheses raised would be the reduction of nervous sympathetic activity: reductions in respiratory frequency of about 6 to 10 breaths per minute, as they increase tidal volume, would stimulate cardiopulmonary stretch receptors, which conversely would reduce the discharge of efferent sympathetic fibers, resulting in decreased systemic vascular resistance and consequent reduction of arterial pressure.9 However, such a hypothesis has not yet been fully confirmed in hypertensive patients who regularly perform this type of intervention.
A study published by our group demonstrated that a single session with device‐guided breathing reduced sympathetic nervous discharges when compared with listening to music in a group of hypertensive patients.10 Recently, it was shown that the acute use of Resperate also reduced peripheral sympathetic nervous activity, despite the fact of not having demonstrated an identical reduction when device‐guided breathing was used regularly for 8 weeks.11
In this sense, one might conclude that the data presented here corroborate the hypothesis that the reduction of arterial pressure observed with the use of device‐guided breathing is explained, at least in part, by the reduction in sympathetic nervous activity.
Support: FAPESP 2010/06921‐2
References
- 1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 2. Gavish B. Device‐guided breathing in the home setting: technology, performance and clinical outcomes. Biol Psychol. 2010;84:150–156. [DOI] [PubMed] [Google Scholar]
- 3. Jones CR, Taylor K, Chowienczyk P, et al. A validation of the Mobil O Graph (version 12) ambulatory blood pressure monitor. Blood Press Monit. 2000;5:233–238. [DOI] [PubMed] [Google Scholar]
- 4. Grossman E, Grossman A, Schein MH, et al. Breathing‐control lowers blood pressure. J Hum Hypertens. 2001;15:263–269. [DOI] [PubMed] [Google Scholar]
- 5. Rosenthal T, Alter A, Peleg E, Gavish B. Device‐guided breathing exercises reduce blood pressure: ambulatory and home measurements. Am J Hypertens. 2001;14:74–76. [DOI] [PubMed] [Google Scholar]
- 6. Viskoper R, Shapira I, Priluck R, et al. Nonpharmacologic treatment of resistant hypertensives by device‐guided slow breathing exercises. Am J Hypertens. 2003;16:484–487. [DOI] [PubMed] [Google Scholar]
- 7. Schein MH, Gavish B, Baevsky T, et al. Treating hypertension in type II diabetic patients with device‐guided breathing: a randomized controlled trial. J Hum Hypertens. 2009;23:325–331. [DOI] [PubMed] [Google Scholar]
- 8. Brook RD, Appel LJ, Rubenfire M, et al. American Heart Association Professional Education Committee of the Council for High Blood Pressure Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, and Council on Nutrition, Physical Activity. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the American Heart Association. Hypertension. 2013;61:1360–1383. [DOI] [PubMed] [Google Scholar]
- 9. Narkiewicz K, Van de Borne P, Montano N, et al. Sympathetic neural outflow and chemoreflex sensitivity are related to spontaneous breathing rate in normal men. Hypertension. 2006;47:51–55. [DOI] [PubMed] [Google Scholar]
- 10. Oneda B, Gusmão JL, Ortega KC, et al. Sympathetic nerve activity is decreased during device‐guided slow breathing. Hypertens Res. 2010;33:708–712. [DOI] [PubMed] [Google Scholar]
- 11. Hering D, Kucharska W, Kara T, et al. Effects of acute and long‐term slow breathing exercise on muscle sympathetic nerve activity in untreated male patients with hypertension. J Hypertens. 2013;31:739–746. [DOI] [PubMed] [Google Scholar]


