Abstract
Pulse wave velocity (PWV) is used for evaluating atherosclerosis; however, it is far from routine use. The authors validate a new device measuring PWV independently in each limb and explore its usefulness. Validity was studied in 40 patients. PWV was compared with endovascular measurements and comparisons were made between PWV in the extremities in 220 patients. The correlation between brachial PWV and endovascular catheter was (r=0.83, P<.001). The correlation coefficients for interobserver and intraobserver were r=0.87 and r=0.91, respectively. The sum of PWV in limbs allowed better stratification of patients according to cardiovascular risk. The validity and reproducibility of PWV measured with this device was good. The sum of PWV in extremities was a good index for stratifying patients according to vascular risk. These results suggest that the device is useful in the evaluation of arteriosclerosis and could possibly replace measurement devices available today.
During the past few decades there has been a substantial decrease in cardiovascular (CV) morbidity and mortality.1 Despite these achievements, however, CV disease remains the leading cause of death worldwide. Management of traditional CV risk factors and novel therapeutic agents are insufficient to control CV diseases; therefore, other actions are necessary.2, 3 The measurement of subclinical atherosclerosis using noninvasive procedures has promise for the identification of patients who are at intermediate or high risk for developing coronary artery disease (CAD),4 although it can also provide reliable intermediate endpoints of use in evaluating therapeutic strategies.5 Thus, procedures of this kind offer great potential as part of our routine diagnostic repertoire for the assessment of individual CV risk and for designing customized therapies.6
Arterial stiffness is a major contributor to CV disease, and is becoming a focal point in the early detection and prevention of CV disease. Pulse wave velocity (PWV) is known to be an indicator of arterial stiffness, and high PWV has been correlated with increased risk of damage to the target end organs and has been regarded as a marker reflecting vascular damage, as well as a prognostic predictor.7, 8 The predictive value of aortic stiffness, measured as carotid‐femoral PWV (cf‐PWV), is now considered the gold standard for arterial stiffness assessment.7, 9 There are different methods for assessing aortic PWV, including Doppler ultrasound, mechanoelectric pulse transduction, tonometry, impedance, and oscillometry. The Complior (Alam Medical, Paris, France) and SphygmoCor (AtCor Medical, West Ryde, Australia) systems have been the most commonly used devices.7 Complior Analyse uses noninvasive pressure sensors to measure PWV and transit time (TT). Sphygmocor Technology focuses on an algorithm that derives the pressure wave in the ascending aorta of an external measurement taken in the radial artery and, although based in the central blood pressures (BPs), can also measure arterial stiffness. However, difficulties such as equipment costs, personnel training, skill development in the location of the arterial pulse, and the availability of sufficient time for exploration, which are usually absent in clinical practice, limit the use of these techniques. Additionally, some patients may feel uncomfortable exposing the inguinal area during the acquisition of femoral pressure waveform.10, 11, 12 Until now, some automated devices for measuring PWV have been marketed, but the complexity of their use and the cost and time required for execution have been of limited success.13, 14
We have developed a simple device using an oscillometric method (referred to as VOPITB: Velocidad Onda de Pulso Indice Tobillo Brazo, or pulse wave velocity ankle‐brachial index) that independently measures PWV in the arm and leg relative to the electrocardiogram (ECG). We hypothesize that the sum of PWV measures in the arms and legs reflects the stiffness of the majority of great arteries and could constitute a marker of vascular risk. The present study was conducted to evaluate the validity and reproducibility of PWV measurement using VOPITB. Moreover, we examined PWV in the summation of 4 extremities, assessing their clinical usefulness in CV risk stratification.
Methods
Instruments
The new device, VOPITB, was developed by the University of Extremadura and Fundesalud, Extremadura (Spain). VOPITB calculates PWV from the heart to the arm and leg. The system is designed to measure TT from outlet of the pulse wave from the heart to the measurement points of the extremities. PWV is calculated as the distance or path length from the heart to the measurement point divided by TT. The path length is obtained by the operator from superficial measurement with a measuring tape (precision 1 mm). The distance in meters from the suprasternal notch to the midpoint of the brachial cuff is considered. In simplified terms, VOPITB is a 5‐channel real‐time PC‐based simultaneous acquisition and analytical system. The acquisition rate is 200 samples per second, which is sufficient, since the significant frequency range of the pressure as well as of the ECG waveform is not more than 40 Hz. According to Nyquist's criteria,15 the minimum sampling rate should be 80 samples per second. Therefore, a sampling rate of 200 samples per second can be regarded as adequate. The system makes use of a digital signal‐processing algorithm to calculate all the results. It is equipped with a dedicated hardware module connected to 3 ECG electrodes and two BP–measuring cuffs. The VOPITB is user‐friendly and fully automatic. The device allows testing without requiring additional interventions, although the operator has the option of performing each function independently using a simple dialog that is displayed on the monitor screen. Once started, the test recording is completed with direct display of the results. The report comprises 6‐second traces of ECG lead I, all pulse pressure waveforms, and all the calculated results. The device has a built‐in database that can be used to store patient files for further referrals at any point in time. This system measures BP by detecting artery pulsation as the pressure oscillation in the cuff. When the cuff around the upper arm is fully inflated, blood flow and arterial pulsation stops. As the pressure in the cuff is slowly decreased, arterial pulsation appears again, causing oscillation of pressure in the cuff. The amplitude of this oscillation gradually increases and eventually reaches a peak. Further reductions in cuff pressure cause the oscillation amplitude to decrease. Cuff pressure when the oscillation reaches a peak is taken as the mean arterial pressure (MAP). As this maximum amplitude oscillation in each limb is detected, the pulse waveforms, along with ECG lead I, are stored simultaneously in the PC memory. These waveforms are used to detect all PWVs as described below. PWV is the speed at which the BP pulse travels from the heart to the peripheral artery after blood is ejected during systole. With VOPITB, the pulse TT values of both the arm and leg are calculated from the waveforms taken from each sensor. Oscillometric graphical representations are derived from the oscillations in the artery when the BP cuffs are deflating while taking the BP readings. The pulse wave and ECG are recorded simultaneously—the mean time between each maximum R (R‐top) wave of ECG lead I and the “foot” of the pulse waveform being considered for the calculation of pulse TT. PWV on each member was determined with the following formula: PWV=distance (m)/TT (s). Where distance is measured from the supraclavicular notch to the midpoint of each cuff and manually entered into the system. For this, the arm is placed at an angle of 90 degrees to the trunk and the sleeve on the arm near the elbow (as BP usually is taken). In the same way, the standing leg is near the ankle, about 2 cm above, while the legs are lightly touching. The TT was calculated by the system as previously described.
Assessment of the Validity of Brachial PWV
To validate the measurement of PWV with the VOPITB system, we established comparisons vs PWV calculated by endovascular catheterization of the arm. Forty patients (mean age 61±7 years [range, 42–63 years]; 23 men and 17 women] with cardiac catheterization including coronary angiography for the diagnosis of coronary artery disease were recruited (20 patients were diagnosed with organic coronary artery stenosis, while 20 presented no significant coronary stenosis). Coronary angiography was performed using a trans‐radial approach with a 6F catheter. For brachial PWV recording, a catheter tip was positioned under radioscopic guidance at the elbow joint interlinear level, and pressure waveforms were recorded using a catheter tip manometer (Sentron; AC Roden, Amsterdam, The Netherlands). Brachial PWV was measured by an experienced observer and was obtained by calculating the index length/time. The time of the brachial PWV was calculated as the time delay between the R tops of ECG lead I and the respective rise (“foot”) in the brachial pressure wave. The distance between the two pressure sites was calculated as follows: with the catheter tip positioned at the top of the aortic root, a mark was made at the point where the catheter exited the wrist. The catheter tip was then withdrawn until radioscopy showed it to be located at the joint interline of the elbow, and a second mark was then made. The distance or path length between the two marks was considered. About 15 minutes after the procedure, the brachial PWV was recorded with the VOPITB device as previously described.
Assessment of Interobserver and Intraobserver Reproducibility
The 40 patients who underwent catheterization were evaluated for the study of the reproducibility of the device. For each patient, PWV was measured in each limb by two blinded observers. Each observer was unaware of the values obtained by the other, registered the day before, and the patient's clinical information. Measurements were performed by an experienced observer (observer 1) and by an inexperienced observer (observer 2), in random order. A minimum of 5 minutes was allowed between measurements, and the cuffs were rewrapped at the second measurement. Both observers measured PWV twice for each participant, with an interval of 1 day between the two measurements. In patients with coronary artery disease, medications were not changed between the two measurements, and were withheld on the morning of the measurement day. A systolic BP (SBP) recording was required for each individual patient in all the measurements (SBP difference <15 mm Hg).
Ultrasound Evaluation and cf‐PWV
Ultrasound evaluation of the common carotid artery (CCA) was performed with a Philips Sonos 5500 Doppler ultrasound system (Philips Healthcare, Best, The Netherlands) using a 7.5‐MHz probe equipped with a Doppler system, as previously described.15 After the patients had rested in the supine position for at least 10 minutes, their neck was placed in a slightly hyperextended position and then optimal bilateral visualization of the carotid artery was performed. Based on multiple visualizations, plaque formation was identified as the presence of wall thickening at least 50% greater than the thickness of the surrounding wall. The intima‐media thickness (IMT) of the far wall was measured in the CCA at sites 1 cm and 2 cm proximal to the bulb from the anterior, lateral, and posterior approaches, and the results were averaged in order to obtain the mean IMT values. No measurements were carried out at the level of discrete plaques. The IMT of the far wall of the CCA was measured.18
The cf‐PWV was measured with an automated system (Complior SP), using the foot‐to‐foot method. Waveforms were acquired simultaneously with two pressure‐sensitive transducers, and the TTs between the carotid and femoral pulses were measured with the electronic calipers of the machine. The distance between the two arterial sites was measured on the body using a measuring tape, and PWV was calculated as the distance divided by time (m/s).
Assessment of Alterations in PWV in Patients With Vascular Disease
All patients studied were older than 40 years, healthy, and with low CV risk selected from among employees undergoing their annual health examination of the University of Extremadura (Spain), together with individuals selected from among patients subjected to follow‐up on an outpatient basis by the Cardiovascular Risk Unit of San Pedro de Alcántara Hospital (Cáceres, Spain). Patients with cardiac arrhythmias such as atrial fibrillation and aortic stenosis were excluded.
Three groups of patients were established according to their CV risk based on the European Risk Score: low in 58 patients (26%), moderate in 108 (49%), and high in 54 (24%). The patients with low or moderate risk were reclassified according to the results of subclinical atherosclerosis studies involving carotid ultrasound and cf‐PWV. Those with common carotid IMT above 75% for age and sex16 or with ≥1 plaques were reclassified as being at high risk. Likewise, patients with cf‐PWV above 75%17 were reclassified as being at high CV risk. Thus, the patients were grouped as follows: low CV risk group (72 patients [33%]), moderate CV risk group (58 patients [26%]), and high CV risk group (90 patients [41%]). All the patients gave informed consent for participation in the study, which was approved by the ethics committee of San Pedro de Alcántara Hospital (Cáceres, Spain). Before inclusion in the study, all patients underwent thorough clinical evaluation to confirm the inclusion criteria.
In all patients, body mass index (BMI), SBP, DBP, serum levels of total cholesterol, triglycerides (TGs), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), creatinine (Cr), uric acid (UA), fasting plasma glucose, and glycosylated hemoglobin (HbA1c) as metabolic risk factors, family history (myocardial infarction, chest pain or sudden death), and history of smoking as CV risk factors were measured. All blood samples were drawn in the morning after the patients had fasted overnight. Laboratory data were determined using enzymatic methods. BP was determined as the mean of two measurements obtained in an office setting by the conventional cuff method using an oscillometric sphygmomanometer. Patient characteristics regarding a history of hypertension, dyslipidemia, diabetes mellitus, smoking, and medication use were recorded. Patients with current SBP/DBP ≥140/90 mm Hg or who were receiving antihypertensive treatment were considered to have arterial hypertension. Patients with LDL‐C ≥140 mg/dL, TGs ≥150 mg/dL, and/or HDL‐C <40 mg/dL, or who were receiving lipid‐lowering treatment, were considered to have dyslipidemia. Diabetes mellitus was defined according to American Diabetes Association criteria.16 BMI was calculated as weight (kg)/height (m)2. The sum of PWV in the 4 extremities was considered the study variable. For this purpose, the PWV was measured independently in each limb, as previously described. The readings were taken by observer 1.
Statistical Analysis
Data were expressed as mean±standard deviation or as relative frequency (percentage). Pearson correlation coefficients were calculated for assessing validity and reproducibility, while intraobserver reproducibility was assessed using Bland‐Altman plots. Intraclass correlation coefficients and coefficients of variation were calculated, as previously described by others. Differences across groups were assessed by the unpaired Student t test and the Pearson chi‐square test referred to means and proportions. PWV index differences according to CV risk groups were assessed with the one‐way analysis of variance test, and comparison between groups was assessed with unpaired Student t test. All analyses were performed using the SPSS version 16.0 statistical package (SPSS Inc, Chicago, IL). Statistical significance was considered for P<.05.
Results
Accuracy and Reproducibility
Figure 1 shows the relationship between brachial PWV obtained with the endovascular catheter and brachial PWV determined by VOPITB. A good correlation was observed between the two measurements (r=0.83, P<.0001). In the case of the measurement summation of PWV in the 4 extremities, coefficients of variation for interobserver and intraobserver reproducibility were 6.2% and 7.5%, respectively. Figure 2 shows the corresponding Bland‐Altman plots.
Figure 1.
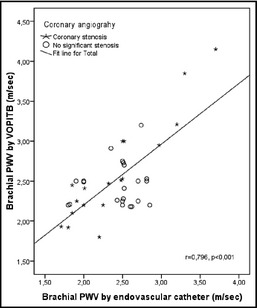
Relationship between brachial pulse wave velocity (PWV) obtained using a catheter‐tip manometer and brachial PWV obtained by the Velocidad Onda de Pulso Indice Tobillo Brazo, or pulse wave velocity ankle‐brachial index (VOPITB) device.
Figure 2.
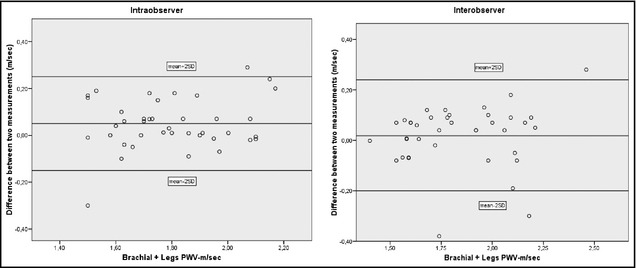
The left panel shows a Bland‐Altman plot depicting the difference between two occasionally different measurements of pulse wave velocity (PWV) brachial and leg sum by one observer, and the right panel shows the difference between measurements by two observers.
Characteristics of the Study Participants
The main characteristics of the patients after reclassification according to subclinical atherosclerosis are shown in the Table1. IMT and cf‐PWV increased in correspondence to CV risk in each group. The variables influencing CV risk were more frequent in the high CV risk groups.
Table 1.
Characteristics of the Study Participants According to Cardiovascular Risk Recoded by Subclinical Atherosclerosis
| Characteristics | Low (n=72) | Moderate (n=58) | High (n=90) | Total (N=220) |
|---|---|---|---|---|
| Male sex, No. (%) | 41 (57) | 34 (60) | 73 (81) a | 148 (68) |
| Age, y | 47±15 | 57±9.8 | 63±9.2 a | 61±11 |
| Current smoker, No. (%) | 0 | 15 (26) | 22 (24) a | 37 (17) |
| Diabetes, No. (%) | 0 | 0 | 56 (62) a | 56 (25) |
| Hypertension, No. (%) | 12 (17) | 42 (74) | 66 (73) a | 120 (55) |
| Hypercholesterolemia, No. (%) | 23 (32) | 42 (74) | 72 (76) a | 137 (63) |
| CVD in relatives, No. (%) | 31 (43) | 26 (46) | 37 (41) | 94 (43) |
| BMI, kg/m2 | 26±4.2 | 31±4.9 | 30±5.1 | 29±4.7 |
| CVD, No. (%) | 0 | 0 | 50 (56) a | 50 (23) |
| CHD | 0 | 0 | 25 (28) a | 25 (11) |
| Stroke | 0 | 0 | 13 (15) a | 13 (5.9) |
| PAD | 0 | 0 | 29 (32) a | 29 (13) |
| Systolic BP, mm Hg | 128±15 | 136±16 | 138±16 a | 135±16 |
| Diastolic BP, mm Hg | 74±6.0 | 77±14 | 79±14 b | 78±12 |
| Total cholesterol, mg/dL | 188±26 | 197±55 | 184±45 | 187±42 |
| LDL cholesterol, mg/dL | 108±20 | 112±53 | 103±34 | 105±34 |
| HDL cholesterol, mg/dL | 59±18 | 51±14 | 49±12 a | 51±14 |
| Triglyceride, mg/dL | 88±34 | 139±108 | 151±96 a | 135±84 |
| Plasma glucose, mg/dL | 88±9.2 | 118±52 | 121±44 a | 113±38 |
| HbA1c, % | 5.7±0.6 | 5.8±1.2 | 6.7±1.5 a | 6.3±1.3 |
| Creatinine, mg/dL | 0.9±0.2 | 0.9±0.2 | 1.0±0.2 a | 0.9±0.2 |
| Atherosclerosis burden | ||||
| IMT, mm | 0.704±0.139 | 0.773±0.121 | 0.879±0.156 a | 0.824±0.147 |
| cf‐PWV, m/s | 7.96±1.91 | 9.13±2.37 | 10.8±2.91 a | 9.92±2.61 |
| Medication, No. (%) | ||||
| ARBs | 0 | 0 | 73 (81) a | 73 (33) |
| Calcium channel blockers | 0 | 6 (10) | 31 (34) c | 37 (17) |
| Diuretics | 0 | 6 (10) | 42 (47) a | 48 (22) |
| Antithrombotics | 0 | 0 | 59 (66) a | 59 (27) |
| Hypoglycemics | 0 | 6 (10) | 42 (47) a | 48 (22) |
| Insulin | 0 | 0 | 11 (12) a | 11 (.5) |
| Statins | 0 | 29 (50) | 83 (92) a | 112 (51) |
Abbreviations: ARBs, angiotensin receptor blockers; BMI, body mass index; BP, blood pressure; cf‐PWV, carotid‐femoral pulse wave velocity; CHD, coronary heart disease; CVD, cardiovascular disease; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; IMT, intima‐media thickness; LDL, low‐density lipoprotein; PAD, peripheral artery disease. Values are expressed as mean±standard deviation unless otherwise indicated. a P<.001. b P<.05. c P<.01.
Comparison of Brachial and Leg PWV Summation in Relation to CV Risk Classification
The sum of both brachial PWV and leg PWV (m/s) showed significant differences between all groups according to recoded CV risk (Figure 3). The comparison between each group was: mean (95% confidence interval), low 18.1 (17.7–18.5), moderate 19.3 (18.8–19.7), and high 21.8 (21.1–22.6); P<.001 for comparisons between each group.
Figure 3.
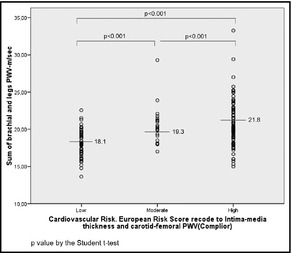
Sum of brachial and leg pulse wave velocity (PWV) obtained by the Velocidad Onda de Pulso Indice Tobillo Brazo, or pulse wave velocity ankle‐brachial index (VOPITB) of people grouped according to their cardiovascular risk. P value by unpaired Student t test.
Discussion
The present study shows that PWV measurement in the limbs with the VOPITB method is simple, reliable, and reproducible. The sum of PWV measurement in the 4 limbs, seems be suitable for stratifying patients according to CV risk.
Increased arterial stiffness is a well‐recognized phenomenon associated with CV disease and an increased risk of CV events.19 In elastic arteries, the pulse wave travels slowly, while in stiff arteries it travels faster. The higher the PWV, the stiffer the artery.20 These changes in pressure components increase left ventricular afterload and myocardial oxygen demand.21 cf‐PWV is used as a reference measure of aortic stiffness.9 Many methods have been designed to assess arterial stiffness but pose the inconvenience of requiring specific devices. It is sometimes impossible to accurately perform the measurements because of difficulties in recording pulse waves. Although these methods are useful in research and clinical laboratories, there is increasing interest in simplifying the procedure for routine clinical measurements, obviating the necessary technical skill required for recording the femoral pulse, particularly in obese patients or individuals with excessive abdominal fat that conditions operator‐dependent measurements. Furthermore, the time required for the exploration is not negligible. As a result of these issues, new automated instruments have been in development for arterial stiffness measurement. Recently, brachial‐ankle pulse wave velocity (ba‐PWV), using a volume‐rendering method, has been shown to be a simple and noninvasive technique that correlates well with arterial stiffness. ba‐PWV could be a useful tool for identifying individuals at increased risk for CV events.22 This technique has become popular in Japan for evaluating arterial stiffness as a marker of vascular damage.23 Although ba‐PWV measurement appears to reflect the stiffness of central arteries, it has been criticized due to the fact that the pulse wave does not travel directly from the brachial arteries to the post‐tibial arteries in the same arterial tree. Furthermore, questions have been raised regarding the fact that TT does not correspond with any virtual brachial‐ankle distance.24 Finally, the sensitivity and specificity of this parameter in predicting coronary artery disease were only 62% and 29%, respectively.25, 26 More recently, the cardio‐ankle vascular index (CAVI), using the plethysmographic volume method, has been developed as a new index of overall stiffness of the artery from the origin of the aorta to the ankle. CAVI seems to be independent of BP at the time of measurement, although it is currently in the evaluation stage.14, 27, 28
We have proposed a new index (sum of PWV in 4 limbs) determined with a simple new device (VOPITB). This method does not require any specialized technique, and the examiner only has to wrap cuffs on all the limbs and place electrodes for ECG recording. The validity and reproducibility of the measurement of PWV were high. The brachial PWV–measured VOPITB can be used interchangeably with VOP evaluated by endovascular catheter. When evaluating the validity of noninvasive PWV measurements, comparisons should be made with PWV obtained by the catheter method using a catheter tip manometer in the arm and leg. However, this procedure is too invasive for clinical application. Therefore, we compared noninvasive PWV with PWV obtained by invasive measurements only in the humeral artery. Brachial PWV estimated with the VOPITB method correlated well with brachial PWV measured with the endovascular catheter. These results provided acceptable validation of noninvasive PWV measurement extrapolated to other arterial territories.29 Furthermore, as shown in Bland‐Altman plots, the reproducibility of the measurements made with VOPITB PWV are good. The coefficients of intraobserver and interobserver variation were similar regardless of the experience of the observer, which suggests that special skill is not necessary to perform this test.
A device similar to the VOPITB system was developed by Naidu and colleagues.30 However, these authors did not establish a good discriminative PWV measure for stratifying patients according to CV risk. Other devices have used a similar TT approach with ECG as a reference, although the sensors employed to detect the pulse wave make use of a different type of technology requiring a measure of skill in locating the pulse, and simultaneous PWV measurement in the arms and legs is not possible, thereby introducing error for comparisons of both extremities. With the VOPITB device, we can analyze different indexes simultaneously, and have established a new variable with a good discriminative capacity. In this context, the sum of PWV measurement in brachial and legs potentially allow us to evaluate the stiffness of the whole accessible arterial tree, avoiding measurements of deep arteries more difficult to reach that necessarily require more complex techniques. This makes the VOPITB a very simple device that can be used by anyone without prior training. The proposed system seems to have a greater ability to correctly classify patients according to their CV risk, improving the accuracy of the European Risk Score, because, in our study, the stratification of this comparison was enhanced with testing for assessing subclinical atherosclerosis, IMT, and cfPWV, which, combined, have proven to be useful in predicting future CV events.
Study Limitations
Our study has some limitations. We follow the Artery Society Guidelines,31 while to assess the validity of noninvasive hemodynamic measurement devices do not make invasive studies mandatory, seeking a higher accuracy we compared VOPITB with endovascular studies. Although the number and age of the patients were not exactly adapted to these guidelines, we believe they are adequate. Another concern is that to calculate PWV, the electromechanical coupling and isovolumetric time are added to the TT and this may lead to inaccurate PWV; however, we feel that this imprecision is clinically negligible. Likewise, these two arterial circulations do not share similar properties. The aorta is a central elastic artery, while in the case of the peripheral arteries (brachial, radial or femoral arteries), the muscular component is greater. In addition, most vessels are not linear, and therefore this measurement alters accuracy of such measures. Another possible bias was to consider a delay of arterial pulse wave due to the inclusion in the legs. However, the records are not different in the TT. Suggesting tree arterial occlusions can affect the perfusion pressure but not the speed of wave propagation through the artery wall. On the other hand, while age and sex are important determinants of PWV, their influence is more pronounced in the case of the central elastic arteries than in the peripheral muscular arteries.32, 33 Thus, normal values in different groups are necessary.
Conclusions
The results of the present study indicate that the sum of brachial and leg PWV determined with the VOPITB device may be a promising index for assessing arterial stiffness in humans. Probably the greatest advantage of brachial and leg PWV sum is that it can be easily measured by simply wrapping the 4 extremities with BP cuffs. Further studies to examine the relationship between the sum of brachial and leg PWV and CV disease morbidity and mortality are warranted.
Disclosures
All authors have submitted a patent application for VOPITB. Dr Juan F. Sánchez: Speaker and consultant for MSD, Astra, Pfizer, Boehringer Ingelheim, Almirall, Bayer. Research grants: Pfizer, Boehringer Ingelheim, Roche. Dr Jorge Vega: Speaker and consultant for MSD, Boehringer Ingelheim, Ferrer, Almirall.
Acknowledgments
The authors are grateful to Sebastian Romani, Raul Rodriguez‐Carreras, and Javier Portales, from Interventional Cardiology, Hospital San Pedro de Alcántara, Cáceres (Spain), for endovascular studies. This study was supported by grant PRIS09005, Plan de Investigación de la Junta de Extremadura 2009.
J Clin Hypertens (Greenwich). 2014;16:378–384. DOI: 10.1111/jch.12304. ©2014 Wiley Periodicals, Inc.
References
- 1. National Heart, Lung, and Blood Institute . NHLBI Fact Book, Fiscal Year 2010. Bethesda, MD: National Heart, Lung, and Blood Institute; 2010. http://www.nhlbi.nih.gov/about/factpdf.htm. Accessed February 25, 2014. [Google Scholar]
- 2. Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. [DOI] [PubMed] [Google Scholar]
- 3. Braunwald E. Shattuck lecture: cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. [DOI] [PubMed] [Google Scholar]
- 4. Peters SA, den Ruijter HM, Bots ML, Moons KG. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart. 2012;98:177–184. [DOI] [PubMed] [Google Scholar]
- 5. Kobayashi K, Akishita M, Yu W, et al. Interrelationship between non‐invasive measurements of atherosclerosis: flow‐mediated dilation of brachial artery, carotid intima‐media thickness and pulse wave velocity. Atherosclerosis. 2004;173:13–18. [DOI] [PubMed] [Google Scholar]
- 6. Taylor AJ. Atherosclerosis imaging to detect and monitor cardiovascular risk. Am J Cardiol. 2002;90:8L–11L. [DOI] [PubMed] [Google Scholar]
- 7. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 8. Vlachopoulos C, Aznaouridis K, Stefanadis C. Predicition of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 9. Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S–10S. [DOI] [PubMed] [Google Scholar]
- 10. Van Bortel LM, Stephane L, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30:445–448. [DOI] [PubMed] [Google Scholar]
- 11. Sigrist MK, Chiarelli G, Levin A, et al. Pulse wave velocity measurements are reproducible in multiple trained observers: a short report. Nephron Clin Pract. 2010;116:c60–c64. [DOI] [PubMed] [Google Scholar]
- 12. Rajzer MW, Wojciechowska W, Klocek M, et al. Comparison of aortic pulse wave velocity measured by three techniques: Complior, SphygmoCor and Arteriograph. J Hypertens. 2008;26:2001–2007. [DOI] [PubMed] [Google Scholar]
- 13. Tomiyama H, Koji Y, Yambe M, et al. Brachial‐ankle pulse wave velocity is a simple and independent predictor of pronognosis in patients with acute coronary syndrome. Circ J. 2005;69:815–822. [DOI] [PubMed] [Google Scholar]
- 14. Shirai K, Hiruta N, Song M, et al. Cardio‐ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. 2011;18:924–938. [DOI] [PubMed] [Google Scholar]
- 15. Meyer EB. Audibility of a CD‐standard A/D/A loop inserted into high‐resolution audio playback. J Audio Eng Soc. 2007;55:775–779. [Google Scholar]
- 16. American Diabetes Association . Standards of medical care in diabetes. 2012. Diabetes Care. 2012;35:S11–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The European cardiovascular disease risk assessment model. Low risk charts score. http://www.escardio.org/communities/EACPR/toolbox/health-professiona. Accessed February 1, 2012.
- 18. Meijer R, Grobee DE, Bots ML. Mannheim consensus on carotid intima‐media thickness: opposite and complementary points of view. Cerebrovasc Dis. 2006;21:415–416. [DOI] [PubMed] [Google Scholar]
- 19. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 20. Kullo IJ, Malik AR. Arterial ultrasonography and tonometry as adjuncts to cardiovascular risk stratification. J Am Coll Cardiol. 2007;49:1413–1426. [DOI] [PubMed] [Google Scholar]
- 21. London GM, Marchais SJ, Guerin AP, Pannier B. Arterial stiffness: pathophysiology and clinical impact. Clin Exp Hypertens. 2004;26:689–699. [DOI] [PubMed] [Google Scholar]
- 22. Yamashima A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of non‐invasive brachial‐ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. [DOI] [PubMed] [Google Scholar]
- 23. Amoh‐Tonto CA, Malik AR, Kondragunta V, et al. Brachial‐ankle pulse wave velocity is associated with walking distance in patients referred for peripheral arterial disease evaluation. Atherosclerosis. 2009;206:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Safar ME, O′Rourke MF. The brachial‐ankle pulse wave velocity. J Hypertens. 2009;27:1960–1961. [DOI] [PubMed] [Google Scholar]
- 25. Matsushima Y, Kawano H, Koide Y, et al. Relationship of carotid intima‐media thickness, pulse wave velocity, and ankle brachial index to the severity of coronary artery atherosclerosis. Clin Cardiol. 2004;27:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koyoshi R, Miura S, Kumagai N, et al. Clinical significance of flow‐mediated dilation, brachial intima‐media thickness and pulse wave velocity in patients with and without coronary artery disease. Circ J. 2012;76:1469–1475. [DOI] [PubMed] [Google Scholar]
- 27. Nye ER. The effect of blood pressure alterations on the pulse wave velocity. Br Heart J. 1964;266:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shirai K, Utino J, Otsuka K, Takada M. A novel blood pressure‐independent arterial wall stiffness parameter; cardio‐ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13:101–107. [DOI] [PubMed] [Google Scholar]
- 29. Bjarnegård N, Ahlgren AR, Sandgren T, et al. Age affects proximal brachial artery stiffness; differential behavior within the length of the brachial artery? Ultrasound Med Biol. 2003;29:1115–1121. [DOI] [PubMed] [Google Scholar]
- 30. Naidu MU, Reddy BM, Yashmaina S, et al. Validity and reproducibility of arterial pulse wave velocity measurement using new device with oscillometric technique: a pilot study. Biomed Eng Online. 2005;23:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilkinson IB, McEniery CM, Schillaci G, et al. ARTERY Society guidelines for validation of non‐invasive haemodynamic measurement devices: part 1, arterial pulse wave velocity. Artery Res. 2010;4:34–40. [Google Scholar]
- 32. Van der Heijden‐Spek JJ, Staessen JA, Fargard RH, et al. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: a population study. Hypertension. 2000;35:637–642. [DOI] [PubMed] [Google Scholar]
- 33. Chirinos JA. Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res. 2012;5:243–255. [DOI] [PubMed] [Google Scholar]


